
Liver resection for hepatocellular carcinoma, are we going to dismiss the traditional approach?
Introduction
Hepatocellular carcinoma (HCC) is the most common primary liver cancer and one of the most common malignant tumors worldwide (1). A myriad of possibilities of therapeutic options are currently available for HCC patients, which include loco-regional treatment of small and single tumor, liver resection and orthotropic liver transplantation (LT) (2). Despite less than 30% of patients with HCC are eligible for surgical resection, surgery is still the most available curative treatment (3), with a post-operative mortality rate of 5%, even in patients undergoing major hepatectomy on cirrhotic liver disease (4), and a 5-year survival of 70% (5). The extent of hepatectomy results from the balance of complete tumor removal and preservation of functional future liver remnants (FLR). In spite of this, in the majority of cases, HCC is growing from a liver with an underlying disease, reducing liver function reserve and compromising the potential regeneration (6). In this case, postoperative liver failure and post operative complication risk are higher rather than in healthy liver (7). Gruttadauria et al. suggested that FLR should be >20–25% in patients with normal liver parenchyma, >30% in case of steatosis, hepatitis and in patients submitted to chemotherapy, and >40% in cases of chronic liver disease (8). Minimally invasive surgery has recently demonstrated results comparable to traditional surgery for recurrence-free or overall survival (9), even in cirrhotic patients (10). Furthermore recent meta-analysis concluded that laparoscopic liver resection (LLR) for HCC is superior to open approach in terms of its perioperative results and does not compromise the oncological outcomes (11). LT seems to be the ideal treatment for both tumor(s) and the underlying liver disease. However, numbers of liver graft is still low inducing to consider resection as a first option and LT in case of HCC recurrence (12,13).
The aim of our study is to analyze the evolution of traditional and minimally invasive liver resection for HCC in our Center since 2001.
Methods
Since 2001, all resected liver patients in our department were included in a prospectively maintained database. Between 2001 to December 2015, 1,463 liver resections were performed. Of them, 429 were HCC. Liver resection was performed if there was no extrahepatic metastasis observed at preoperative imaging. Evidence of tumor thrombosis was not an absolute contraindication. Liver segment resection was defined according to the Brisbane classification (14). A major resection was defined as a resection of three or more segments, and a minor resection as a resection of two or fewer segments. Surgical complications were classified as described by Dindo and colleagues (15). Major complications (Clavien-Dindo 3 and 4) and operative mortality (Clavien-Dindo 5) were considered when they occurred within 90 days after surgery or at any time during postoperative hospitalization. Liver-specific complications such ascites was defined as >10 mL/kg/day of drainage output from the abdomen after postoperative day 5 and bile leakage was defined as a bilirubin concentration in the drain fluid at least three times that of serum bilirubin on or after postoperative day 3 or as the need for radiological or operative intervention resulting from biliary collections or bile peritonitis. We divided the cohort into two groups: group 1, patients between 2001 and 2007 and group 2 patients between 2008 and 2015. All surgical procedures were performed by senior surgeon specialized in hepatobiliary surgery.
Results
Patients characteristics
Since 2001, 429 patients were resected in our department, of them 335 (78%) were male and 94 (22%) were female. Mean age was 66-year-old (range: 32–89) and the mean body mass index was 25.9. Baseline alpha-fetoprotein was 2,431.8 UI (range: 0.8–100,000), in 20 cases Child Pugh score B was observed, mean MELD score was 7.96 (6-16). A HCV related underling liver disease was observed in 308 (71.8%), a HBV in 47 (11%), alcohol in 31 (7.2%).
Of 166 patients in the group 1, 134 (80%) were male. Mean age was 66-year-old and the mean body mass index was 25.3. Baseline alpha-fetoprotein in group 1 was 2,431.7 UI, in 12 cases Child Pugh score B was observed, mean MELD score was 8 (range: 6–15).
In the second group, 201 patients (76.4%) were male. Mean age was 66-year-old and the mean body mass index was 25.8. Baseline alpha-fetoprotein in group 2 was 1,132.4 UI (range: 0.8–44,052), in 8 cases Child Pugh score B was observed, mean MELD score was 7.93 (range: 6–16).
The overall and groups patients characteristics are resumed in Table 1.
Table 1
| Characteristics | Total | 2001–2007, group 1 (n=166) | 2008–2015, group 2 (n=263) |
|---|---|---|---|
| Male gender, n (%) | 335 (78.1) | 134 (81.0) | 201 (76.4) |
| Age (years), mean [range] | 66 [32–89] | 66 [32–88] | 66 [38–89] |
| BMI | 25.9 (17.7–41.5) | 25.3 (17.7–36.2) | 25.8 (15.3–41.5) |
| AFP (ng/mL), mean [range] | 2,431.8 [0.8–100,000] | 2,431.7 [1–100,000] | 1,132.4 [0.8–44,052] |
| Child-Pugh class B, n (%) | 20 (4.6) | 12 (7.2) | 8 (3.04) |
| Etiology, n (%) | |||
| HBV | 47 (11.0) | 24 (14.6) | 23 (8.8) |
| HCV | 308 (71.8) | 116 (69.8) | 192 (73.0) |
| Alcohol | 31 (7.2) | 13 (7.8) | 18 (6.8) |
| Other | 43 (10.0) | 13 (7.8) | 30 (11.4) |
| MELD score, mean [range] | 7.96 [6–16] | 8 [6–15] | 7.93 [6–16] |
| Tumor size (mm), mean [range] | 48.7 [4–300] | 52.9 [4–300] | 46 [4–230] |
Operative finding
In group 1, we performed 42 major hepatectomies (25.3%) and 124 minor hepatectomies (74.7%). In group 2, we respectively performed 49 (18.6%) major hepatectomies and 214 (81.4%) minor hepatectomies. We had no difference between the two groups for major or minor surgery (P=0.09). On the other hand, an important difference was observed between the laparoscopic approaches in the two groups (P<0.00001). In group 1, 3% of patients and 44.5% in group 2 were treated by LLR. Despite this important difference, surgical time was no different, but blood transfusion has improved between the two period (P=0.02). The surgical procedure is resumed in Table 2.
Table 2
| Surgical procedure | Total | 2001–2007 | 2008–2015 |
|---|---|---|---|
| Hepatectomies, n (%) | |||
| Major | 91 (21.2) | 42 (25.3) | 49 (18.6) |
| Minor | 338 (78.8) | 124 (74.7) | 214 (81.4) |
| Laparoscopy, n (%) | 122 (28.4) | 5 (3.0) | 117 (44.5) |
| Major | 1 | 0 | 1 |
| Minor | 121 | 5 | 116 |
| Operative time, min, mean [range] | 197.6 [36–540] | 196.8 [55–540] | 198.1 [36–500] |
| Estimated blood loss, mL, mean [range] | 188.7 [10–1,000] | 198.9 [20–1,000] | 182.6 [10–1,000] |
| Transfusion, n (%) | 21 (5.1) | 13 (8.4) | 8 (3.0) |
| Pedicle clamping, n (%) | 82 (19.0) | 53 (33.0) | 29 (11.0) |
| Intraoperative complications rate (%) | 19 (4.4) | 7 (4.3) | 12 (4.6) |
We observed 6 deaths since the first liver resection for HCC. All of them during the first period analyzed (group 1). This results was support by an improvement of morbidity between the two groups (P<0.001). Post operative results with complication are resumed in Table 3.
Table 3
| Complication | Total | 2001–2007 | 2008–2015 |
|---|---|---|---|
| Mortality, n (%) | 6 (1.3) | 6 (3.6) | 0 (0) |
| Clavien-Dindo (%) | |||
| 0 | 145 (33.7) | 51 (30.7) | 94 (35.7) |
| I | 182 (42.4) | 61 (36.7) | 121 (46.0) |
| II | 67 (15.6) | 32 (19.2) | 35 (13.3) |
| IIIa/IIIb | 11 (2.5) | 8 (4.8) | 3 (0.05) |
| IV | 5 (1.1) | 2 (1.2) | 3 (0.05) |
| V | 6 (1.3) | 6 (3.6) | 0 (0) |
| Overall morbidity > II, n (%) | 89 (20.7) | 48 (28.9) | 41 (15.5) |
| Intensive care unit stay, days, mean [range] | 0.48 [0–14] | 0.57 [0–14] | 0.43 [0–9] |
| Hospital stay, days, mean [range] | 10 [0–37] | 12 [0–37] | 8 [2–33] |
Discussion
The number of LLR has increased since 2001. We observed an increasing of laparoscopic approach in the second group until reaching a stable number of LLR each year (Figure 1). Furthermore, we observed an improvement of morbidity between the two groups associated with no death in the second group. These results were obtained maintaining stable operative time. According with the second consensus conference held on Morioka (16), laparoscopic minor resection is often performed in our unit for HCC. Our previous study demonstrates the feasibility of a laparoscopic approach in HCC patients with chronic liver disease, even in selected Child B patients (17). On the other hand, the new loco-regional treatment, gastrointestinal therapy and anesthesiologist supports, allow to operate initial unresectable HCC. Nonetheless, surgical procedure as associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) authorize surgeon to propose curative resection in patients with previously considered unresectable HCC. The presence of macroscopic tumor thrombosis contraindicates LT and laparoscopic resection. Nevertheless, in those patients the ALPPS procedure has been described to be an effective curative treatment, in these cases with a traditional open surgery (18,19). Minimally invasive surgery is growing every year with impressive technical supports. Not last, the use of a robotic system can improve certain steps of minimally invasive major hepatectomy (20). Robotic ALPPS may have a place for HCC patients (21). Totally laparoscopic ALPPS is described as feasible but must be performed by experienced hands (22,23). Furthermore, performing the initial HCC resection by laparoscopy could facilitate a subsequent LT (24). On top, LLR thanks to a reduction of post-operative liver failure and ascites development in comparison to standard open (25). Moreover, LLR could be proposed in patients with clinical signs of mild portal hypertension (25). Technical difficulty for LLR seems to be overpass, in our experience we treated with laparoscopic approach HCC in each one liver segment (17). Therefore, limitation of the posterior localization of the HCC has been now overpassed (26).
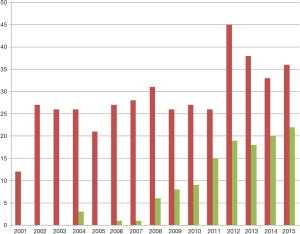
However, the complexity of HCC resection on patients with cirrhosis seems to leave a place to the traditional approach.
Conclusions
Laparoscopic approach in HCC is gaining more places even in patients with cirrhosis. Major LLR in those patients is still selected cases. In our experience, the traditional surgical approach has still a place for the major resection in patients with HCC.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Laparoscopic and Endoscopic Surgery for the series “Laparoscopic Liver Surgery”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2017.02.15). The series “Laparoscopic Liver Surgery” was commissioned by the editorial office without any funding or sponsorship. Levi Sandri GB served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Annals of Laparoscopic and Endoscopic Surgery from Aug 2016 to Jul 2018. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study is approved by the institutional ethical committee (2016/43) and obtained the informed consent from every patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lai Q, Lerut JP. Hepatocellular cancer: how to expand safely inclusion criteria for liver transplantation. Curr Opin Organ Transplant 2014;19:229-34. [Crossref] [PubMed]
- Rahbari NN, Mehrabi A, Mollberg NM, et al. Hepatocellular carcinoma: current management and perspectives for the future. Ann Surg 2011;253:453-69. [Crossref] [PubMed]
- Forner A, Reig ME, de Lope CR, et al. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis 2010;30:61-74. [Crossref] [PubMed]
- Capussotti L, Muratore A, Massucco P, et al. Major liver resections for hepatocellular carcinoma on cirrhosis: early and long-term outcomes. Liver Transpl 2004;10:S64-8. [Crossref] [PubMed]
- Eguchi S, Kanematsu T, Arii S, et al. Recurrence-free survival more than 10 years after liver resection for hepatocellular carcinoma. Br J Surg 2011;98:552-7. [Crossref] [PubMed]
- Fan ST. Liver functional reserve estimation: state of the art and relevance for local treatments: the Eastern perspective. J Hepatobiliary Pancreat Sci 2010;17:380-4. [Crossref] [PubMed]
- Poon RT, Fan ST, Lo CM, et al. Extended hepatic resection for hepatocellular carcinoma in patients with cirrhosis: is it justified? Ann Surg 2002;236:602-11. [Crossref] [PubMed]
- Gruttadauria S, Vasta F, Minervini MI, et al. Significance of the effective remnant liver volume in major hepatectomies. Am Surg 2005;71:235-40. [PubMed]
- Gaillard M, Tranchart H, Dagher I. Laparoscopic liver resections for hepatocellular carcinoma: current role and limitations. World J Gastroenterol 2014;20:4892-9. [Crossref] [PubMed]
- Twaij A, Pucher PH, Sodergren MH, et al. Laparoscopic vs open approach to resection of hepatocellular carcinoma in patients with known cirrhosis: systematic review and meta-analysis. World J Gastroenterol 2014;20:8274-81. [Crossref] [PubMed]
- Zhou YM, Shao WY, Zhao YF, et al. Meta-analysis of laparoscopic versus open resection for hepatocellular carcinoma. Dig Dis Sci 2011;56:1937-43. [Crossref] [PubMed]
- Cucchetti A, Vitale A, Del Gaudio M, et al. Harm and benefits of primary liver resection and salvage transplantation for hepatocellular carcinoma. Am J Transplant 2010;10:619-27. [Crossref] [PubMed]
- Fuks D, Dokmak S, Paradis V, et al. Benefit of initial resection of hepatocellular carcinoma followed by transplantation in case of recurrence: an intention-to-treat analysis. Hepatology 2012;55:132-40. [Crossref] [PubMed]
- The Terminology Committee of the IHPBA. The Brisbane 2000 terminology of hepatic anatomy and resections. HPB 2000;2:333-9.
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Wakabayashi G, Cherqui D, Geller DA, et al. Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg 2015;261:619-29. [PubMed]
- Ettorre GM, Levi Sandri GB, Santoro R, et al. Laparoscopic liver resection for hepatocellular carcinoma in cirrhotic patients: single center experience of 90 cases. Hepatobiliary Surg Nutr 2015;4:320-4. [PubMed]
- Vennarecci G, Laurenzi A, Levi Sandri GB, et al. The ALPPS procedure for hepatocellular carcinoma. Eur J Surg Oncol 2014;40:982-8. [Crossref] [PubMed]
- Levi Sandri GB, Lai Q, Rayar M, et al. ALPPS procedure for hepatocellular carcinoma with macrovascular thrombosis: a new opportunity? J Hepatol 2015;62:241-2. [Crossref] [PubMed]
- Giulianotti PC, Sbrana F, Coratti A, et al. Totally robotic right hepatectomy: surgical technique and outcomes. Arch Surg 2011;146:844-50. [Crossref] [PubMed]
- Levi Sandri GB, Guerra F. Are We Ready to Perform Fully Minimally Invasive ALPPS? Ann Surg 2015; [Epub ahead of print]. [Crossref] [PubMed]
- Xiao L, Li JW, Zheng SG. Totally laparoscopic ALPPS in the treatment of cirrhotic hepatocellular carcinoma. Surg Endosc 2015;29:2800-1. [Crossref] [PubMed]
- Schelotto PB, Gondolesi G. Laparoscopy in ALPPS Procedure: When We Can Do It? Ann Surg 2015; [Epub ahead of print]. [Crossref] [PubMed]
- Levi Sandri GB, Vennarecci G, Santoro R, et al. Laparoscopic left liver lobectomy for hepatocellular carcinoma in a cirrhotic patient: a video report. Chin J Cancer Res 2014;26:735-6. [PubMed]
- Belli A, Cioffi L, Russo G, et al. Liver resection for hepatocellular carcinoma in patients with portal hypertension: the role of laparoscopy. Hepatobiliary Surg Nutr 2015;4:417-21. [PubMed]
- Levi Sandri GB, de Werra E, Mascianà G, et al. Laparoscopic and robotic approach for hepatocellular carcinoma-state of the art. Hepatobiliary Surg Nutr 2016;5:478-84. [Crossref] [PubMed]
Cite this article as: Levi Sandri GB, de Werra E, Mascianà G, Ferraro D, Colasanti M, Vennarecci G, Ettorre GM. Liver resection for hepatocellular carcinoma, are we going to dismiss the traditional approach? Ann Laparosc Endosc Surg 2017;2:34.


