
Mesh technology
Inguinal hernia repair is one of the most routinely performed procedure in surgical practice. Today, mesh repair for management of inguinal hernia is considered standard in most countries and almost universally accepted to be superior to primary suture repair. Surgeons and researchers continued to evolve the technique to look for ways to improve the surgical outcomes. Over a period of time the surgical techniques have reasonably standardized. Most of the research and innovation in hernia repair worldwide now focusses on improvement in mesh technology. Today, the surgical community is faced with the difficulty of choosing from a variety of prosthetic materials from an array of manufacturers. Every year new meshes are introduced, making it difficult for the surgeon to decide the most suitable prosthetic to be used.
History of mesh use in inguinal hernia repair
Over last 6 decades our understanding of the biological basis for development of hernia has improved. This has led to advances in the type of surgical repair used and more importantly the widespread use of prosthetics in hernia repair. When introduced for the first time, hernia prosthetics were made up of metal. Phelps used meshes braided with silver wires in 1894, followed by Goepel, Witzel and Perry in 1900 (1-4). These meshes were not ideal and produced stiffness. A toxic compound silver sulfate was reported to form on its surface. As a result, these meshes were modified and replaced by braided stainless steel meshes (5-7). In the latter half of 19th century prosthetics made of tantalum gauze, prefabricated nylon and perlon meshes were used by different surgeons (8-13). However, problems were reported in all these meshes. While tantalum gauze reported higher rates of infection, nylon was reported to break apart, an intense inflammatory reaction was reported in perlon and hence were abandoned.
In the 20th century, multiple biomaterials were used such as nylon mesh, silicon, polyvinyl sponges, orlon cloth and teflon meshes but were abandoned due to sub-optimal results or complications (14). The start of 2nd world war made steel and tantalum costly and scarce due to its use for military equipment. This combined with the development of plastic industry pushed fabricators and hernia surgeons towards considering new prosthetic materials. This ultimately led to the development of polypropylene, polyester and the expanded polytetrafluoroethylene (ePTFE). Most available prosthetic meshes in use today are based on these materials (13,15,16).
Why do we need a mesh for inguinal hernia repair?
The two basic principles in inguinal hernia repair are to restore normal anatomy and avoid tension during this process. Main long-term goal is to avoid recurrence. Mesh or prosthetic material acts as a scaffold which provides mechanical strength to the fascial and muscle tissues. Detailed knowledge of wound healing, the reasons for hernia formation and the physiologic response to prosthetic material is needed to fully understand the role of mesh.
Biologic response to mesh
Wound healing is a complex and dynamic process, and hence understanding the same is important to any surgeon attempting hernia repair. Wound healing involves a sequence of events. Once implanted there is protein adsorption around the prosthesis forming a coagulum. This is formed by combining together of albumin, immunoglobulins, plasminogen, fibrinogen, complement factors (17,18). This coagulum causes platelet adherence, which attracts polymorphonucleocytes (PMNs), fibroblasts, macrophages and other platelets by releasing chemoattractants. This process is affected by a number of patient factors, type of prosthesis, medicines, presence of infection and some unknown factors. An intense foreign body reaction follows ultimately leading to collagen deposition in the extracellular matrix. The collagen undergoes transformation from immature to mature collagen over a period of time increasing its strength. The strength of collagen increases gradually till 6 months, achieving 70–80% of strength of native connective tissue (19,20). It never completely regains the original strength of native tissue, and hence the basis for use of prosthesis which provides permanent support.
Classification of meshes
Variety of factors assist a surgeon while choosing a mesh or prosthetic. Mesh can be classified based upon the material used and mesh design. Based on the material used and biological activity mesh can be broadly classified into synthetic and biologic. Synthetic mesh can be further classified into synthetic non-absorbable, coated non-absorbable and partially absorbable.
In 1997, Amid (21) classified biomaterials based on the porosity of mesh into 4 types:
- Macroporous >75 micrometer;
- Macro with microporous;
- Microporous;
- Submicronic pores.
But in late nineties as multiple materials and meshes came into the market researchers moved away from the concept of porosity towards the concept of weight of mesh and density. Coda et al. (22) classified meshes based on weight into:
- Ultralight ≤35 gm/m2;
- Light weight 35–70 gm/m2;
- Standard 70–140 gm/m2;
- Heavy ≥140 gm/m2.
But biological and host response to mesh and the material used are far more complex. Architecture of mesh and its design also plays a vital role in this response based upon factors such a film, fleece and filament structure. Weyhe et al. in 2006 compared heavy and light weight meshes (23). They concluded that less weight does not essentially mean less biological response. Similarly, some heavy weight meshes showed good biocompatibility, probably due to large pore size by avoiding bridging effect of scar. Reduced bridging was associated with reduced mesh contraction (24,25). As our understanding of the biologic response improved it was clear that multiple factors interact to modify the biological response to mesh and in turn host compatibility. In 2012, Deeken et al. further sub-classified meshes having additional barrier function, mainly for intra-abdominal use (26). Researchers have also tried to classify meshes based on their biomechanical stability and elasticity. But there are limitations as these shows marked anisotropy i.e., different values are seen in different axis. This in turn prevents reliable comparison between different meshes (27-30).
To overcome these limitations, Klinge et al. in 2012 (31) grouped meshes into different classes based on textile data taking into consideration porosity and effect of anisotropy over the same. They put forward the concept of effective porosity and classified meshes as follows:
- Large pore mesh (textile porosity of >60% or effective porosity of >0%). They further sub-classified it into:
- Monofilament;
- Multifilament;
- Mixed structure or polymer i.e., combination of absorbable with non-absorbable or different varieties of non-absorbable materials.
- Small pore mesh (textile porosity of <60% and without any effective porosity). Further sub-classified it into:
- Monofilament;
- Multifilament;
- Mixed structure or polymer.
- Meshes with special features e.g., covered meshes or composite meshes for intra-abdominal use or meshes with surface coatings.
- Meshes with films e.g., meshes without porosity, submicronic pore size
- 3D meshes e.g., Pre-shaped or 3D devices. These were separated from flat meshes.
- Biological meshes: these can be further classified onto absorbable or non-absorbable and based on source into synthetic or biological. They further sub-grouped into
- Non-cross linked;
- Cross linked;
- Special features.
Different materials used for preparing meshes
Before deciding on the type of mesh to use, the surgeon needs to have an understanding of the different raw materials that are used for preparing meshes. Factors such as bioreactivity and ease of handling a mesh play a vital role particularly in laparoscopic repair of inguinal hernia where mesh handling characteristics may affect operative times and results.
Synthetic non-absorbable
Most commonly used meshes for inguinal hernia repair are made up of polypropylene, polyester or polytetrafluoroethylene (PTFE). Based on the polymer used the biological response induced varies (32). Polypropylene, a polymerized ethylene was developed by Giolo Natta, an Italian scientist in 1954 is the most commonly used polymer in surgical practice. The strength of the prosthesis depends upon the position of methyl group attached to the ethylene during the process of polymerization. When all the methyl group are attached on the same side it provides maximum strength (33). The biological response induced by the mesh further varies depending upon the pore size, type of filament, weight in addition to host response. Polypropylene is hydrophobic by nature, neutral electrostatically and shows significant resistance to biological degradation. Biological reactivity induced will vary according to the weight, effective porosity, size and type of filament, mesh design, presence of coating and individual host response (32). In order to reduce the inflammatory reaction, most surgeons prefer to use more porous light weight meshes. This also helps in preventing bridging of scar (34,35). However, the debate is far from settled regarding the most ideal prosthesis and most surgeons have individual preferences during selection. Polypropylene induces an intense biological response causing protein adherence and leading to scar tissue formation. The scar formation also leads to prosthetic contraction. The same response leads to adhesions at the interface between the mesh and host tissue. Intense adhesions and scar formation are common reasons for operative site discomfort and chronic pain after inguinal hernia repair (36,37). When in contact with intra-abdominal contents e.g., bowel these adhesions can lead to intestinal obstruction or fistulisation in the worst-case scenario. Hence the need to use composite or meshes with a barrier when used intra-abdominally. Properties of the mesh material and individual host response play a vital role (38-41).
Polyethylene terephthalate, commonly called as polyester is made up of ethylene glycol and terephthalic acid. Polyester is hydrophilic in nature and hence a propensity to swell when in contact with tissue fluids. Though it induces similar biological response as polypropylene, polyester is known to degrade with time. This effect is accentuated in an infected environment (42).
Other material more commonly used for ventral hernia as compared to inguinal hernia is polytetrafluoroethylene (PTFE). It was discovered in 1938 by Roy Plunkett, accidentally while researching on a gas refrigerant—tetrafluoroethylene. They found the gas to polymerize spontaneously into a wax like powdery material. It was not until 1958 when William Gore applied it for manufacturing PTFE meshes. PTFE has poor tissue incorporation and a tendency to be encapsulated by the scar tissue. The microporous nature prevents passage of protein coagulum but allows bacteria to pass through, and hence once infected it is difficult to clear the bacteria. Hence, an infected PTFE mesh should be explanted. As a result now PTFE is expanded to produce a uniform structure called ePTFE. Though not commonly used for inguinal hernia repair, ePTFE is preferred for intra-abdominal use. Particularly when doing combined repair of inguinal and lower abdominal ventral hernia.
Other materials experimented on are polymers like carbon fibres and polyvinylidenflouride, but never gained popularity for commercial use.
Coated non-absorbable meshes
In order to improve the host compatibility to mesh and improve the tensile strength of the prosthesis, many commercially available meshes today provide an absorbable or non-absorbable coating over polyester or polypropylene meshes (43). The coating is thought to reduce protein coagulum adherence leading to a reduced inflammatory response. This in turn should reduce mesh to tissue adhesions and hence lower the incidence of chronic pain. Also by reducing mesh shrinkage it is thought to reduce recurrence rates (44).
A variety of meshes are available worldwide. Different coating layers such as omega 3 fatty acids (O3FA), titanium, monocryl, PVDF or hyaluronate are used (45). But all these meshes have their own set of complications (46).
- C-QurTM and C-QURTM Centrifix mesh (Atrium medical) is an O3FA coated filament polypropylene mesh. The O3FA is derived from a highly purified pharmaceutical grade fish oil, which is a blend of triglycerides and O3FA. In roughly 3 months about 70% coating is thought to get absorbed and hence potentially a reduced biological response. Comes in weight of 50 or 85 gm/m2.
- Titanized polypropylene meshes: two meshes are marketed for surgical use. TiMesh (GFE Medizintechnik, Nurnberg, Germany) and TiO2 Mesh (Biocer, Bayreuth, Germany). The polypropylene filament in TiMesh (47 gm/m2) is coated by a 30 micrometer thick titanium layer using plasma activated chemical vapor deposition technique. Titanium is known to have good biocompatibility and should lead to reduced adhesion. It comes in different tensile strength based on filament size (strong—120 micrometer, light—90 micrometer and extra-light—65 micrometer). In TiO2 mesh the polypropylene filament is coated with titanium dioxide. This coating is thought to be hydrophilic and hence provides a self-adhesive effect. It is large pored and provides tensile strength of about 55 N/cm. But most clinical studies in literature have been performed on TiMesh.
- Glucamesh (Brennen Medical, St. Paul, Minnesota) is a polypropylene mesh (50 gm/m2) coated with oat beta glucan, a complex absorbable carbohydrate.
- Dynamesh (FEG Textiltechnik, Aachen, Germany) is a polypropylene mesh covered with polyvinylidene fluoride (PVDF) filament.
- Other meshes mainly used for intra-abdominal use can be occasionally used for inguinal hernia repair are made of ePTFE (two-sided DualMesh, IntrameshT1, Dulex and Composix). DualMesh coated with silver-chlorhexidine film which acts as an anti-microbial agent is also available.
- Parietene Composite (Covidien, Mansfield, USA) has woven polypropylene covered with oxidized collagen film over one side to protect the viscera. VentralightTM (Bard, Davol Inc., Warwick, RI, UK), uses a hydrogel barrier and bioabsorbable polyglycolic acid to cover the polypropylene mesh.
Partially absorbable meshes
In order to reduce the amount of foreign body and in turn the inflammatory response, researchers came out with meshes made up of mixed polymers which contain both absorbable and non-absorbable components. Challenge is to maintain the tensile strength of the mesh once the absorbable component degrades. The other advantages of partially absorbable mesh are lighter weight and larger pores post degradation of absorbable component. Though most clinical studies have shown conflicting results, both animal as well as human studies have not documented reduced inflammatory or biological response when partially absorbable meshes were compared with absorbable counterparts (46-48). In fact, O’Dwyer et al. in a randomized trail comparing partially absorbable polypropylene-polyglactin (Ethicon Vypro II) to standard polypropylene (Atrium Medical) during standard open Lichtenstein repair reported a significant increase in the hernia recurrence rates (5.6% to 0.4% respectively) in the partially absorbable mesh. Most other clinical parameters were found to be comparable at 12 months (46). Further randomized control trials with larger sample size and longer follow up periods would be needed before reaching any definitive conclusions on partially absorbable meshes.
It is important to achieve an ideal balance between the weight, density and porosity with respect to the tensile strength of mesh post partial absorption. The most commonly used partially absorbable meshes are Vypro II (Ethicon, Johnson and Johnson) and UltraPro (Ethicon, Johnson & Johnson) which is made up of polypropylene and poliglecaprone (Monocryl). These meshes are used widely with suggested advantages like large pores, light weight, reduced biologic response hence greater elasticity and flexibility (49). This should also theoretically lead to lesser shrinkage, less pain and possible less infection when compared to their heavier counterparts. But partially absorbable meshes still show complications like recurrence, infection and adhesion formation (45).
Biological meshes
The intent to further reduce the complications associated with polymers and also in special scenario e.g., in presence of infection/contamination, lead to the development of biological meshes. Usually these consist of collagen scaffolds, which are supposed to help surrounding connective tissue and cells to bridge and cover the hernia defect. Meshes are made from a variety of tissues like decellularized human, porcine or bovine dermis, porcine small bowel submucosa, bovine pericardium. Most of these meshes will get absorbed by 3 months. During commercial production, these can be further subjected to chemical crosslinking which increases the mesh persistence, and hence mesh may take more time, sometimes up to a year for complete re-absorption. Any newly formed connective tissue matrix will only regain 70–80% of native strength and hence a theoretically high risk of recurrence. High cost is a major issue with biological meshes. As a result, use of these meshes is limited to selected case scenario and in investigational studies.
Commonly available biological meshes are
- Surgisis (Cook Biomedical, Bloomington, USA) derived from submucosa of porcine small bowel;
- Permacol (Covidien, Norwalk, CT, USA) consist of porcine dermis which is processed with diisocyanate and sterilized by gamma irradiation;
- Collamend (Davol Inc., Warwick, RI, USA) consists of freeze dried porcine dermis;
- XenMatrix (Brennen Medical, St. Paul, MN, USA) consists of non-crosslinked porcine dermis which is sterilized by E-beam radiation;
- Tutopatch (Tutogen, Alachua, FL, USA) is derived from bovine pericardium which is subjected to multiple chemical process and gamma irradiated for sterilization. Similarly, Veritas (Synovis Surgical innovations, St. Paul, MN, USA) pericardium of young cows;
- Cadaveric allografts are also commercially available after processing. Alloderm (LifeCell Corporation, Branchburg, NJ, USA) is derived from cadaveric skin. The skin is then processed taking care not to damage the extracellular matrix. Deoxycholate is used to remove remnant cells. After cleaning and lyophilization what remains is sheet of extracellular matrix mainly made up of collagen, elastin and laminin. This is preserved by freeze drying. So before use we need to soak it in saline to defreeze. Allomax (Tutogen Medical Inc., Alachua, FL, USA) and marketed by Davol, processes human dermis to produce an acellular sheet which acts as a scaffold;
- Researchers have also used scaffold made up from musculoskeletal tissue. Use of various growth factors e.g., fibroblast growth factors to stimulate collagen synthesis has been attempted in animal studies.
Meshes based of design
The search for an ideal mesh continued, along with the search of an ideal technique for hernia repair. With advent and acceptability of laparoscopy for hernia repair, researchers and manufacturers have now turned their attention towards the mesh design. Two main factors taken in to consideration were mesh handling during laparoscopy, ease in positioning of mesh and if possible reduce or completely avoid the need for using fixation devices. This also lead to advent of different mesh designs for open inguinal hernia repair, particularly meshes which can be inserted through smaller incisions and avoid extensive dissection.
Meshes with special design used in laparoscopy
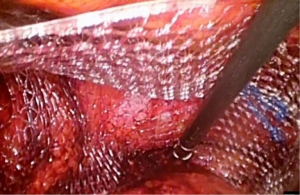
With greater acceptability and popularity of laparoscopy in recent years most research on meshes in inguinal hernia repair has focused on mesh design. Meshes with anatomical design to conform to the shape of inguinal anatomy were manufactured. 3D MaxTM mesh (Bard Davol, USA) is made up of polypropylene light weight monofilament with large pore size (Figure 1). It has anatomical design with sealed edges and a medial orientation marker to facilitate placement. It comes in three different sizes medium, large and extra-large. Its contour is supposed to minimize buckling and possibly reduce need for fixation. Design is different for right and left side, and so marketed as side specific. C-Qur TM CentriFX (Atrium medical, USA) also made from polypropylene has a light spray coating of O3FA to reduce inflammation (Figure 2). This has an anatomical contour but with invertible design, hence the same mesh can be used on both sides. Similar to 3D MaxTM mesh (Bard Davol, USA) it is supposed to ease handling and possibly reduce need for fixation.

Covidien came out with different designs of polyester meshes specially designed for laparoscopic use. ParietexTM (Covidien) anatomical mesh designed to fit the contour of the inguinal canal. ParietexTM ADP2 (Covidien) mesh again made up of polyester, side specific design. It had a lateral slit and prefixed suture so as to encircle the cord structures.
With the intent to reduce chronic groin pain, both after open as well as laparoscopic approach, possibly by avoiding need for mesh fixation, self-gripping mesh was devised. EaseGripTM (Covidien) is a three-dimensional elliptical shaped mesh made up of polyester. It has a lateral slit with an adjustable self-gripping flap. They also manufacture conventional self-gripping flat mesh. Parietex ProgripTM (Covidien) made up of polyester monofilament and polylactic acid is a lightweight self-gripping mesh. Initially devised for use in open Lichtenstein repair, its use has gained popularity in laparoscopic inguinal hernia repair. Though technically challenging to handle laparoscopically, most surgeons with experience have devised strategies to safely place the mesh (50). Safety of self-gripping meshes have been proved in different studies (51,52). To improve ease of handling and placement of mesh Parietex ProGrip LaparoscopicTM (Sofradim production, Covidien) was released. It is made up of monofilament polyethylene terephthalate, which is covered with two layers. The first layer is microgrids made from polylactic acid which is slow absorbing. While the second layer is fast absorbing made from a combination of collagen and glycerol which is supposed to get absorbed in a day.
Special mesh designs for open inguinal hernia repair
Flat meshes are used commonly in standard open Lichtenstein inguinal hernia repair. But as some surgeons prefer placing prosthesis in the posterior space, leading to development of multiple prosthesis which are “plug and patch” devices. This technique where partial posterior repair is combined with anterior was popularized by Rutkow and Robbins (53). Similarly some surgeons use self-gripping flat meshes so as to avoid fixation stiches and also reduce operative time. The Perfix plug (CM Bard, New Jersey) was made up of two components, a conical plug which is placed over the defect and a flat mesh which is placed like any other flat mesh in a standard anterior repair. Prepared from polypropylene and heavy, there were reports of mesh contraction with possible recurrence, plug migration, adhesions with intra-peritoneal structures (54-56). This leads to an array of new meshes in the market. ProLoop Ultra plug (Atrium Medical) a light weight polypropylene with large pores, had a design which help to grip the tissue with supposedly less migration or contraction. Gore Bio-absorbable plug (W.L. Gore and Associates) used an absorbable plug with a non-absorbable flat mesh made up of combination of polyglycolic acid and trimethylene carbonate. The absorbable plug avoided fear of adhesions with intra-peritoneal contents.
Ethicon introduced the Prolene Hernia System (PHS), made from heavy polypropylene. It had three components, an anterior oval shaped flat mesh, a flat round shaped pre-peritoneal mesh and both are connected to each other by a cylindrical column. The UltraPro Hernia System (Ethicon) has similar design as PHS but is lightweight and partially absorbable. For surgeons who prefer to place mesh in the preperitoneal plane during open mesh repair, particularly for large inguinal hernia or bilateral hernia. The Giant prosthetic reinforcement of the visceral sac technique wherein any flat mesh made from polymer of choice is used. Posterior space is created through a Pfannenstiel or a lower midline incision. The Bard Kugel Hernia Patch (CM, Bard) is made from heavyweight polypropylene. It has two layers, a polypropylene covered with another recoil ring also made of polypropylene.
Prosthesis with emphasis on cost
Cost is a major factor and has a significant impact on the healthcare expenses in developing countries. Efforts are underway to create a cheap alternative which is safe, easily available and feasible for mass production. Use of sterilized mosquito nets for inguinal hernia repair has been reported from India as well as Africa, with acceptable clinical outcomes (57-62). Nylon is the most common material used in these nets. One such low-cost mesh made from polyethylene is marketed by Amsa Plastics (Karur, India).
Future Research in hernia repair and mesh technology
Future research in hernia repair will continue to focus on new prosthesis as well as surgical technique. Theodore Billroth once said to his pupil Czerny, in 1878 that, “If we can artificially produce tissues of the density and toughness of fascia and tendon the secret of the radical cure of hernia would be discovered”.
Any hernia occurs as a result of a mechanical disparity between the intra-abdominal pressure and the resistance of the abdominal musculature. Variety of biological mechanisms are involved, leading to changes in fascial pathology or failure of the surgical wound are involved.
Literature review shows us that certain genetic or systemic disorders involving extracellular matrix and connective tissue abnormalities may predispose patients to develop a hernia. It may not be wrong to assume that while primary hernia may occur due to congenital defects in extracellular matrix in certain patients, those with failed laparotomy who develop incisional hernia and hernia repairs who develop recurrence may have an acquired defect in extracellular matrix. These acquired defects can lead to secondary fascial pathology like wound healing failure, abnormal fibroblast production, errors in wound remodelling and wound ischaemia. Acquired collagen defects are also known to occur due to smoking, obesity, collagen deficiency disorders, nutritional conditions e.g., Lathyrism, vitamin, zinc and copper deficiency, factors which affect tissue perfusion with resultant reduced tissue oxygenation. Acquired deficiency of extra-cellular matrix leads to hernia formation as well as recurrence post-surgery by affecting wound healing and synthesis of extra-cellular matrix. Recurrence of hernia involves combination of technical failure and biological factors.
Understanding of this complex mechanism is paramount and will probably be the most important key to solve the problem of recurrence and provide a better repair in patient with abdominal wall hernia. It is with this aim that we need to invest more in the understanding of tissue matrix biology which may help us to improve results after hernia repair surgery. Future research in hernia should focus on modifying these factors. New mesh technology needs to focus on prosthesis which least affects or which can augment extra-cellular matrix synthesis.
Factors which should influence mesh choice
Though an ideal mesh is far from reality, most surgeons should take into consideration following factors before using any prosthesis
- Ease of handling—particularly useful during laparoscopy;
- Strength of the mesh—any prosthesis should be strong enough to resist about 16 N/cm force so as to avoid disruption (63);
- Large pore size of about 600–800 micrometer, so as to avoid scar bridging and as a result reduced contraction (34);
- Density—though lacks definite consensus, it needs to be between 28 to 90 gm/m2 (46);
- Architecture of the polymer—solid or woven;
- Clinical scenario—elective or emergency, presence of infection, size of hernia;
- Cost.
Conclusions
As our search for an ideal mesh continues, the most important goal is to maximize benefit to the patient. Focus on prosthesis which will produce expected biological response, withstand disruptive force, minimise contraction and at the same time are durable. Adequate funding to researchers needs to be arranged. Laboratory with animal models which will mimic human tissue needs to be developed. Any animal study will never truly recreate human tissue and so before human studies or using any new prosthesis on patients for investigational purposes, adequate safeguards in the form of ethics committee and institutional review boards at the hospital level and government agency or authorities need to be in place. Adequate counselling and discussion with patients before use of any new prosthesis is important. Lastly, surgical skills and principles in any hernia repair are paramount. An ideal prosthesis if improperly placed, small sized, repair under tension and improper fixation will lead to recurrence and complications.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Laparoscopic and Endoscopic Surgery for the series “Inguinal Hernia Repair”. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2017.07.04). The series “Inguinal Hernia Repair” was commissioned by the editorial office without any funding or sponsorship. DL served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Annals of Laparoscopic and Endoscopic Surgery from Jun 2016 to May 2018. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Goepel R. Uber die Verschliessung Von Bruchpforten Durch Einheilung Geflochtener Fertiger Silberdrahtnetze. Verh Dtsch Ges Chir 1900;29:4.
- Perry HB. Implantations of silver filigree for cure of large ventral hernia; report of two cases. Boston Med Surg J 1904;151:97-9. [Crossref]
- Witzel O. Uber den Verschluss van Bauchwunden und Bruchpforten durch versenkte Silberdrahtnetze. Zetralbl Chir 1900;10:257-60.
- Phelps AM. A new operation of hernia. N Y Med J 1894;60:291-6.
- Preston DJ, Richards CF. Use of wire mesh prostheses in the treatment of hernia. 24 years’ experience. Surg Clin North Am 1973;53:549-54. [Crossref] [PubMed]
- Mathieson AJ, James JH. A review of inguinal hernia repair using stainless steel mesh. J R Coll Surg Edinb 1975;20:58-62. [PubMed]
- Thomeret G, Dubost C, Pillot P. The use of inoxydizable steel gauze in the treatment of eventrations of hernias. Mem Acad Chir (Paris) 1960;86:500-7. [PubMed]
- Douglas DM. Repair of large herniae with tantalum gauze; an experimental and clinical study. Lancet 1948;1:936-9. [Crossref] [PubMed]
- Throckmorton TD. Tantalum gauze in the repair of hernias complicated by tissue deficiency; a preliminary report. Surgery 1948;23:32-46. [PubMed]
- Koontz AR, Kimberly RC. Tantalum and marlex mesh (with a note on marlex thread):an experimental and clinical comparison – preliminary report. Ann Surg 1960;151:796-804. [Crossref] [PubMed]
- Cumberland VH. A preliminary report on the use of prefabricated nylon weave in the repair of ventral hernia. Med J Aust 1952;1:143-4. [PubMed]
- Doran FS, Gibbins RE, Whitehead R. A report on 313 inguinal herniae repaired with nylon nets. Br J Surg 1961;48:430-4. [Crossref] [PubMed]
- Usher FC, Wallace SA. Tissue reaction to plastics; a comparison of nylon, orlon, Dacron, Teflon, and Marlex. AMA Arch Surg 1958;76:997-9. [Crossref] [PubMed]
- DeBord JR. The historical development of prosthetics in hernia surgery. Surg Clin North Am 1998;78:973-1006. [Crossref] [PubMed]
- Usher FC, Ochsner J, Tuttle LL. Jr. Use of marlex mesh in the repair of incisional hernias. Am Surg 1958;24:969-74. [PubMed]
- Adloff M, Arnaud JP. Surgical management of large incisional hernias by an intraperitoneal Mersilene mesh and an aponeurotic graft. Surg Gynecol Obstet 1987;165:204-6. [PubMed]
- Tang L, Ugarova TP, Plow EF, et al. Molecular determinates of acute inflammatory response to biomaterials. J Clin Invest 1996;97:1329-34. [Crossref] [PubMed]
- Busuttil SJ, Ploplis VA, Castellino FJ, et al. A central role for plasminogen in the inflammatory response to biomaterials. J Thromb Haemost 2004;2:1798-805. [Crossref] [PubMed]
- Woloson SK, Greisler HP. Biochemistry, Immunology, and Tissue Response to Prosthetic Material. In: Bendavid R, Abrahamson J, Arregui ME, et al. editors. Abdominal Wall Hernias. New York: Springer, 2001:201-7.
- Diegelmann RF. Collagen metabolism. Wounds 2000;13:177-82.
- Amid P. Classification of biomaterials and their related complications in abdominal wall surgery. Hernia 1997;1:15-21. [Crossref]
- Coda A, Lamberti R, Martorana S. Classification of prosthetics used in hernia repair based on weight and biomaterial. Hernia 2012;16:9-20. [Crossref] [PubMed]
- Weyhe D, Schmitz I, Belyaev O, et al. Experimental comparison of monofile light and heavy polypropylene meshes: less weight does not mean less biological response. World J Surg 2006;30:1586-91. [Crossref] [PubMed]
- Klinge U, Klosterhalfen B, Ottinger AP, et al. PVDF as a new polymer for the construction of surgical meshes. Biomaterials 2002;23:3487-93. [Crossref] [PubMed]
- Berger D, Bientzle M. Polyvinylidene fluoride: a suitable mesh material for laparoscopic incisional and parastomal hernia repair! Hernia 2009;13:167-72. [Crossref] [PubMed]
- Deeken CR, Faucher KM, Matthews BD. A review of the composition, characteristics, and effectiveness of barrier mesh prostheses utilized for laparoscopic ventral hernia repair. Surg Endosc 2012;26:566-75. [Crossref] [PubMed]
- Deeken CR, Abdo MS, Frisella MM, et al. Physicomechanical evaluation of polypropylene, polyester, and polytetrafluoroethylene meshes for inguinal hernia repair. J Am Coll Surg 2011;212:68-79. [Crossref] [PubMed]
- Saberski ER, Orenstein SB, Novitsky YW. Anisotropic evaluation of synthetic surgical meshes. Hernia 2011;15:47-52. [Crossref] [PubMed]
- Anurov MV, Titkova SM, Oettinger AP. Impact of position of light mesh endoprosthesis with anisotropic structure for the efficiency of anterior abdominal wall reconstruction. Bull Exp Biol Med 2010;149:440-4. [Crossref] [PubMed]
- Cobb WS, Peindl RM, Zerey M, et al. Mesh terminology 101. Hernia 2009;13:1-6. [Crossref] [PubMed]
- Klinge U, Klosterhalfen B. Modified classification of surgical meshes for hernia repair based on the analyses of 1,000 explanted meshes. Hernia 2012;16:251-8. [Crossref] [PubMed]
- Langenbach MR, Schmidt J, Zirngibl H. Comparison of biomaterials in the early postoperative period. Surg Endos 2003;17:1105-9. [Crossref] [PubMed]
- Kossovy N, Freiman CJ, Howarth D. Biomaterials pathology. In: Bendavid R, Abrahamson J, Arregui ME, et al. editors. Abdominal wall hernias, principles and management. New York: Springer, 2001:221-34.
- Schumpelick V, Klinge U, Rosch R, et al. Light weight meshes in incisional hernia repair. J Minim Access Surg 2006;2:117-23. [PubMed]
- Novitsky YW, Harrell AG, Hope WW, et al. Meshes in hernia repair. Surg Technol Int 2007;16:123-7. [PubMed]
- Cobb WS, Kercher KW, Heniford BT. The argument for lightweight polypropylene mesh in hernia repair. Surg Innov 2005;12:63-9. [Crossref] [PubMed]
- Champault G, Barrat C. Inguinal hernia repair with beta glucan-coated mesh: results at two-year follow up. Hernia 2005;9:125-30. [Crossref] [PubMed]
- Losanoff JE, Richman BW, Jones JW. Entero-colocutaneous fistula: a late consequence of polypropylene mesh abdominal wall repair: case report and review of the literature. Hernia 2002;6:144-7. [Crossref] [PubMed]
- Chuback JA, Sigh RS, Sill D, et al. Small bowel obstruction resulting from mesh plug migration after open inguinal hernia repair. Surgery 2000;127:475-6. [Crossref] [PubMed]
- Bellón JM, Rodríguez M, García-Honduvilla N, et al. Peritoneal effects of prosthetic meshes use to repair abdominal wall defects: monitoring adhesions by sequential laparoscopy. J Laparoendosc Adv Surg Tech A 2007;17:160-6. [Crossref] [PubMed]
- Langer C, Schwartz P, Krause P, et al. In-vitro study of the cellular response of human fibroblasts cultured on alloplastic hernia meshes. Influence of mesh material and structure. Chirurg 2005;76:876-85. [Crossref] [PubMed]
- Gumargalieva KZ, Mosieev YV, Daurova TT, et al. Effects of infections on the degradation of polyethylene terephthalate implants. Biomaterials 1982;3:177-80. [Crossref] [PubMed]
- Klinge U, Klosterhalfen B, Muller M, et al. Influence of polyglactin-coating on functional and morphologic parameters of polypropylene-mesh modifications for abdominal wall repair. Biomaterials 1999;20:613-23. [Crossref] [PubMed]
- Scheidbach H, Tamme C, Tanapfel A, et al. In vivo studies comparing the biocompatibility of various polypropylene meshes and their handling properties during endoscopic total extraperitoneal (TEP) patchplasty. An experimetnal study in pigs. Surg Endosc 2004;18:211-20. [Crossref] [PubMed]
- Brown CN, Finch JG. Which mesh for hernia repair? Ann R Coll Surg Engl 2010;92:272-8. [Crossref] [PubMed]
- O'Dwyer PJ, Kingsnorth AN, Molloy RG, et al. Randomized clinical trial assessing impact of a lightweight or heavyweight mesh on chronic pain after inguinal hernia repair. Br J Surg 2005;92:166-70. [Crossref] [PubMed]
- Junge K, Klinge U, Rosch R, et al. Functional and morphologic properties of a modified mesh for inguinal hernia repair offer advantages over nonabsorbable meshes. Am J Surg 2002;26:1472-80.
- Bellón JM, Rodríguez M, García-Honduvilla N, et al. Partially absorbable meshes for hernia repair offer advantages over nonabsorbable meshes. Am J Surg 2007;194:68-74. [Crossref] [PubMed]
- Klinge U. Mesh for hernia repair. Br J Surg 2008;95:539-40. [Crossref] [PubMed]
- Pielaciński K, Dabrowski W, Szczepanik AB, et al. Self-fixating Progrip implant used in the laparoscopic totally extraperitoneal technique for inguinal hernia repair. Pol Merkur Lekarski 2011;31:345-7. [PubMed]
- Birk D, Hess S, Garcia-Pardo C. Low recurrence rate and low chronic pain associated with inguinal hernia repair by laparoscopic placement of Parietex ProGrip(TM) Mesh: clinical outcomes of 220 hernias with mean follow-up at 23 months. Hernia 2013;17:313-20. [Crossref] [PubMed]
- Fang Z, Zhou J, Ren F, et al. Self-gripping mesh versus sutured mesh in open inguinal hernia repair: system review and meta-analysis. Am J Surg 2014;207:773-81. [Crossref] [PubMed]
- Rutkow IM, Robbins AW. The Marlex mesh Perfix plug groin hernioplasty. Eur J Surg 1998;164:549-52. [Crossref] [PubMed]
- Murphy JW, Misra DC, Silverglide B. Sigmoid colonic fistula secondary to Perfixplug, left inguinal hernia repair. Hernia 2006;10:436-8. [Crossref] [PubMed]
- Moorman ML, Price PD. Migrating mesh plug: complication of a well-established hernia repair technique. Am Surg 2004;70:298-9. [PubMed]
- Mayagoitia JC, Prieto-Diaz Chavez E, Suarez D, et al. Predictive factors and comparison of complications and recurrences in three tension-free herniorrhaphy techniques. Hernia 2006;10:147-51. [Crossref] [PubMed]
- Tongaonkar RR, Reddy BV, Mehta VK, et al. Preliminary multicentric trial of cheap indigenous mosquito-net cloth for tension-free hernia repair. Indian J Surg 2003;65:89-95.
- Stephenson BM, Kingsnorth AN. Inguinal hernioplasty using mosquito net mesh in low income countries: an alternative and cost effective prosthesis. BMJ 2011;343:d7448. [Crossref] [PubMed]
- Clarke MG, Oppong C, Simmermacher R, et al. The use of sterilised polyester mosquito net mesh for inguinal hernia repair in Ghana. Hernia 2009;13:155-9. [Crossref] [PubMed]
- Sanders DL, Kingsnorth AN, Stephenson BM. Mosquito net mesh for abdominal wall hernioplasty: a comparison of material characteristics with commercial prosthetics. World J Surg 2013;37:737-45. [Crossref] [PubMed]
- Sørensen CG, Rosenberg J. The use of sterilized mosquito nets for hernioplasty: a systematic review. Hernia 2012;16:621-5. [Crossref] [PubMed]
- Löfgren J, Nordin P, Ibingira C, et al. A randomized trial of low-cost mesh in groin hernia repair. N Engl J Med 2016;374:146-53. [Crossref] [PubMed]
- Klosterhalfen B, Klinge U, Hermanns B, et al. Pathology of traditional surgical nets for hernia repair after long-term implantation in humans. Chirurg 2000;71:43-51. [Crossref] [PubMed]
Cite this article as: Salgaonkar H, Lomanto D. Mesh technology. Ann Laparosc Endosc Surg 2017;2:116.


