
Roux-en-Y gastric bypass for the treatment of Asian type II diabetes
IntroductionOther Section
- Introduction
- Technical aspects
- Operative risk
- Weight loss outcome
- Remission of T2DM
- Recurrence of T2DM
- Comparison of RYGB with other procedures
- Conclusions
- Acknowledgments
- Footnote
- References
Gastric bypass surgery was invented by Mason and Ito at 1966 to replace intestinal bypass as a bariatric procedure (1). The procedure consisted by a proximal transverse gastric pouch and a loop gastroenterostomy. Because a loop bypass close to esophgo-gastric junction would cause intractable bile reflux, the procedure was then modified to Roux-en-Y (RY) gastroenterostomy, so called Roux-en-Y gastric bypass (RYGB), by Griffen at 1977 (2,3). At 1991, vertical banded gastroplasty (VBG) and RYGB are the only two procedures approved by the NIH (4). Because gastric bypass has been demonstrated to result in a better weight reduction than VBG in several randomized trials (5-8), RYGB gradually become the most commonly performed surgical procedures for obesity treatment and regarded as a gold standard bariatric/metabolic procedure (9,10). Laparoscopic RYGB (LRYGB) was then developed in 1993 by Wittgrove and Clark (11). Although the learning curve is very long, LRYGBP was rapidly adopted and emerged as the preferred bariatric procedure worldwide (12,13).
Traditional RYGB has been shown to be effective in achieving significant and durable long-term weight loss as well as improving medical co-morbidities in morbidly obese patients. The initiation of using bariatric surgery for the treatment of T2DM started from the report by Pories et al. in 1987 (14). Strong evidences have shown that RYGB is an effective treatment for severe obesity (BMI >35 kg/m2) and result in marked improvement of T2DM control (15-17). Recently, several randomized control trial have demonstrated the feasibility and efficacy using LRYGB as a metabolic surgery for the treatment of T2DM in low BMI patients (BMI <35 kg/m2) (18-23). However, data of the results for the treatment of T2DM in Asian is less known. The aim of this study is to analyze the update results of RYGB on weight reduction and the efficacy on T2DM treatment in Asian.
Technical aspectsOther Section
- Introduction
- Technical aspects
- Operative risk
- Weight loss outcome
- Remission of T2DM
- Recurrence of T2DM
- Comparison of RYGB with other procedures
- Conclusions
- Acknowledgments
- Footnote
- References
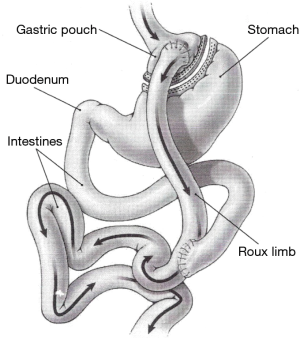
The operation consisted of two components, including, first, a small proximal gastric pouch, usually less than 50 cc, second, a Roux-en-Y gastrojejunostomy with a 75 to 150 cm alimentary or Roux limb and 50 to 100 cm bilio-pancreatic limb (Figure 1). The gastro-jejonostomy is recommended to be smaller than 2cm in diameter (13). The anastomosis can be created using circular stapler, linear stapler or pure hand sewn techniques (11-13). The alimentary bring up was started from retro-colic and retro-gastric to ante-colic and ante-gastric. However, omentum bivalve was recommended in ante-colic and ante-gastric way to avoid the tension of gastro-jejunal anastomosis. The most specific and notorious complication of LRYGB was the development of internal hernia (24). Closure of mesenteric defects can markedly reduce the incidence of internal hernia after LRYGB and should be routinely performed (25,26).
Operative riskOther Section
- Introduction
- Technical aspects
- Operative risk
- Weight loss outcome
- Remission of T2DM
- Recurrence of T2DM
- Comparison of RYGB with other procedures
- Conclusions
- Acknowledgments
- Footnote
- References
LRYGB is regarded as one of the most technically demanding advanced laparoscopic surgeries with a very steep learning curve (27-31). The estimated learning curve period of LRYGB is stated to be 100 to 500 cases (27,32). The reported conversion rate of LRYGB varied from 0.8% to 11.8%, the major complication rate from 3.3% to 15% and the late complication rate from 2.2% to 27%. Although bariatric surgery, especially performed by laparoscopic surgery, is one the most common complex laparoscopic operations, the safety of laparoscopic bariatric surgery improved very rapidly. The 30-day operation mortality of LRYGB was reported to be 2% in 2004 and decreased to 0.2% in 2009 through the program of high quality bariatric center program in USA (33, 34). Well experienced surgeons, fully trained in laparoscopic technique and proctorship, team work and adequate volume are important for a high quality bariatric surgical center (35). Improvement of technology, operative technique, results of clinical trials and accumulation of experience all contributed to this progress (36). In the most recently publication, the 30-day mortality of LRYGB from European center of excellence program was reported to be only 0.012% (37). In conclusion, LRYGB is the leading bariatric/metabolic surgery in the past decade but the operation is one hundred times safer now.
Weight loss outcomeOther Section
- Introduction
- Technical aspects
- Operative risk
- Weight loss outcome
- Remission of T2DM
- Recurrence of T2DM
- Comparison of RYGB with other procedures
- Conclusions
- Acknowledgments
- Footnote
- References
LRYGB has been shown to be effective in achieving significant and durable long-term weight loss. The Long-term (>10 years) weight loss after LRYGB was reported to be around 25–30% total weight loss (%TWL) and 55 to 70% excess weight loss (%EWL) (38-43). Table 1 showed the long-term outcome of LRYGB at different reports. The mean %WL at 10-y are 28.8% and %EWL are 60.9%. Up to 20% of RYGB patients may require a revision surgery for various complications or weight regain. The most commonly reoperation indication is intestine obstruction, including internal herniation. Weight regain was common after RYGB and usually related to dilatation of gastric pouch and anastomosis (44,45). Endoscopic treatment was developed recently and was recommended for the first-line treatment for those with weight regain after RYGB (46). Other options included band replacement, converting to distal gastric bypass or duodenal switch (47-49).
Table 1
| Author | Case no | Age (years) | BMI (Kg/m2) | %TWL | %EWL | Revision rate % |
|---|---|---|---|---|---|---|
| Higa (38) | 65 | 41.5 | 47.8 | – | 57.1 | 32.0 |
| Lee (39) | 46 | 35.4 | 38.5 | 26.7 | 60.1 | 5.1 |
| Angrisani (40) | 24 | 34.7 | 43.8 | 29.6 | 69 | 28.6 |
| Edholm (41) | 40 | 37.9 | 44.5 | NR | 67.3 | 2.1 |
| Obeid (42) | 134 | 42.9 | 46.6 | 31.6 | 58.9 | 23.5 |
| Mehaffey (43) | 651 | 41.4 | 53.3 | 27.3 | 52.5 | – |
| Mean | 160 | 39.0 | 45.8 | 28.8 | 60.9 | 18.3 |
Data are presented in mean (SD). LRYGB, Laparoscopic Roux-en-Y gastric bypass; –, not reported; BMI, body mass index; TWL, total weight loss; EWL, excess weight loss.
Remission of T2DMOther Section
- Introduction
- Technical aspects
- Operative risk
- Weight loss outcome
- Remission of T2DM
- Recurrence of T2DM
- Comparison of RYGB with other procedures
- Conclusions
- Acknowledgments
- Footnote
- References
T2DM remission rate was reported up to 82.9% during 10- to 14-year follow-up in the historical landmark article by Pories et al. (15). Another meta-analysis by Buchwald et al. reported RYGB achieved a high T2DM remission rate up to 80.3% (81.6% in less than 2 years and 70.9% in more than 2 years) but these data generally used loose and heterogeneous criteria (50). Table 2 listed the reported T2DM remission rate in randomized clinical trial (RCT) (19-23,51-54). The prolong T2DM complete remission (5-y) rate was less than 50% in RCT trials. Generally speaking, T2DM remission rate was higher in morbid obese patients (BMI >35 Kg/m2) than in low BMI group (BMI <35 Kg/m2). Complete T2DM remission rate (HbA1c <6% without medication) usually sit at 50% in low BMI patients and might decrease to less than 30% after 5-y [prolong remission (55)]. However, studies from Asia usually reported a higher T2DM remission rate that those from Western countries (56-74). Table 3 listed the reported T2DM remission rate from Asia. The reason might be the patients in Asia were relatively young with shorter duration of T2DM than in other part of the world (73).
Table 2
| Author | Year | No | Pre-op BMI [range] | Pre-op HbA1c% | F/u year | Post HbA1c% | T2DM 1-y CR | T2DM 1-y PR | T2DM 5-y PCR | T2DM 5-y PCR |
|---|---|---|---|---|---|---|---|---|---|---|
| Schauer (18,51) | 2017 | 50 | 36 [28–42] | 9.4% | 5 | 7.3% | 42% | – | 26.4% | – |
| Ikramuddin (19,52) | 2017 | 60 | 34.6 [30–40] | 9.6% | 5 | 6.7% | 44% | – | 27% | – |
| Mingrone (53,54) | 2015 | 20 | 48.7 [>35] | 8.7% | 5 | 6.7% | – | 75% | 37% | 42% |
| Liang (20) | 2013 | 31 | 30 [28–35] | 10.5% | 1 | 6.0% | 90% | – | – | – |
| Courcoulas (21) | 2014 | 21 | 35.5 [30–40] | 7.9% | 1 | 6.4% | 27% | 50% | – | – |
| Halperin (22) | 2014 | 19 | 36.3 [30–40] | 8.5% | 1 | 6.7% | – | 58% | – | – |
| Cummings (23) | 2016 | 15 | 37.8 [30–45] | 7.5% | 1 | 6.4% | 60% | 60% | – | – |
| Mean | – | – | – | – | – | – | 42% | 66.6% | 30.1% | 42% |
T2DM, type 2 diabetes mellitus; LRYGB, laparoscopic Roux-en-Y gastric bypass; CR, complete remission A1c <6.0%; PR, partial remission A1c <6.5%; PCR, prolog complete remission: CR more than 5 years; –: no data.
Table 3
| Author | Year | No | Pre-op BMI | Pre-op HbA1c% | F/u year | Post HbA1c% | T2DM 1-y CR | T2DM 1-y PR | T2DM 5-y PCR | T2DM 5-y PCR |
|---|---|---|---|---|---|---|---|---|---|---|
| Kim (56) | 2011 | 50 | 34.5 | 8.6% | 1 | – | 68% | – | – | – |
| Huang (57) | 2011 | 221 | 30.8 | 9.2% | 1 | 6.4% | 63.6% | 50% | – | – |
| Lee (58) | 2012 | 205 | 40.7 | 8.3% | 1 | – | 78% | – | – | – |
| Zhu (59) | 2012 | 30 | 26.2 | 8.2% | 1 | 5.6% | 30.0% | – | – | – |
| Dixon (60) | 2013 | 154 | 37.2 | 9.1 | 1 | 6.7% | 69.5% | 75% | – | 42% |
| Lee (61) | 2013 | 176 | 36.7 | 8.6% | 1 | 6.0% | 65.3% | NA | – | – |
| Yan (62) | 2013 | 99 | 26.3 | 9.1% | 1 | 6.7% | NA | 80% | – | – |
| Lakdawalab(63) | 2013 | 52 | 32.6 | 8.8% | 5 | 6.2% | NA | 73.1% | – | 57.7% |
| Malapan (64) | 2014 | 29 | 24.4 | 10.0% | 1 | 6.4% | 37.9% | 60% | – | – |
| Yang (65) | 2015 | 30 | 32.3 | 8.9% | 3 | – | – | – | 85.2%(3-y) | 92.6%(3-y) |
| Tang Q (66) | 2015 | 38 | 37.8 | 7.4% | 2 | – | 38.6% | 57.6% | – | – |
| Liang (67) | 2015 | 86 | 24.7 | 6.8% | 1 | – | 23.3% | – | – | – |
| Park (68) | 2016 | 134 | 37.9 | 8.0% | 1 | 6.1% | 46.1% | 61.8% | – | – |
| Wang (69) | 2016 | 78 | 28.3 | 8.2 | 2 | 7.1% | – | 43.8% | – | – |
| Zhang (70) | 2017 | 120 | 33.9 | 8.3% | 3 | – | 76.4% | – | 62.2%(3-y) | – |
| Du (71) | 2017 | 64 | 31.2 | 9.3% | 3 | NA | - | 75% | – | 59.5%(3-y) |
| Haruta (72) | 2017 | 13 | 42 | NA | 1 | NA | 92% | – | – | – |
| Chong (30) | 2017 | 14 | 31.9 | 9.6 | 2 | 6.4% | 29% | 57% | – | – |
| Lee (74) | 2017 | 157 | 34.5 | 8.6% | 5 | 6.5% | 55.4% | – | 39.4% | – |
| Mean | – | – | – | – | – | – | 55.2% | 60.7% | 62.3% | 62.9% |
T2DM, type 2 diabetes mellitus; LRYGB, laparoscopic Roux-en-Y gastric bypass; CR, complete remission A1c <6.0%; PR, partial remission A1c <6.5%; PCR, prolog complete remission: CR more than 5 years; –: no data.
Recurrence of T2DMOther Section
- Introduction
- Technical aspects
- Operative risk
- Weight loss outcome
- Remission of T2DM
- Recurrence of T2DM
- Comparison of RYGB with other procedures
- Conclusions
- Acknowledgments
- Footnote
- References
Studies have illustrated some patients whose T2DM remission after RYGB experienced a recurrence of their disease over times (75-77). DiGiorgi et al. have shown that beyond 3 years after RYGB, 24% of patients with initial remission of their T2DM had re-emergence of diabetes (76). Recurrence was related to weight regain and longer duration of T2DM. The variance of T2DM remission rate and high recurrent rate of T2DM highlights the importance of patient selection and understanding the mechanism of T2DM remission by metabolic surgery. We need information of pre-operative predictors to identify the best candidates to achieve this goal, durable or prolonged T2DM remission. A simple scoring system combined age, BMI, C-peptide and duration of disease to become an ABCD score which is a very useful in selecting suitable patients for metabolic surgery (60). Studies have shown this multi-dimension scoring system ABCD score, is the only predictor of prolonged T2DM remission after metabolic surgery (69,71,78).
Comparison of RYGB with other proceduresOther Section
- Introduction
- Technical aspects
- Operative risk
- Weight loss outcome
- Remission of T2DM
- Recurrence of T2DM
- Comparison of RYGB with other procedures
- Conclusions
- Acknowledgments
- Footnote
- References
RYGB had a better weight loss than pure restrictive bariatric procedures, including VBG and adjustable gastric banding (5-8,40). However, weight loss after LRYGB was found to be similar or slightly better than LSG in many RCTs but with more nutritional deficiencies in LRYGB (79-82). That’s why that LSG is becoming the leading bariatric/metabolic procedure now (83,84). However, LRYGB still have a better glycemic and lipid control than LSG, probably because of the duodenum exclusion effect (85-87). On the other hand, mal-absorptive procedures including bilio-pancreatic diversion/duodenal switch (BPD/DS), laparoscopic single anastomosis (Mini-) gastric bypass (LSAGB) and single anastomosis duodeno-ileostomy (SADI), had a better weight loss and glycemic control than RYGB but had higher incidence of malnutrition (39,50,53,54,88). Therefore, the surgeon should counsel the patients for metabolic surgery and choice of surgical procedure to construct a personalized treatment according to individual situation and update evidence.
ConclusionsOther Section
- Introduction
- Technical aspects
- Operative risk
- Weight loss outcome
- Remission of T2DM
- Recurrence of T2DM
- Comparison of RYGB with other procedures
- Conclusions
- Acknowledgments
- Footnote
- References
LRYGB is a safe and durable primary bariatric procedure with overall 28.8% TWL and 60.9%EWL at 10 years and satisfactory resolution of obesity related co-morbidities. LRYGB has become the most favorable metabolic procedure for T2DM treatment and resulted in a better glycemic control than intensive medical control. However, prolong complete T2DM remission rate was less than 50% 5-y after LRYGB. We need to understand the mechanism of metabolic system and more information of pre-operative predictor in order to identify the best candidates from T2DM patients for this procedure.
AcknowledgmentsOther Section
- Introduction
- Technical aspects
- Operative risk
- Weight loss outcome
- Remission of T2DM
- Recurrence of T2DM
- Comparison of RYGB with other procedures
- Conclusions
- Acknowledgments
- Footnote
- References
Funding: None.
FootnoteOther Section
- Introduction
- Technical aspects
- Operative risk
- Weight loss outcome
- Remission of T2DM
- Recurrence of T2DM
- Comparison of RYGB with other procedures
- Conclusions
- Acknowledgments
- Footnote
- References
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Laparoscopic and Endoscopic Surgery for the series “Laparoscopic Metabolic Surgery for the Treatment of Type 2 Diabetes in Asia”. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2017.07.09). The series “Laparoscopic Metabolic Surgery for the Treatment of Type 2 Diabetes in Asia” was commissioned by the editorial office without any funding or sponsorship. Lee WJ served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Annals of Laparoscopic and Endoscopic Surgery from Jun 2016 to May 2018. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
ReferencesOther Section
- Introduction
- Technical aspects
- Operative risk
- Weight loss outcome
- Remission of T2DM
- Recurrence of T2DM
- Comparison of RYGB with other procedures
- Conclusions
- Acknowledgments
- Footnote
- References
- Mason EE, Ito C. Gastric bypass in obesity. Surg Clin North Am 1967;47:1345-51. [Crossref] [PubMed]
- Griffen WO, Young VL, Stevenson CC. A prospective comparison of gastric and jejunoileal bypass procedures for morbid obesity. Ann Surg 1977;186:500-9. [Crossref] [PubMed]
- McCarthy HB, Rucker RD Jr, Chan EK, et al. Gastritis after gastric bypass surgery. Surgery 1985;98:68-71. [PubMed]
- NIH conference. Gastrointestinal surgery for severe obesity. Consensus Development Conference Panel. Ann Int Med 1991;115:956-61. [Crossref] [PubMed]
- Freeman JB, Burchett HJ. A comparison of gastric bypass and gastroplasty for morbid obesity. Surgery 1980;88:433-44. [PubMed]
- Laws HL, Piantadosi S. Superior gastric reduction procedure for morbid obesity: a prospective, randomized prospective, randomized trial. Ann Surg 1981;193:334-40. [Crossref] [PubMed]
- Lechner GW, Callender AK. Subtotal gastric exclusion and gastric partitioning: a randomized prospective comparison of one hundred patients. Surgery 1981;90:637-44. [PubMed]
- Näslund I, Wickbom G, Christoffersson E, et al. A prospective randomized comparison of gastric bypass and gastroplasty; complications and early results. Acta Chir Scand 1986;152:681-9. [PubMed]
- Buchwald H, Williams SE. Bariatric surgery worldwide 2003. Obes Surg 2004;14:1157-64. [Crossref] [PubMed]
- Buchwald H, Oien DM. Metabolic/bariatric surgery worldwide 2008. Obes Surg 2009;19:1605-11. [Crossref] [PubMed]
- Wittgrove AC, Clark GW. Laparoscopic gastric bypass. Roux-en-Y-500 patients:technique and results,with3-60 month follow-up. Obes Surg 2000;10:233-9. [Crossref] [PubMed]
- Schauer PR, Ikranuddin S, Gourash W, Ramanathan R, Luketich J. Outcomes after laparoscopic gastric bypass for morbid obesity. Ann Surg 2000;232:515-29. [Crossref] [PubMed]
- Higa KD, Boone KB, Ho T, et al. Laparoscopic Roux-en-Y gastric bypass for morbid obesity. Arch Surg 2000;135:1029-33; discussion 1033-4. [Crossref] [PubMed]
- Pories WJ, Caro JF, Flickinger EG, et al. The control of diabetes mellitus(NIDDM) in the morbidly obese with the Greenville gastric bypass. Ann Surg 1987;206:316-23. [Crossref] [PubMed]
- Pories WJ, Swanson MS, MacDonald KG, et al. Who would have thought it? An operation proves to be most effective therapy for adult-onset diabetes mellitus. Ann Surg 1995;222:339-50. [Crossref] [PubMed]
- MacLean LD, Rhode BM, Nohr CW. Late outcome of isolated gastric bypass. Ann Surg 2000;231:524-8. [Crossref] [PubMed]
- Schauer PR, Burguera B, Ikramuddin S, et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg 2003;238:467-84; discussion 84-5. [PubMed]
- Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med 2012;366:1567-76. [Crossref] [PubMed]
- Ikramuddin S, Korner J, Lee WJ, et al. Roux-en-Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: The diabetes surgery study randomized clinical trial. JAMA 2013;309:2240-9. [Crossref] [PubMed]
- Liang Z, Wu Q, Chen B, et al. Effect of laparoscopic Roux-en-Y gastric bypass for type 2 diabetes mellitus with hypertension: a randomized controlled trial. Diabetes Res Clin Pract 2013;101:50-6. [Crossref] [PubMed]
- Courcoulas AP, Goodpaster BH, Eagleton JK, et al. Surgical vs medical treatments for type 2 diabetes mellitus: a randomized clinical trial. JAMA Surg 2014;149:707-15. [Crossref] [PubMed]
- Halperin F, Ding SA, Simonson DC, et al. Roux-en-Y gastric bypass surgery or life style with intensive medical management in patients with type 2 diabetes: Feasibility and 1-year results of a randomized clinical trial. JAMA Surg 2014;149:716-26. [Crossref] [PubMed]
- Cummings DE, Arterburn DE, Westbrook EO, et al. Gastric bypass surgery vs intensive lifestyle and medical intervention for type 2 diabetes: the CROSSROADS randomized controlled trial. Diabetologia 2016;59:945-53. [Crossref] [PubMed]
- Paroz A, Calmes JM, Giusti V, et al. Internal hernia after laparoscopic Roux-en-Y gastric bypass for morbid obesity: A continuous challenge in bariatric surgery. Obes Surg 2006;16:1482-7. [Crossref] [PubMed]
- Chowbey P, Baijal M, Kantharia N, et al. Mesenteric defect closure decreases the incidence of internal hernias following laparoscopic Roux-en-Y gastric bypass: a retrospective cohort study. Obes Surg 2016;26:2029-34. [Crossref] [PubMed]
- Kristensen SD, Jess P, Floyd AK, et al. Internal herniation after laparoscopic antecolic Roux-en-Y gastric bypass: A nationwide Danish study based on the Danish National Patient Register. Surg Obes Relat Dis 2016;12:297-303. [Crossref] [PubMed]
- Schauer P, Ikramuddin S, Hamad G, Gourash W. The learning curve for laparoscopic Roux-en-Y gastric bypass is 100 cases. Surg Endosc 2003;17:212-5. [Crossref] [PubMed]
- DeMaria EJ, Sugerman HJ, Kellum JM, et al. Results of 281 consecutive total laparoscopic Roux-en-Y gastric bypasses to treat morbid obesity. Ann Surg 2002;235:640-5; discussion 645-7. [Crossref] [PubMed]
- Westling A, Gustavsson S. Laparoscopic vs Open Roux-en-Y gastric bypass: a prospective, randomized trial. Obes Surg 2001;11:284-92. [Crossref] [PubMed]
- Reddy RM, Riker A, Marra D, et al. Open Roux-en-Y gastric bypass for the morbidly obese in the era of laparoscopy. Am J Surg 2002;184:611-5; discussion 615-6. [Crossref] [PubMed]
- Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the global burden of disease study 2013. Lancet 2014;384:766-81. [Crossref] [PubMed]
- El-Kadre L, Tinoco AC, Tinoco RC, et al. Overcoming the learning curve of laparoscopic Roux-en-Y gastric bypass: a 12-year experience. Surg Obes Relat Dis 2013;9:867-72. [Crossref] [PubMed]
- Flum DR, Dellinger EP. Impact of gastric bypass operation on survival: a population-based analysis. J Am Coll Surg 2004;199:543-51. [Crossref] [PubMed]
- Longitudinal Assessment of Bariatric Surgery (LABS) Consortium. Peri-operative safety in the longitudinal assessment of bariatric surgery. N Engl J Med 2009;361:445-54. [Crossref] [PubMed]
- Nguyen NT, Paya M, Sterens M, et al. The relationship between hospital volume and outcome in bariatric surgery at academic medical center. Ann Surg 2004;240:586-93; discussion 593-4. [PubMed]
- Buchwald H, Estok R, Fahrback K, et al. Trends in mortality in bariatric surgery a systematic review and meta analysis. Surgery 2007;142:621-32. [Crossref] [PubMed]
- Melissas J, Stavroulakis K, Tzikoulis V, et al. Sleeve gastrectomy vs Roux-en-Y bypass. Data from IFSO-European chapter of excellence program. Obes Surg 2017;27:847-55. [Crossref] [PubMed]
- Higa K, Ho T, Tercero F, et al. Laparoscopic Roux-en-Y gastric bypass: 10-year follow-up. Surg Obes Relat Dis 2011;7:516-25. [Crossref] [PubMed]
- Lee WJ, Ser KH, Lee YC, et al. Laparoscopic Roux-en-Y vs. mini-gastric bypass for the treatment of morbid obesity: a 10-year experience. Obes Surg. 2012;22:1827-34. [Crossref] [PubMed]
- Angrisani L, Cutolo PP, Formisano G, et al. Laparoscopic adjustable gastric banding versus Roux-en-Y gastric bypass: 10-year results of a prospective, randomized trial. Surg Obes Relat Dis 2013;9:405-13. [Crossref] [PubMed]
- Edholm D, Sevensson F, Naslund I, et al. Long-term results 11 years after primary gastric bypass in 384 patients. Surg Obes Relat Dis 2013;9:708-13. [Crossref] [PubMed]
- Obeid NR, Malick W, Concors SJ, et al. Long-term outcomes after Roux-en-Y gastric bypass:10- to 13-year data. Surg Obes Relat Dis 2016;12:11-20. [Crossref] [PubMed]
- Hunter Mehaffey J, Turrentine FE, Miller MS, et al. Roux-en-Y gastric bypass 10-year follow-up: the found poupulation. Surg Obes Relat Dis 2016;12:778-82. [Crossref] [PubMed]
- Abu Dayyeh BK, Lautz DB, Thompson CC. Gastrojejunal stoma diameter predicts weight regain after Roux-en-Y gastric bypass. Clin Gastroenterol Hepatol 2011;9:228-33. [Crossref] [PubMed]
- Hamdi A, Julien C, Brwon P, et al. Mid-term outcomes of revisional surgery for gastric pouch and gastrojejunal anastomotic enlargement in patients with weight regain after gastric bypass for morbid obesity. Obes Surg 2014;24:1386-90. [Crossref] [PubMed]
- Dakin GF, Eid G, Mikami D, et al. Endoluminal revision of gastric bypass for weight regain--a systematic review. Surg Obes Relat Dis 2013;9:335-42. [Crossref] [PubMed]
- Irani K, Youn H, Ren-Fielding C, et al. Midterm results for gastric banding as salvage procedure for patients with weight loss failure after Roux-en-Y gastric bypass. Surg Obes Relat Dis 2011;7:219-24. [Crossref] [PubMed]
- Rawlins ML, Teel D, Hedgeorth K, et al. Revision of Roux-en-Y gastric bypass to distal bypass for failed weight loss. Surg Obes Relat Dis 2011;7:45-9. [Crossref] [PubMed]
- Parikh M, Pomp A, Gagner A. Laparoscopic conversion of failed gastric bypass to duodenal switch: technical considerations and preliminary outcomes. Surg Obes Relat Dis 2007;3:611-8. [Crossref] [PubMed]
- Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med 2009;122:248-56.e5. [Crossref] [PubMed]
- Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes – 5-year outcomes. N Engl J Med 2017;376:641-51. [Crossref] [PubMed]
- Ikramuddin S, Korner J, Lee WJ, et al. Durability of addition of Roux-en-Y gastric bypass to life style intervention and medical management in achieving primary treatment goals for uncontrolled type 2 diabetes in mild to moderate obesity: A randomized control trial. Diabetes Care 2016;39:1510-8. [Crossref] [PubMed]
- Mingrone G, Panunz S, De Gaetamo A, et al. Bariatric-metabolic surgery versus conventional medical treatment for type 2 diabetes. N Engl J Med 2012;366:1577-85. [Crossref] [PubMed]
- Mingrone G, Panunz S, De Gaetamo A, et al. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-center, randomized controlled trials. Lancet 2015;386:964-73. [Crossref] [PubMed]
- Buse JB, Laughlin S, Caprio S, et al. How do we define cure of diabetes? Diabetes Care 2009;32:2133-5. [Crossref] [PubMed]
- Kim MK, Lee HC, Kwon HS, et al. Visceral obesity is a negative predictor of remission of diabetes 1 year after bariatric surgery. Obesity (Silver Spring) 2011;19:1835-9. [Crossref] [PubMed]
- Huang CK, Shabbir A, Lo CH, et al. Laparoscopic Roux-en-Y gastric bypass for the treatment of type II diabetes mellitus in Chinese patients with body mass index of 25-35. Obes Surg 2011;21:1344-9. [Crossref] [PubMed]
- Lee WJ, Hur KY, Lakadawala M, et al. Gastrointestinal metabolic surgery for the treatment of diabetic patients: a multi-institutional international study. J Gastrointest Surg 2012;16:45-51; discussion 51-2. [Crossref] [PubMed]
- Zhu L, Mo Z, Yang X, et al. Effect of laparoscopic Roux-en-Y gastroenterostomy with BMI<35 kg/m(2) in type 2 diabetes mellitus. Obes Surg 2012;22:1562-7. [Crossref] [PubMed]
- Dixon JB, Chuang LM, Chong K, et al. Predicting the glycemic response to gastric bypass surgery in patients with type 2 diabetes. Diabetes Care 2013;36:20-6. [Crossref] [PubMed]
- Lee WJ, Hur KY, Lakadawala M, et al. Predicting success of metabolic surgery: age, body mass index, C-peptide, and duration score. Surg Obes Relat Dis 2013;9:379-84. [Crossref] [PubMed]
- Yan H, Tang L, Chen T, et al. Defining and predicting complete remission of type 2 diabetes: a short-term efficacy study of open gastric bypass. Obesity Facts 2013;6:176-84. [Crossref] [PubMed]
- Lakdawala M, Shaikh S, Bandukwala S, et al. Roux-en-Y gastric bypass stands the test of time: 5-year results in low body mass index (30-35 kg/m(2)) Indian patients with type 2 diabetes mellitus. Surg Obes Relat Dis 2013;9:370-8. [Crossref] [PubMed]
- Malapan K, Goel R, Tai CM, et al. Laparoscopic Roux-en-Y gastric bypass for nonobese type II diabetes mellitus in Asian patients. Surg Obes Relat Dis 2014;10:834-40. [Crossref] [PubMed]
- Yang J, Wang C, Cao G, et al. Long-term effects of laparoscopic sleeve gastrectomy versus roux-en-Y gastric bypass for the treatment of Chinese type 2 diabetes mellitus patients with body mass index 28-35 kg/m(2). BMC Surg 2015;15:88. [Crossref] [PubMed]
- Tang Q, Sun Z, Zhang N, et al. Cost-Effectiveness of Bariatric Surgery for Type 2 Diabetes Mellitus: A Randomized Controlled Trial in Chin. Medicine (Baltimore) 2016;95:e3522 [Crossref] [PubMed]
- Liang H, Guan W, Yang Y, et al. Roux-en-Y gatric bypass for Chinese type 2 diabetes mellitus patients with a BMI < 28 Kg/m2: a multi-institutional study. J Biomed Res 2015;29:112-7. [PubMed]
- Park JY, Kim YJ. Prediction of diabetes remission in morbidly obese patients after Roux-en-Y gastric bypass. Obes Surg 2016;26:749-756. [Crossref] [PubMed]
- Wang G, Zhu L, Li W, et al. Can low BMI Chinese patients with type 2 diabetes benefit from laparoscopic Roux-en-Y gastric bypass surgery? Surg Obes Relat Dis 2016;12:1890-5. [Crossref] [PubMed]
- Zhang H, Han X, Yu H, et al. Effect of Roux-en-Y gastric bypass on remission of T2D: Medium-term follow-up in Chinese patients with different BMI. Obes Surg 2017;27:134-42. [Crossref] [PubMed]
- Du X, Zhou HX, Zhang SQ, et al. A comparative study of the metabolic effects of LSG and LRYGB in Chinese diabetes patients with BMI < 35 Kg/m2. Surg Obes Relat Dis 2017;13:189-97. [Crossref] [PubMed]
- Haruta H, Kasama K, Ohta M, et al. Long-term outcome of bariatric and metabolic surgery in Japan: Results of a multi-institutional surgery. Obes Surg 2017;27:754-62. [Crossref] [PubMed]
- Chong K, Ikramuddin S, Lee WJ, et al. National Differences in Remission of Type 2 Diabetes Mellitus After Roux-en-Y Gastric Bypass Surgery-Subgroup Analysis of 2-Year Results of the Diabetes Surgery Study Comparing Taiwanese with Americans with Mild Obesity (BMI 30-35 kg/m2). Obes Surg 2017;27:1189-95. [Crossref] [PubMed]
- Lee WJ, Chong K, Aung L, et al. Metabolic surgery for diabetes treatment: sleeve gastrectomy or gastric bypass? World J Surg 2017;41:216-23. [Crossref] [PubMed]
- Sjöström L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 2004;351:2683-93. [Crossref] [PubMed]
- DiGiorgi M, Rosen DJ, Choi JJ, et al. Re-emergence of diabetes after gastric bypass in patients with mid- to long-term follow-up. Surg Obes Relat Dis 2010;6:249-53. [Crossref] [PubMed]
- Chikunguwo SM, Wolfe LG, Dodson P, et al. Analysis of factors associated with durable remission of diabetes after Roux-en-Y gastric bypass. Surg Obes Relat Dis 2010;6:254-59. [Crossref] [PubMed]
- Lee MH, Lee WJ, Chong K, et al. Predictors of long-term diabetes remission after metabolic surgery. J Gastrointest Surg 2015;19:1015-21. [Crossref] [PubMed]
- Peterli R, Borbely Y, Ker B, et al. Early results of the Swiss multicentre bypass or sleeve study (SM-BOSS): A prospective randomized trial comparing laparoscopic sleeve gastrectomy and Roux-en-Y gastric bypass. Ann Surg 2013;258:690-4; discussion 695. [Crossref] [PubMed]
- Zhang Y, Zhao H, Cao Z, et al. A randomized clinical trial of laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy for the treatment of morbid obesity in China: a 5-year outcome. Obes Surg 2014;24:1617-24. [Crossref] [PubMed]
- Vix M, Liu KH, Diana M, et al. Impact of Roux-en-Y gastric bypass versus sleeve gastrectomy on vitamin D metabolism: short-term results from a prospective randomized clinical trial. Surg Endosc 2014;28:821-6. [Crossref] [PubMed]
- Ignat M, Vix M, Imad I, et al. Randomized trial of Roux-en-Y gastric bypass versus sleeve gastrectomy in achieving excess weight loss. Br J Surg 2017;104:248-56. [Crossref] [PubMed]
- Ponce J, DeMaria E, Nguyen NT, et al. American society for metabolic and bariatric surgery estimation of bariatric surgery procedures in 2015 and surgeon workforce in the United States. Surg Obes Relat Dis 2016;12:1637-9. [Crossref] [PubMed]
- Angrisani L, Santonicola A, Iovino P, et al. Bariatric surgery worldwide 2013. Obes Surg 2015;25:1822-32. [Crossref] [PubMed]
- Lee WJ, Chong K, Ser KH, et al. Gastric bypass vs sleeve gastrectomy for type 2 diabetes mellitus: a randomized controlled trial. Arch Surg 2011;146:143-8. [Crossref] [PubMed]
- Lee WJ, Chong K, Lin YH, et al. Laparoscopic sleeve gastrectomy versus single anastomosis (mini-) gastric bypass for the treatment of type 2 diabetes mellitus: 5-year results of a randomized trial and study of incretin effect. Obes Surg 2014;24:1552-62. [Crossref] [PubMed]
- Lee WJ, Almulaifi AM, Tsou JJ, et al. Duodenal–jejunal bypass with sleeve gastrectomy versus the sleeve gastrectomy procedure alone: the role of duodenal exclusion. Surg Obes Relat Dis 2015;11:765-70. [Crossref] [PubMed]
- Sánchez-Pernaute A, Herrera MA, Pérez-Aguirre ME, et al. Single anastomosis duodeno-ileal bypass with sleeve gastrectomy (SADI-S). one to three-year follow-up. Obes Surg 2010;20:1720-6. [Crossref] [PubMed]
Cite this article as: Almalki OM, Lee WJ. Roux-en-Y gastric bypass for the treatment of Asian type II diabetes. Ann Laparosc Endosc Surg 2017;2:113.


