Laparoscopic extended right hemicolectomy with D3 lymphadenectomy
Introduction
Laparoscopic extended right hemicolectomy with D3 lymphadenectomy has been recognized as the most challenging surgery among all colon procedures. This surgery involves an extensive scope and a number of important organs, where a clear understanding of the superior mesenteric vein (SMV) anatomy is needed to complete the thorough dissection. It is not only demanding for the surgeon, but also associated with a high requirement for the cooperation by the assistant and camera-holder. The slightest mistake may result in failure to achieve complete mesocolic excision (CME) and D3 lymphadenectomy, or even damage to the ureter, duodenum and other vital organs, or worse, superior mesenteric arteriovenous damage directly leading to fatal bleeding. We have accumulated the experience of experts from multiple centers across the country, and summed up with some of our own experience in this study to share with you.
Position
The patient is first placed supine with legs apart. For those with a small statue, both hands should be placed close to the body. Postural changes are required for a few times according to surgical needs (see below). The basic principle is to avoid the interference of the small intestine with the operative field by adjusting the position.
Operator positions
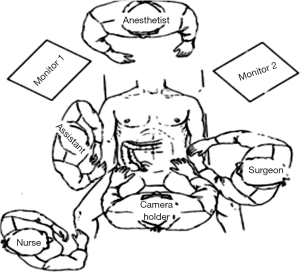
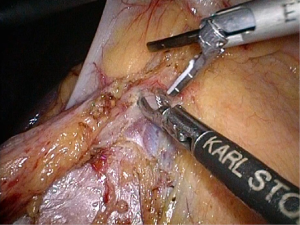
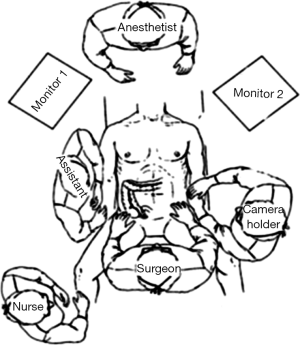
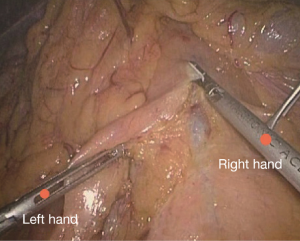
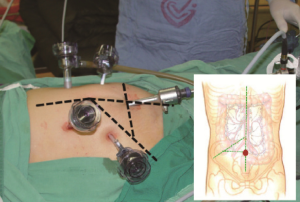
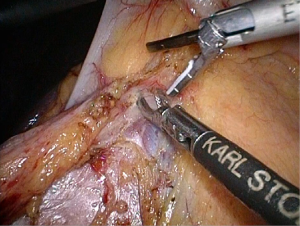
Positioning: in our center, the surgeon stands to the left of the patient; the primary assistant stands to the right, while the camera-holder stands between the patient’s legs (Figure 1). This positioning will facilitate the exposure and freeing of the pancreatic head, duodenal C curves and the hepatocolic ligament. However, due to the perpendicular relationship between the ultrasonic scalpel and SMV, it is more difficult to dissect the lymph nodes on its surface. A pair of dissecting forceps in the left hand can be used to aid (Figure 2). The other positioning is that the surgeon standing in the middle between the patient’s legs (Figure 3), which has just the opposite strengths and weaknesses compared to the above (Figure 4). I recommend that beginners can take the second positioning during lymph node dissection, and then change to the left side standing position for dissection of the pancreatic head and duodenum; at last, you can stand between the patient’s legs to continue dissecting the middle colonic vessels. Once you are skilled, the two positioning modes make no difference to the outcome of surgery.
Trocar positions
At present, the traditional five-port method is used.
Observation port
The location depends on the overall vision of the surgery. A rule of thumb is that the farther away from the surgical field, the better exposure of the whole picture is, but caution should still be given to close meticulous operation. If it is placed too close to the operative field, it will increase the difficulty of seeing the whole area, thus increasing the difficulty of operations by surgeons and assistants as well as the risk of injuring adjacent organs. Along the learning curve, we once made the observation port below the umbilicus and two fingers below the umbilicus, ignoring the patient’s body size. As a result, patients with a short abdomen were easy to operate, but for those with a longer abdomen, the camera had to be placed towards the left hand of the surgeon for observing the ileocecal part, interfering with the operation by the surgeon. They even needed to suspend the surgery just to adjust the position of the camera holder. After practice over a long time, we decided to choose the junction of the line connecting both McBurney’s points and the lower abdomen white line (about 4 cm below the umbilicus) as the observation port (Figure 5). In this way, the camera holder does not need to adjust his/her position for complete observation of the field of the right colon, which has less interference on the surgeon. For elderly and female patients, given the loose skin of the lower abdomen, subcutaneous fat thickness, peritoneum relaxation and urachus remains, even if a towel clamp is used to pick up the skin around the puncture point, a deep vertical length is often needed to successfully break through the peritoneum during needle puncture for pneumoperitoneum. Sometimes the entire needle needs to be inserted, and even so, there will be no strong peritoneal breakthrough feel. Inadvertently, there may be puncture outside the peritoneum. In this case, inflation may lead to widespread extraperitoneal emphysema, leading to open surgery. There used to be some cases with increased CO2 partial pressure, resulting in termination of surgery. Our experience is that in the case of exceptionally thick abdominal fat, we will separate the subcutaneous tissue through the white line under direct vision, and clamp the white line with curved forceps during the puncture, which will lead to a higher success rate than pulling the skin using a tower clip. After the puncture needle enters into the abdominal wall, it is directed to the umbilical part. Due to the relatively fixed position of the umbilical peritoneum, it is easier to puncture successfully. After successful pneumoperitoneum, a 10 mm trocar is vertically inserted. Before connecting continued air supply, it is recommended to insert the laparoscopic instrument first to identify whether the trocar has caused any injury in the abdominal cavity, and adjust the optimal position of the trocar by observing the side hole at the end of the trocar. In principle, the shorter the abdominal segment of a trocar, the less interference it will cause to the abdominal operation. It is recommended to make a single stitch on the abdominal wall to fix the trocar so that the movement of the camera will not bring the trocar away and consequently affect the surgery.
Working port
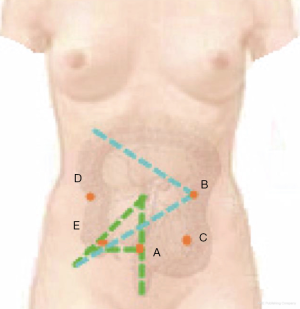
This is made at the conjunction between the left clavicular midline and the line connecting the hepatic flexure to the midpoint of the ileocecal part (B in Figure 6). This position makes it comfortable for both operations at the ileocecal region and the hepatic flexure. An auxiliary port is made 5 cm below the right subclavian midline, along the middle point at the line between bilateral anterior superior iliac spines and the umbilicus. The assistant port is basically opposite to the surgeon, located in the right abdomen. Each port should be at least four fingers away from each other to reduce mutual interference.
Surgical approach
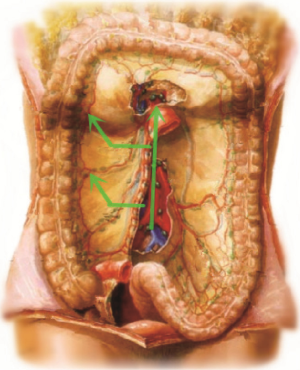
The procedure is approached from inside to outside and from bottom up (Figure 7). In other words, lymph nodes No. 3 on SMV are first treated and the vessels ligated, and the right mesocolon is freed outwards in a fanned shape. After that, the right part of the greater omentum is resected. Finally, the right paracolic sulcus is freed from up to down to meet the starting point. All operations for malignant tumors should follow the principle of non-tumor below. As for transverse colon tumors at or close to the hepatic flexure, D3 extended radical mastectomy is recommended (see the appendix).
Surgical procedures
We intentionally divide the surgery into the following 11 steps.
Step 1: abdominal exploration
The abdomen is explored from distant to proximal ends, with focus on the liver. If necessary, laparoscopic ultrasound probe can be used to identify the presence of any possible liver metastases, and frozen pathology can be employed to diagnose liver metastases. Finally, the primary lesion is explored.
Step 2: identifying anatomical projection
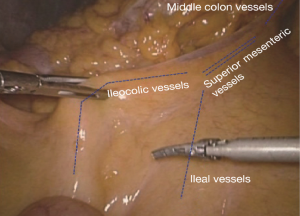
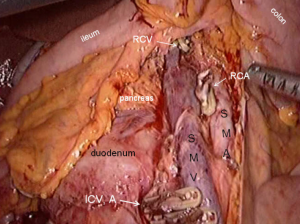
The operating table is adjusted so that the patient is tilted backwards with legs higher than the head. In this position, the small intestine gathers at the lower left quadrant, reducing interference to the operative field. The camera holder keeps the camera at 30° forward and downward, overlooking the upper abdomen from a far distance to expose the transverse colon and omentum so that the assistant and the surgeon can flip the omentum onto the top of the transverse colon to expose the surgical field, including the blood vessels in the colon, inferior mesenteric veins, projection of ileocolic vessels, horizontal part of the duodenum and so on (Figure 8).
The assistant uses his/her left hand to drag the transverse mesocolon to the head site. (The optimal clamped site is the mesangial avascular zone around the right branch of the middle colon vessels, so that the omentum can be blocked and the middle colon vessels can be pulled to maintain tension between the middle colon vessels and the superior mesenteric vessels). The right hand is used to clamp the ileocolic vessels, and pull, flatten and pick them towards the right bottom direction so that the ileocolic vessels—superior mesenteric vessels—middle colon vessels form a plane like a piece of canvas, in which a compact tension is maintained (Figure 9).
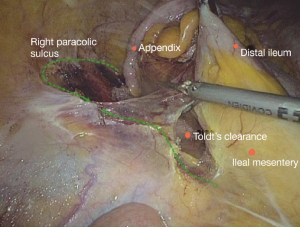
Step 3: looking for Toldt’s clearance, ligating the ileocolic artery (ICA/ICV) and dissecting lymph nodes No. 203
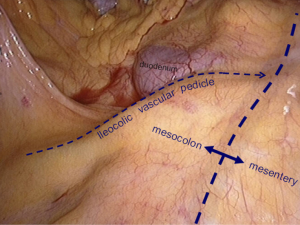
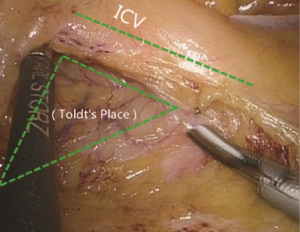
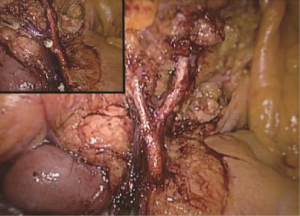
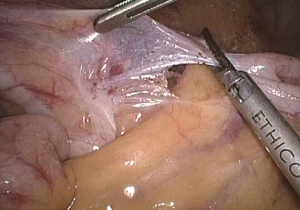
The conjunction between ileocolic vessels and SMV is exposed via step 1 (Figure 10). The ileocecal mesentery is cut with an ultrasonic scalpel from the distal to proximal end to enter the loose Toldt’s space (located between the right prerenal fascia and the ascending colic mesentery). Upon entering the correct Toldt’s space, the camera holder gradually adjusts the vision with the ureter at a horizontal position (Figure 11). During lymph node dissection and vascular separation, the vision can be adjusted with the SMV at the neutral position (6 to 12 o’clock). The assistant maintains stable traction, or insert the clamp in the left hand into the Toldt’s space to pick it up to further expose the gap (“mischief”). The surgeon follows the principle of priority on a plane, first bluntly separates towards the lateral paracolic sulcus and properly extends upwards and downwards while ensuring the intactness of the ascending mesocolon and anterior fascia of the right kidney. For some skinny patients, the right ureter and reproductive vessels may be visible but should not be separated, unless it is needed to confirm the presence of side injury. With continued separation along the Toldt’s plane towards the ICV and SMV angles, the ICV and ICA are skeletonized one after another (in which it is important to note if the relationship between ICV and ICA is variated, see upper right part of Figure 8), until the they enter SMV. The No. 203 lymph adipose tissue at the arteriovenous root of the colon is dissected and the SMV end needs to be completely revealed until the right wall of the SMA is visible. The roots of ICV and ICA are cut off with a biological clamp (Figure 12).
Step 4: expanding Toldt’s clearance
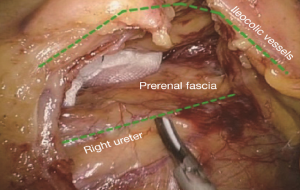
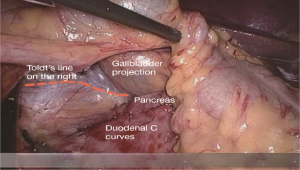
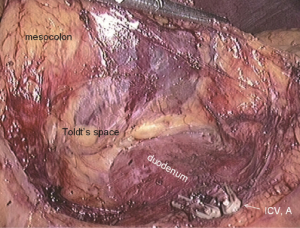
After cutting the ICA/ICV, the Toldt’s gap is revealed more fully, so that the mesangial ascending colon is gradually separated from the renal front fascia, duodenum, head of the pancreas and other tissue. The camera holder maintains the vision at right ureter horizontal position, adjusts along with the surgeon’s operation and gradually transfers it to the pancreas horizontal position. The fiber angle can be adjusted to facilitate the surgeon’s operation, but the camera body should always be remained at the retroperitoneal level. The assistant can insert clamps in both hands into the Toldt’s space and pick up the mesocolon in a reverse direction. The surgeon further expands the whole Toldt’s space down to the iliac artery, external to the Toldt’s line (i.e., the right paracolic sulcus), and up to the outside edge of the duodenal C loop for the moment, as long as the gallbladder can be vaguely seen through the mesocolon at this point (Figure 13). Now one or two pieces of gauze can be placed in the Toldt’s gap to stop bleeding with compression and separate vital organs such as the ureter, duodenum and head of the pancreas, to prevent injury later when separating the paracolic sulcus.
Step 5: transecting right colonic vessels (RCV) and middle colonic vessels (MCV), and dissecting the right side
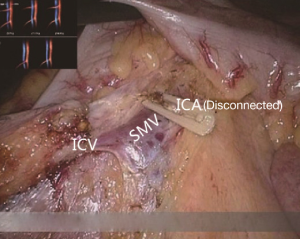
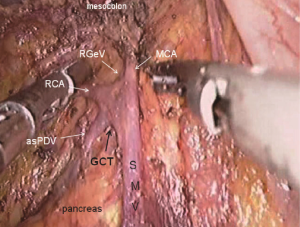
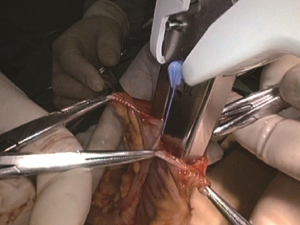
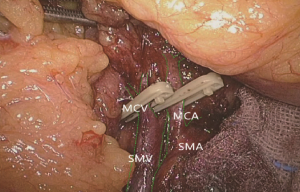
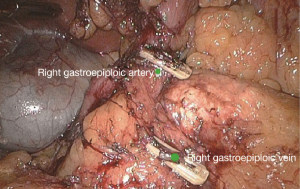
The camera holder adjusts the field of view to the SMV neutral position. The primary assistant pulls the transverse mesocolon with the left hand, and pulls the separated ascending mesocolon with a clamp in the right hand to fully expose the SMV and MCV junction area. During the surgery, holding a clamp in the right hand can help the surgeon to perform fine separation. Holding a separation clamp in the left hand is conducive to fine separation by the surgeon. When separating the SMV vascular sheath, blunt dissection can be done down to up with dissection forceps in the left hand, with the right hand holding an ultrasonic scalpel to cut the tissue in the middle of the clamp teeth (Figure 14). The protective tip of the ultrasonic scalpel should be placed close to vessels while the working surface away from them to avoid accidental injury. Using this method, the surgeon can separate upwards closely along the SMV anterior part until the Henle stomach and colon common joint and have it skeletonized. Meanwhile, the lymph nodes around the surgical root are dissected to expose the right colon vein and right gastroepiploic vein, as well as various SMV branches, and the root of the right colon vein is clamped and cut off. The superior mesenteric lymph nodes are dissected left to the vein at the level of the right colic artery branch where the superior mesenteric artery is originated, and the arterial root is clamped and cut off at this level.
Step 6: dissecting lymph nodes at the root of the middle colonic artery, and cutting off the right branch of the vessel
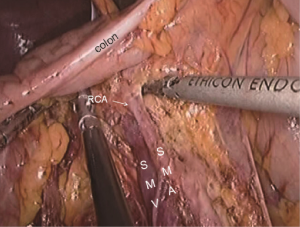
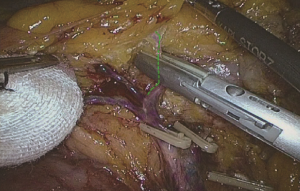
The camera holder adjusts the vision at the pancreas horizontal position. The primary assistant pulls the transverse mesocolon towards the head side, holds a clamp in left hand to pull the left side of the mesocolon, and pull its right side with a clamp in the right hand, flatten it so that the mesocolon maintains tension, revealing the roots of the transverse mesocolon. The surgeon continues to divide tissue upward along the SMV, the purpose being to find the middle colic artery and vein as well as their right and left, and to dissect the lymph nodes to the right of the vascular root. Due to the length of the transverse mesocolon, it is difficult to expose the laparoscopic surgical vision, and there are multiple starting points and variations of the middle colonic artery (Figure 15). In laparotomy, the arterial pulse can be felt through tactile sensation to determine the position of the vessel, and the course of the vessel is visible through light projection, and the omental bursa can also be cut to determine the relationship between the pancreas and middle colonic blood vessels. However, with the laparoscopic approach, the superior mesenteric artery is the only thing that can be relied on to predict the location of the root of middle colonic blood vessels, which are sometimes hardly distinguishable from the RCV (Figure 16), leading to mistaken litigation. It will be easy for surgery in skinny patients if colonic vessels can be directly seen through the mesentery, but for obese patients, particularly when lymph nodes are enlarged and fused here, it is difficult to distinguish the starting point and course of the middle colonic vessels. This is an inherent “flaw” of laparoscopic surgery! Therefore, many young doctors often stop here or just blindly separate until injury occurs, and have to perform regular dissection of the right half colon without being able to retain the left branch of the middle colonic vessels, leading to compromised blood supply to the left colon and excessive bowel resection. We usually use separate against the SMV until MCV with a separation clamp (Figure 14). When lymph nodes are enlarged, however, it is not easy to do so. We explore the following surgical methods. Since the duodenum and head of the pancreas are basically exposed, we usually do not hurry to separate the middle colonic vessels along the superior mesenteric artery vein. Instead, we separate the lower edge of the pancreas towards the left Treitz’s ligament first. Since the ligament is loose and structurally constant, there is no interference from major blood vessels or enlarged lymph nodes. The lower edge of the pancreatic body can be easily exposed along the jejunum wall. With exposure to the pancreatic body and head of the pancreas, the location of the middle colonic vessels becomes very clear. At the same time, clear exposure of the pancreas can also help to block the superior mesenteric artery beneath, reducing the risk of injury. By this “encircling” approach with three-dimensional separation of the roots of the colon lymphatic vessels in adipose tissue, the third group of lymph nodes can be successfully dissected in the middle colonic vessels while retaining its left branch (Figure 17). After ligation of the left branch of the middle colic artery, the Toldt’s space in the left upper quadrant can be further extended until the dark green gallbladder can be seen through the transverse mesocolon (Figure 18). One to two pieces of gauze can be placed in the gap to stop bleeding by compression and also as a marker of the correct layer to reduce accidental injury in the separation of the hepatocolic ligament.
Step 7: cutting off the gastrocolic ligament and hepatocolic ligament
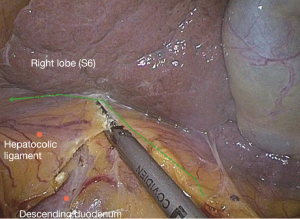
Next, turn to the superior transverse colon area so that the transverse colon is hanging downwards to the left naturally. The camera holder uses a far vision maintaining the hepatic horizontal position or horizontal transverse colon position. The primary assistant uses both hands alternately to stretch the greater curvature to the left, to find the “avascular zone” between the left and right gastroepiploic arteries. The assistant uses a clamp in both hands to lift the gastric wall to the head side, with the left hand holding the distal wall and right hand the proximal side, and expand it to maintain tension. The surgeon uses the left hand to control the omentum, and identify from outside the vascular arch, the weakest point of the gastrocolic ligament to cut into the omental bursa, and then “pull while cutting” from left to right along the greater curvature of the stomach to cut off the right gastrocolic ligament (Figure 19). After entering the omental sac, the right transverse mesenterium is cut at the root of the pancreas. Gauze placed before can be seen, and cross from left to right the descending duodenum to the hepatocolic ligament. It is recommended to cut the hepatocolic ligament close to the hepatic edge, particularly when resecting a hepatic flexure tumor.
Step 8: freeing the hepatic flexure of the colon and ascending paracolic sulcus (i.e., Toldt’s line)
Because the ascending mesocolon has been isolated by separation of Toldt’s space, it is easier to free the hepatic flexure and descending paracolic sulcus.
When freeing the hepatic flexure, the camera holder should insert the camera to the right upper quadrant, with the fiber dialed at 1 to 2 o’clock position, keeping the liver in the horizontal position as much as possible. When reaching the descending paracolic sulcus, the vision can be adjusted to the horizontal position of the paracolic sulcus. The primary assistant needs to pull the hepatic flexure inwards and downwards to expose the hepatocolic ligament and right phrenicocolic ligament and maintain tension. The surgeon divides the hepatocolic ligament and right phrenicocolic ligament along the lower edge of the liver and the anterior renal fascia surface, separate the hepatic flexure and cut off the side peritoneum (Figure 20). For patients with hepatic flexure tumors, if the serous membrane is already involved, the anterior part of the right renal fat capsule should be cut until the thin and tough fibrous membrane covering the kidney is exposed. Because the Toldt’s space posterior to the right mesocolon has been completely exposed and marked with gauze, this steps is aimed at opening the above “membranous” colon fixation device, so that the whole process can be done “in one snap” (Figure 20).
Step 9: cutting the ileocecal part and peritoneal return outside the ascending colon, as well as the upper and lower bout
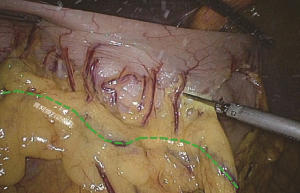
The patient should be adjusted to a position with the head lower than the legs and tilted left. The intestine is then gathered to the left upper abdomen to facilitate exposure of the surgical field. Under direct vision of the ileocecal region, the camera holder adjusts the fiber to 10 to 11 o’clock in favor of observing the ascending paracolic sulcus. The primary assistant needs to operate in a mirror reverse way with clamps in both hands retracting the ileocecal region, revealing the ascending paracolic sulcus while maintaining tension. The surgeon cuts the lateral side of the peritoneum at the ascending ileocecal region (Figure 21). The ascending colon is pulled to the left side to the middle line, and the lateral peritoneum is separated along the right paracolic sulcus from the iliac fossa to the hepatic flexure, joining the upper part. So far, all the surgical dissection has been completed and “harvested”. All right colon fixation ligaments are cut (Figure 22), and it is ready for the next step of vitro resection and anastomosis.
Step 10: resecting the right colon and creating an ileotransverse anastomosis
The pneumoperitoneum is removed and the patient adjusted to the supine position. A vertical incision is made through the rectus abdominis in the right upper quadrant based on tumor size, just allowing the removal of the tumor.
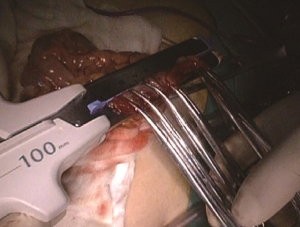
A plastic sleeve is placed to protect the incision so that tumor cells may not fall in it and grow. The right colon is dragged out of the body. It is recommended to remove the omentum first, which will be more conducive to removing the tumor. Try not to rotate the intestine to avoid reverse during anastomosis. Under direct vision, transect the transverse colon, and ensure that the bowel resection margin is >10 cm from the edge of the lesion, including all dissected D3 lymph nodes. Remove the right colon, including 10–15 cm distal ileum, the right colon where the tumor is located, colorectal mesentery and adequate bowel segments, and remove specimens. If the tumor is large, a cutter can be used to cut the intestines in the body, thus reducing the length of the abdominal incision. In our center, we often use a linear stapler for functional anastomoses between the ileum and the colon, which does not damage the vascular arcades at the bowel edge, and will not lead to anastomotic stricture. More importantly, the differences in the ileum and colon diameters do not matter. After completion of the anastomosis, it is recommended to add one to two stitches in the intestine junction 2 to relieve creeping tension of the anastomosis. Caution should be made to the risk of anastomotic bleeding. For high-risk groups, it is recommended to use the 8-shape whole layer suture to prevent anastomotic bleeding. The free edge of the transverse mesocolon and the ileal mesentery can be either closed or remained open. Finally, check the anastomotic patency and presence of torsion (Figures 23-25).
Step 11: washing, checking, placing and drainage
Close the small incision, re-establish pneumoperitoneum, wash the abdominal cavity with saline, and check if there is bleeding from the wound, bowel or without tension, anastomotic leak, and so on. Pay attention to place the intestines properly to prevent intestinal hernia. After confirming there is no active bleeding, place a drainage tube in the right paracolic sulcus led out from the pole in the right lower quadrant (Figures 26,27). At present, due to implementation of ERAS, if the surgery is satisfactory and there is no contamination of the surgical field, the laparoscopic drainage tube can be waived.
Summary
All laparoscopic colon surgery should follow the four key principles: identifying blood vessels, dissecting lymph nodes, going through the gap, and separating the colon. The key points in the implementation of D3 colon surgery include: non-contact operation, transection at vascular roots, main lymph node dissection, precise gap separation and adequate bowel resection. In the right laparoscopic colon surgery, we must adhere to the principle “from the center to the surrounding, from lymph nodes to vessels, and from veins to arteries”. First, transecting the draining lymph nodes of the bowel tumor segment followed by vessels helps to reduce tumor blood and lymphatic metastases during the surgical procedure due to squeezing. Intrathecal separation along the vascular sheath in operation is safe and thorough. In summary, a deep understanding of the fascial anatomy, especially the integration gap, skillful mastery of surgery, and tacit cooperation of the surgical team can make laparoscopic colorectal surgery smoother. With the integration of modern surgical equipment, high-definition cameras and development of energy platforms, laparoscopic colorectal surgery can become a pleasing art.
Supplementary
Laparoscope-assisted extended right hemicolectomy with D3 lymphadenectomy
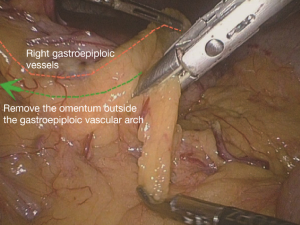
Extended D3 resection is needed for right colon resection in transverse colon cancer distal to the hepatic flexure. On the basis of the above standard right colon resection, the following three main steps are added: (I) ligation of the starting point of the middle colic vein and artery at the root (Figure S1); (II) No. 6 lymph node dissection, ligation of right gastroepiploic artery and vein. When we find the colonic vein along the SMV, the next step is to find the gastrointestinal trunk. In extended radical mastectomy, it is basically need to litigate the right gastroepiploic vein entering the gastrointestinal trunk (Figure S2). Afterwards, when cutting the right omentum, the gastroepiploic artery is litigated at the root (Figure S3); (III) resection of most part of the right greater omentum in the vascular arch of the greater curvature (Figure S4).
Supplementary
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2017.02.24). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
Cite this article as: Hu W, Zheng J, Li Y. Laparoscopic extended right hemicolectomy with D3 lymphadenectomy. Ann Laparosc Endosc Surg 2017;2:120.