Secrets for successful laparoscopic antireflux surgery: preoperative workup
Introduction
Gastroesophageal reflux disease (GERD) is a multifactorial disease, present in 20% of the United States population, and was responsible for almost 9 million ambulatory care visits in 2009 (1-3). Typical symptoms include heartburn, regurgitation and dysphagia, which can be present in 20% to 80% of the patients. In contrast, atypical presentation with symptoms, such as bloating, cough, globus sensation, and hoarseness, is often misdiagnosed, despite being highly prevalent in this population (4-6).
Anti-acid medication is highly prescribed for the treatment of GERD, with an estimated cost of 10 billion dollars per year (7). These drugs account for 60% of the total cost of managing the disease in the United States, and 18% of Americans report taking proton-pump inhibitors (PPI) over the counter for GERD treatment (7). Despite the widespread pharmacologic treatment of GERD, antireflux surgery is currently the most effective and definitive treatment for the disease (2,4). Here, we address the importance of an accurate and appropriate preoperative workup for patients undergoing antireflux surgery.
Patient selection and workup
Indication for surgery
Several factors should be taken into consideration when deciding if the patient is a good candidate for antireflux surgery, including the patient’s previous treatment. The patient’s response to anti-acid medication and therapy duration are significant predictors of surgical success (4,7). Literature supports that patients who exhibited symptom improvement with medication and patients with well-controlled GERD (regardless of PPI dosage) are the best candidates for surgery. Patients who are non-compliant with medical therapy or those with a potential need for lifelong therapy will also benefit from the surgery. In spite of the success of PPIs, up to 45% of patients on PPI-therapy may have refractory symptoms. Any positive response during the treatment course, even if intermittent, can predict benefit from the surgery (3,6).
Presence of extra-esophageal or atypical symptoms should also be meticulously evaluated, since 50% of patients with cough or wheezing may not present with typical symptoms and still have a positive pH test. Antireflux surgery has been shown to improve up to 85% of the respiratory symptoms in these patients (8). Ruling out other primary causes for these symptoms, such as cardiac, respiratory or other intestinal-related diseases, is also crucial, and the appropriate diagnostic workup must be done before making a final decision (3,7).
The pH increase in the stomach due to long-term treatment with PPI can lead to a host of undesired effects, such as clostridium difficile infection or community-acquired pneumonia. The less acidic environment also impairs iron absorption and decreases the absorption of magnesium in the intestine. Patients may have diminished calcium absorption, resulting in bone fractures and osteoporosis, especially in post-menopausal women (5,7,9). Additionally, studies have reported increased risk for dementia, myocardial infarction and chronic kidney disease after long-term PPI therapy (7). The manifestation of any of these side effects should prompt discontinuation of the medication and the patient should be considered for surgical management of GERD (2,4).
Finally, the presence of any GERD complications is another indication for antireflux surgery. These include esophageal strictures, Barrett’s esophagus, ulcerations, and esophagitis, among others (4,6). Moreover, patients with a concomitant hiatal hernia found during an imaging exam, or with a proven defective lower esophageal sphincter, are excellent surgical candidates. Elevated body mass index, greater than 35, is not a contraindication for antireflux surgery. However, suboptimal results have been reported in those patients, and bariatric surgery should be discussed prior to GERD treatment (2,4,5).
Preoperative workup
The purpose of the preoperative workup of a candidate for antireflux surgery is to determine if GERD is the primary cause of the symptoms, evaluate esophageal motility and LES function, identify possible GERD complications, and determine which type of surgical therapy would be most appropriate for the patient (4,7). Preoperative workup includes symptom evaluation and esophageal testing, including pH monitoring, high-resolution manometry, upper GI endoscopy (EGD), and barium esophagram (5,6). However, whether all or some of these exams should be performed in each patient is still controversial.
Symptom evaluation
The evaluation of symptoms must be the first step in the assessment of a patient undergoing antireflux surgery. The presence of GERD symptoms alone are not sufficient as a formal indicator for surgery, and further preoperative tests are required (3,4,7). Bello et al. found that documented GERD symptoms alone had a positive predictive value for surgery of approximately 50–60% for typical symptoms (10). Validated questionnaires are useful tools for the qualification and quantification of symptoms, and these questionnaires play an important role in appropriately assessing the patient’s response to pharmacologic antireflux therapy. Some of the most commonly utilized questionnaires are: GERD-Q, GERD-HQRL, symptoms scores, and visual severity scale. This assessment is important not only in the preoperative workup, but also for efficacy evaluation during the postoperative follow-up period (4,7,9,10).
Ambulatory pH testing
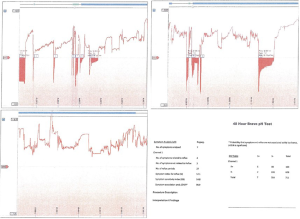
The pH test is currently the gold standard for GERD diagnosis (9), with a sensitivity and specificity that exceeds 90% (8). There are a few variants of the exam. The first version comprises a small monitoring device connected to an intra-nasal catheter, which stays in place for 24 hours. This period was later extended to 48 hours, since increasing the monitoring period improved the accuracy of the exam by 22% (6). With the advance of technology, a wireless system was created, allowing up to 96-hour of recording without causing any discomfort to the patient. Currently, the BRAVO system is the preferred pH test (4,9). This exam consists of a probe-capsule that is placed 6 cm above the gastroesophageal junction, and it records the pH in this area over time, without needing an intranasal catheter. The probe can be placed either endoscopically or guided by manometry, and it confirms the presence of elevated distal esophageal acid exposure (Figure 1) (4,5). A key factor is to discontinue any anti-acid medication, such as H2-blockers or PPI, at least 3 or 7 days prior to the study, respectively (4). The DeMeester score, described by Johnson and DeMeester in 1986, compiles parameters that are analyzed after the pH test, such as acid exposure time, number of episodes of reflux, and duration of those episodes (11). The cutoff score is 14.72, thus any score above that is considered abnormal and positive for GERD (4,9).
Impedance-pH monitoring is another innovation that evaluates the presence of any pathologic reflux, acid or nonacid (4,6). This exam is a good choice for patients who cannot discontinue antireflux medication and would, therefore, have a negative result in the regular pH testing. Moreover, impedance-pH monitoring is indicated for patients presenting with idiopathic cough, and for those with symptoms refractory to anti-acid medication (5,9).
Regardless of the method adopted, the correlation between the reflux episode and symptomatology is crucial. Hence, the patient must be advised to take notes every time he has a symptom presentation, and to take these notes within 2 minutes of the reflux occurrence. Later, this information must be crossed-tabulated with the data results from the pH test (2,6,10).
High-resolution manometry
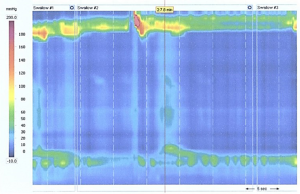
High-resolution manometry is the gold standard test to assess esophageal body motility and LES function. An intra-nasal catheter with 36 circumferential pressure sensors, at 1 cm intervals, is placed along the esophageal length to evaluate the pressure and bolus movement throughout the esophagus during ten consecutive swallows. Parameters such as upper and lower esophageal sphincter location and pressure, and peristalsis strength and speed are measured during the exam (4,9). The interpretation of high-resolution manometry is well-described by the new International Chicago Classification (12). It is important that the patient discontinue any anti-acid medication 3 days prior to the study (2). If a patient with GERD symptoms is found to have impaired peristalsis or defective LES, it may be due to another concomitant esophageal motility disorder, such as achalasia or scleroderma (Figure 2). In this case, a total fundoplication may be contraindicated, and the surgeon should opt for a partial fundoplication, such as Dor or Toupet (3,5,9,13). Manometry also facilitates the proper positioning of the catheter for pH testing, since it identifies the LES location (5,10).
Upper gastrointestinal endoscopy
Despite having a low sensitivity in the diagnosis of GERD, EGD is the most appropriate exam to evaluate GERD complications (2,10). It depicts the degree of reflux severity using the Los Angeles classification of esophagitis, which categorizes the disease into grades A-D, depending on the extension and quantity of mucosal breaks (5,6). At first, it was thought that the presence of an erosive disease would suffice for a surgical indication. However, a pH test is currently required to establish GERD, as many patients with erosion might have a negative pH test, and therefore may not benefit from the surgery (4,9). Also, more than 50% of patients with typical symptoms will have a non-erosive reflux disease, requiring a pH test result to establish the presence of GERD (14).
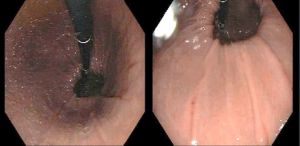
Endoscopy can detect other GERD complications, such as esophageal strictures or Barrett’s esophagus, which is present in up to 14% of patients (5). Furthermore, endoscopic study can identify the presence of hiatal hernias due to its capability of a retroflexed view of the cardia (Figure 3). A hiatal hernia is a well-established indication for antireflux surgery, with no need of further testing, except for high-resolution manometry to determine the appropriate surgical procedure. Finally, EGD should be performed in every patient with long-term PPI therapy, due to the increased risk of complications, as well as in those with history of dysphagia, weight loss, or bleeding. Endoscopy is crucial to rule out disease progression, diagnose non-GERD diseases (such as eosinophilic esophagitis) and, especially, to rule out esophageal cancer (4,9,15). The addition of random biopsies should only be indicated for the diagnosis of patients with an unclear history of symptoms and an apparent normal mucosa (9).
Barium swallow
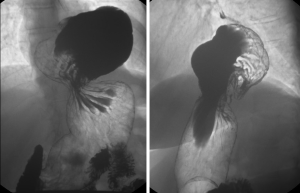
The barium swallow, or esophagram, has a very low sensitivity (34%) and specificity (85%) for diagnosing GERD (4,10). However, it provides a detailed anatomy of the entire esophagus and stomach, and assesses the esophageal body and sphincter function. Likewise, it can diagnose structure abnormalities, such as strictures and diverticula, and the presence of a Schatzki ring. More importantly, it assesses the presence of hiatal hernia, which can be found in up to 90% of GERD patients, as well as gathers information about the hernia size and type (Figure 4) (2,3,5,10). Additionally, 45% of patients will experience disease progression if they have non-treated GERD associated with a type 3 hiatal hernia (6). The advantages of barium swallow over upper endoscopy are that the former is a non-invasive esophageal test. However, it also has some limitations, since it is not possible to evaluate the esophageal mucosa, or perform any biopsies during the exam (4,5).
In summary, a careful preoperative workup done by experts is crucial for good patient outcomes. After a meticulous assessment for surgical candidacy, the first step in the preoperative workup of GERD is to establish GERD as the primary cause of the symptoms. Esophageal motility and LES function must be assessed in order to define the best surgical option, and to rule out other esophageal diseases in all surgical candidates. GERD complications, especially esophageal cancer, must also be excluded in these patients (4).
Acknowledgments
Funding: This study was funded by the Center for Advanced Surgical Technology at the University of Nebraska Medical Center and the Foundation for Surgical Fellows.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Fernando A. M. Herbella) for the series “Secrets for Successful Laparoscopic Antireflux Surgery” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2017.02.34). The series “Secrets for Successful Laparoscopic Antireflux Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- El-Serag HB, Sweet S, Winchester CC, et al. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut 2014;63:871-80. [Crossref] [PubMed]
- Andolfi C, Vigneswaran Y, Kavitt RT, et al. Laparoscopic Antireflux Surgery: Importance of Patient's Selection and Preoperative Workup. J Laparoendosc Adv Surg Tech A 2017;27:101-5. [Crossref] [PubMed]
- Fuchs KH, Babic B, Breithaupt W, et al. EAES recommendations for the management of gastroesophageal reflux disease. Surg Endosc 2014;28:1753-73. [Crossref] [PubMed]
- Oleynikov D. editor. Surgical Approaches to Esophageal Disease, An Issue of Surgical Clinics. Philadelphia: Elsevier Health Sciences, 2015.
- Fisichella PM, Patti MG. GERD procedures: when and what? J Gastrointest Surg 2014;18:2047-53. [Crossref] [PubMed]
- Jobe BA, Richter JE, Hoppo T, et al. Preoperative diagnostic workup before antireflux surgery: an evidence and experience-based consensus of the Esophageal Diagnostic Advisory Panel. J Am Coll Surg 2013;217:586-97. [Crossref] [PubMed]
- Patti MG, Schlottmann F, Farrell TM. Fundoplication for Gastroesophageal Reflux Disease: Tips for Success. J Laparoendosc Adv Surg Tech A 2017;27:1-5. [Crossref] [PubMed]
- Patti MG, Fisichella PM, Perretta S. Preoperative evaluation of patients with gastroesophageal reflux disease. J Laparoendosc Adv Surg Tech A 2001;11:327-31. [Crossref] [PubMed]
- Vela MF. Diagnostic work-up of GERD. Gastrointest Endosc Clin N Am 2014;24:655-66. [Crossref] [PubMed]
- Bello B, Zoccali M, Gullo R, et al. Gastroesophageal reflux disease and antireflux surgery-what is the proper preoperative work-up? J Gastrointest Surg 2013;17:14-20; discussion p. 20.
- Johnson LF, DeMeester TR. Development of the 24-hour intraesophageal pH monitoring composite scoring system. J Clin Gastroenterol 1986;8:52-8. [Crossref] [PubMed]
- Kahrilas PJ, Bredenoord AJ, Fox M, et al. The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil 2015;27:160-74. [Crossref] [PubMed]
- Chan WW, Haroian LR, Gyawali CP. Value of preoperative esophageal function studies before laparoscopic antireflux surgery. Surg Endosc 2011;25:2943-9. [Crossref] [PubMed]
- Yi CH, Liu TT, Chen CL. Atypical symptoms in patients with gastroesophageal reflux disease. J Neurogastroenterol Motil 2012;18:278-83. [Crossref] [PubMed]
- Stefanidis D, Hope WW, Kohn GP, et al. Guidelines for surgical treatment of gastroesophageal reflux disease. Surg Endosc 2010;24:2647-69. [Crossref] [PubMed]
Cite this article as: Armijo PR, Pagkratis S, Krause C, Oleynikov D. Secrets for successful laparoscopic antireflux surgery: preoperative workup. Ann Laparosc Endosc Surg 2017;2:58.