内镜黏膜下剥离术治疗早期胃腺癌:文献回顾
引言
胃癌(gastric cancer)是全球第5大最常见的癌症,2012年新发病例数为952 000[1]。胃癌患病率最高的区域为西太平洋地区,尤其是中国、韩国和日本。这些国家许多已经开展了早期胃癌的筛查,以期降低该疾病的高致病率与死亡率。日本和韩国开展了全国性的早期胃癌筛查计划,即所有年龄>40岁的公民,每2年接受1次上消化道内镜检查或钡餐吞服检查。文献报道表明该计划使胃癌早期诊断率获得提升[2,3]。在日本,约50%的胃癌在确诊时仍为局限于黏膜或黏膜下层的早期胃癌[4]。
既往治疗早期胃癌的主流方法是手术切除,而即使利用现代技术,手术切除仍可能导致较高的围术期和远期并发症[5]。这一难题也促进了内镜切除早期胃癌的技术创新与发展。内镜下黏膜切除(endoscopic mucosal resection,EMR)于20世纪80年代在日本到应用,该技术的目的是完整切除局限于胃黏膜层的小病灶,这些病灶淋巴结转移和远处转移的风险低,因此采用内镜下切除可获得根治性治疗效果[6]。随后,这项技术被扩展为内镜黏膜下剥离术(endoscopic submucosal dissection,ESD),该技术可实现对更深层病灶更精确的剥离,对更大的病灶也可实现更高的整块切除率和完整切除率。随着手术流程与技术的改进,特制的手术器械也随之问世,ESD如今也成功应用于更加复杂的早期胃癌病灶的治疗。
诊断
上消化道内镜检查是早期胃癌的主要诊断模式。对恶性肿瘤病灶进行完整评价的方法包括评价其组织病理学类型、病灶大小、侵袭深度,以及是否存在溃疡性病灶[8]。在一项随机对照研究中,使用日本奥林巴斯的“三重模式”内镜(Olympus Medical System,Tokyo,Japan),即将高分辨率白光(white light imaging,WLI)与窄带光成像(narrow band imaging,NBI)和自体荧光成像(autofluorescent imaging,AFI)两种增强放大成像模式相结合的检查模式。这种增强光学成像技术可针对浅表性胃癌病灶表面的微血管病灶进行分析[9],与其他传统内镜诊断方法相比可获得最高的敏感性(89%)与特异性(100%),并提高早期胃癌的诊断率[10]。其他辅助方法,如染色内镜,通常使用靛蓝胭脂红染色剂(可添加或不添加乙酸),因其在恶性肿瘤病灶表面的洗脱速度更快而被广泛用于识别恶性肿瘤病灶的边界与表面结构[11]。内镜下还可使用活检钳对病灶进行活检,以获取病灶的病理学与组织学诊断。
对病灶侵袭深度的评估可采用白光内镜成像对病灶表面的特点加以判断。表面光滑的外凸型或凹陷型病灶、病灶边缘轻度抬高,以及皱襞光滑变细等特点常提示黏膜性疾病,而表面不光滑、边缘抬高明显、皱襞聚集融合、走形不规则等特点则常提示存在黏膜下层浸润[12]。超声内镜(endoscopic ultrasound,EUS)在鉴别早期胃癌黏膜层与黏膜下层侵袭中的作用目前尚存争议。近期一篇荟萃分析报道EUS评估黏膜层侵袭的敏感性与特异性分别为76%和72%,而评估黏膜下层侵袭的敏感性与特异性分别为62%和78%[13]。另一项研究表明传统内镜相比EUS的诊断效能更高,单纯内镜检查组的诊断准确性为73.7%,EUS组为67.4%(n=955,P<0.001)[14]。在一篇EUS对早期胃癌分级的Cochrane系统综述中,EUS诊断区域性淋巴结转移的敏感性与特异性亦较为局限,分别为83%和67%[15]。是否使用EUS检查最终取决于内镜医生的个人偏好。
切除标准
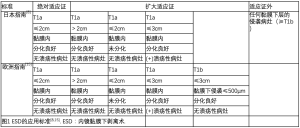
并非所有早期胃癌均可通过ESD切除。日本消化内镜学会(Japan Gastroenterological Endoscopy Society,JGES)与日本胃癌学会(Japan Gastric Cancer Association,JGCA)于2016年对ESD的诊疗指南发表了一项联合声明[8],欧洲胃肠内镜学会(European Society of Gastrointestinal Endoscopy,ESGE)于2015年也发表了类似的声明[15]。这些指南旨在对淋巴结转移风险可忽略不计的病灶进行识别。两项声明均建议将病灶位于黏膜内、分化型、非溃疡性病灶、直径小于2 cm的腺癌作为ESD的绝对适应证,而某些方面的微小差异可作为扩大适应证(图1)。
ESD技术
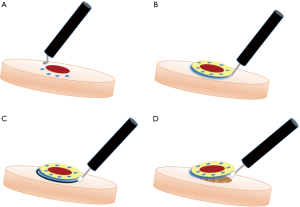
ESD可在全麻或静脉镇静下进行,使用接入能量源的治疗用内镜,注入二氧化碳(相比空气充气患者耐受度更高)[17],按照标准操作步骤进行(图2)[18]。首先,使用电灼器械环形标记待切除病灶的周围,以确定切除范围。然后将靛蓝胭脂红或亚甲基蓝注射于黏膜下层以抬举病灶,以更好地辨别切除平面。随后,使用尖端绝缘的特制手术电刀[如IT刀(Olympus America, Center Valley, USA)]先行环形切除,再深入黏膜下层完整切除病灶。如操作中发生出血,可用电凝或止血夹进行止血。
ESD技术应该需要事先进行特定的培训,以便术者更好地掌握手术操作,确保充分切除的同时减少并发症。日本的一家培训中心表明,对于位置较好的胃远端病灶须有30例成功切除经验才能掌握熟练的操作技术,而对于更复杂的远端病灶或中部至近端胃体的病灶,则需要额外进行进一步训练[19]。ESD的术前培训可使用离体猪或活体猪等模拟训练模型[20,21]。在西方国家,由于早期胃癌较为少见,因此使用这些非人体训练模型是尤其重要的。
结果
ESD与EMR效果对比
许多中心仍然会对<2 cm和分化良好的病灶采用EMR治疗。在一个纳入177名早期胃癌的队列研究中,这些患者同时符合EMR和ESD的切除标准,结果显示对于>5 mm的病灶,ESD的整体切除率、完整切除率及局部复发率结果均占优[22],且并发症发生率未见显著升高。2014年发表的一项荟萃分析纳入了先前的研究和9项额外的回顾性研究,该分析进一步比较了EMR和ESD的上述指标差异,结果表明尽管ESD组穿孔率明显更高(OR 4.67,2.77-7.87),但其余指标差异与前文结论一致[7]。EMR确实仍可用于特定病灶的治疗,但ESD对于更大、更复杂病灶的切除仍是业内主流,而且可获得更精确的切除效果。
ESD与手术效果对比
ESD和手术切除的长期肿瘤学结果是相似的。与手术切除效果相关的最长随访数据来自韩国的一个单中心研究,该研究将611对符合ESD或手术绝对与相对适应证的术后患者进行倾向性得分匹配,完成了长达10年的随访[23]。结果表明,手术组与ESD组的总体10年生存率分别为94.9%和96.7%(P=0.120)。并发症发生率在不同队列中存在差异,但ESD组总体相对更低[24,25]。其他大型研究表明,内镜治疗术后早期并发症发生率相对更高,而手术治疗的并发症发生时间通常更晚[23]。相比手术切除,ESD在各研究中的术后住院天数均更短,ESD组平均术后住院天数为5~7天,而手术组术后住院天数为11~13天[23,26]。
绝对标准与扩大标准效果对比
ESD对符合绝对标准与扩大标准患者的治疗结果是相近的。日本的两项大型研究(纳入超过1000名患者)表明,无论采用何种纳入标准,术后5年总体生存率均>90%[27,28]。另外,多项大型回顾性研究也报道了相似的结果,且无病生存率[28,29]或局部复发率[29,30]均未见差异。多数研究表明,相比扩大标准(汇总结果为64.5%~88.4%),采用绝对标准纳入患者的完整切除率更高(汇总结果为91.1%~95.9%),而日本的一项多中心研究的两组间完整切除率是相似的(93.4% vs 96.4%,P=0.736),整体切除率均>97%(组间P=0.867)[28-31]。局部复发率与异时性复发率在这些研究中均未见明显差异。部分研究表明采用扩大标准进行ESD治疗的穿孔率(6.6% vs 2.4%,P<0.001)与出血率(6.36% vs 3.31%,P=0.020)更高[28,31]。扩大标准所纳入的病灶在技术上可能更难以切除,而这些病灶如能实现完整切除,则其肿瘤学指标相比绝对标准所纳入的病灶是相近的。
淋巴结转移
早期胃癌出现淋巴结转移提示显著更差的生存期[32],因此内镜切除治疗所假定的前提是淋巴结转移的风险是可忽略不计的。一项针对接受胃切除术的T1a或T1b期胃癌患者的荟萃分析表明,对于黏膜层病灶,总体淋巴结转移率为3.2%,而黏膜下层病灶总体淋巴结转移率为19.2%[33]。Gotoda等在2000和2001年的开创性研究[34,35]表明,淋巴血管侵犯、肿瘤病灶>30 mm、溃疡性病灶及组织学未分化是早期胃癌淋巴结转移的独立危险因素。在一项纳入超过5 000例患者的研究中,当上述危险因素不存在,且肿瘤局限于黏膜层或黏膜下浅层(<500 μm)时,淋巴结转移的风险为0。这也构成了被广泛采用的前文所述绝对切除标准与扩大切除标准的基础。
后续研究进一步回顾性分析了既往接受胃切除+标准淋巴结清扫的早期胃癌患者,并证实了上述结果,而这些患者均符合如今行ESD的切除标准。尽管这些病例淋巴结转移的风险不是0,但也是极低的。韩国的一项纳入3,951名患者的研究表明,符合绝对ESD切除标准的患者淋巴结转移风险为0.3%,而符合扩大ESD切除标准的患者淋巴结转移风险为0.4%[32]。另一项纳入超过1 000名患者的回顾性研究纳入淋巴结转移患者18例(1.8%),而未分化型病灶淋巴结侵犯的风险比为6.104(95%置信区间:1.1317-28.284)[36]。为更准确地鉴别局灶性肿瘤,相关学术组织正致力于进行切除标准的更新。
术后复发
ESD治疗后可能出现局部复发与异时性复发。韩国的一项倾向性得分匹配队列研究表明,ESD切除后的局部复发率为1.6%(5/288),而胃切除术后局部复发率为0.6%(1/173),但无统计学差异[37]。ESD切除后发生异时性复发的风险体现为内镜下切除与手术切除之间主要的肿瘤学差异,前者发生异时性复发的风险为5.3%~6.2%,而后者的风险低至几乎可忽略不计[23,26,38]。即便如此,异时性复发病灶也可通过再次内镜下剥离成功治疗,而不会导致生存期的减少[26,39]。
ESD发生局部复发的危险因素也得到了更好的认识。仅有的几项研究表明,即使切缘为阴性,分块切除肿瘤可导致局部复发风险提高[40,41]。然而,即使是整块切除后,仍可能出现局部复发。与局部复发相关的因素包括肿瘤边界不清、切缘距肿瘤距离近(<1 mm)及病灶位于胃体上1/3处。肿瘤体积>3 cm、淋巴血管侵犯、黏膜下层浸润深度>500 μm也与水平切缘阳性及局部复发相关[42,43]。组织学分化程度与同时性及异时性病灶的发生率升高相关[44]。
西方研究结果
西方国家关于ESD的文献数据十分有限。仅有的一项直接比较ESD与胃癌切除术的研究来自加拿大,该小型研究(ESD组n=30,手术组n=37)表明两组间术后2年生存期无统计学差异;然而,由于本研究样本量较小,且ESD组同时纳入了异型增生性病灶(30%),研究结果的证据效力较为有限[45]。德国的一项纳入91名患者的研究表明在西方人群中对早期胃癌及腺瘤进行ESD是可行的,但失败率较高,且整块切除率较低[41]。这可能与西方有相关疾病的患者数量少、培训机会减少相关。
非治愈性切除/适应证外病灶
内镜切除的可行性是基于术前评估加以判断的。最近的一项研究表明ESD术前与术后病理特征存在显著差异,在经ESD切除的所有病灶中,15.9%(120/756)的最终病理结果不符合适应证,30.2%(29/96)初始分级为扩大适应证的病灶最终证实为超适应证[46]。这些最终病理发现为超适应证的患者通常须接受外科手术治疗。
许多学者都在致力于确定接受非治愈性切除术后发生肿瘤复发或淋巴结转移的特定危险因素,以更好地指导后续治疗。目前最大的多中心回顾性研究来自日本,该研究纳入了1 969名接受非治愈性ESD治疗后采取手术治疗或仅观察随访的患者,发现两组间的3年总体生存期存在显著差异(96.7% vs 84.0%,P<0.001);无病生存率差异虽更小,但也具有统计学意义(99.4% vs 98.7%,P=0.012)[47,48]。对总体生存期的解读应更加谨慎,因为采取观察随访的患者年龄更大,合并症也更多。对于那些超适应证但无淋巴血管侵犯或切缘阳性的病灶,淋巴结转移或局部复发的风险并未升高,提示对于经仔细筛选的特定患者,仅采用随访也可能是合适的方案[47,49]。
疾病监测
ESD术后的常规监测对发现局部复发与异时性复发是十分重要的。治愈性切除后的上消化道内镜检查通常在初始治疗后3~6个月进行,随后每6~12个月复查1次[8,16]。回顾性研究表明,即使是符合绝对适应证的病灶,接受初始切除5年后,发生异时性复发的概率仍稳定于每年约3.5%,提示长期监测可能是必要的[50,51]。常规CT复查一般不是必须的,因为该检查发现胃外转移的概率极低,但仍有部分中心常规开具术后CT检查;一项纳入2 182例患者的研究表明ESD切除5年后每年行CT检查仅发现了2例胃外复发[52]。
并发症
ESD术后并发症包括出血(5%)、穿孔(1%~9%)与狭窄(1%~2%)。出血既可发生于术后早期(<48 h),也可为迟发性出血(≥48 h)。出血的危险因素包括患者及肿瘤与操作的相关特点。一项荟萃分析表明,慢性肾病、肿瘤>2 cm、切除标本>3 cm、操作时间>60 min这些因素导致出血风险的风险比均>2.0[53]。肿瘤位于胃体下1/3也是出血的危险因素之一[54]。一些研究者报道了局部喷涂纤维蛋白胶成功预防迟发性出血的经验;一项研究表明使用该方法后,出血率从对照组的5.98%降低至0(P=0.03)[55]。抗血小板与抗凝药物通常在大多数患者的围术期应停用,但持续服用低剂量阿司匹林似乎并未增加出血风险[56]。肝素替代治疗与恢复抗血小板治疗是造成迟发性出血风险显著升高的危险因素[57,58]。降低出血风险的方案,包括围术期应用质子泵抑制药(proton pump inhibitor, PPI)[59]或二次内镜检查并未获得一致的获益结果[60]。然而,已有研究发表了使用PPI,尤其是与黏膜促愈剂(如瑞巴派特,已在日本、韩国和中国获批)合用成功治疗因ESD所致医源性溃疡的成功经验[61,62]。
ESD术后胃穿孔是另一个主要并发症,既可发生于内镜切除时,也可迟发于切除后数天。ESD总体穿孔发生率介于1.2%与9.5%之间[63-65]。危险因素包括肿瘤自身特征(如组织学分化较差、浸润深度较深、病灶位于近端胃、病灶纤维化等),以及患者年龄及操作时间等[63,65,66]。穿孔一般情况下可在内镜下使用标准止血夹或OTSC(over the scope clips,OTSC)吻合夹(Ovesco Endoscopy USA Inc.,Cary,USA)内镜下缝合和(或)仅观察出血情况[64,66]。更复杂的内镜下操作,如圈套器-内镜夹技术,已成功用于直径达2 cm穿孔的修补。这项技术首先使用圈套器夹持穿孔边缘的正常黏膜,随后收紧圈套器并固定,以使缺损边缘重新对合[67]。对于迟发性穿孔,如未并发腹膜炎或脓毒症,可尝试使用鼻胃管减压及抗生素等方法保守治疗[64]。如出现临床失代偿或存在腹膜炎,则应行大网膜修补或手术切除等方法治疗。
ESD术后狭窄是较为罕见的并发症。较大病灶的内镜下切除可导致近环形的溃疡,而位于贲门或胃窦部的病灶发生术后狭窄的风险最高[68,69]。患者可出现吞咽困难或胃流出道梗阻的相关症状。与狭窄相关的症状可通过球囊扩张进行治疗,但这也可能导致胃穿孔而需要进一步的内外科治疗[68,70]。其他治疗方法包括内镜下黏膜内切开以缓解ESD术区张力,或经病灶内或静脉使用类固醇药物以改善肉芽组织生长,并减轻组织纤维化[69,71]。
特殊人群
对于既往接受胃部手术或食管切除胃代食管而出现胃解剖结构改变的胃癌患者,ESD可能带来额外的风险。一些小样本研究也报道了这类患者接受ESD治疗的肿瘤学结果。一项研究表明,对于经胸胃代食管的患者,近半数需要在ESD进镜前对吻合口狭窄段行球囊扩张,但这些病例最终均成功完成操作[72]。值得注意的是,一项研究报道的穿孔率为18%,发生率相比正常解剖结构的患者明显更高,因为这些患者的操作难度更高[74]。
结论
ESD是治疗早期胃癌的一项重要技术,对于有经验的中心,可在成功切除病灶的前提下具有较低的并发症与较好的肿瘤控制效果。对于患者数量低的中心而言,可能存在较长的学习曲线,且治疗效果通常欠佳。最优切除标准与随访计划尚待后续研究进一步完善。
Conclusions
ESD is an important technique which allows for successful curative resection of early gastric cancer with low morbidity and excellent oncologic value in skilled centers. A substantial learning curve persists in low volume areas, and outcomes are worse in those areas. Further investigation is needed to establish optimal resection criteria and surveillance patterns.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2017.01.07). RZ serves as an unpaid editorial board member of Annals of Laparoscopic and Endoscopic Surgery from Jul 2016 to Jun 2018. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Suh M, Song S, Cho HN, et al. Trends in Participation Rates for the National Cancer Screening Program in Korea, 2002-2012. Cancer Res Treat 2016; [Epub ahead of print]. [Crossref] [PubMed]
- Allemani C, Weir HK, Carreira H, et al. Global surveillance of cancer survival 1995-2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet 2015;385:977-1010. [Crossref] [PubMed]
- Noguchi Y, Yoshikawa T, Tsuburaya A, et al. Is gastric carcinoma different between Japan and the United States? Cancer 2000;89:2237-46. [Crossref] [PubMed]
- Lu W, Gao J, Yang J, et al. Long-term clinical outcomes of laparoscopy-assisted distal gastrectomy versus open distal gastrectomy for early gastric cancer: A comprehensive systematic review and meta-analysis of randomized control trials. Medicine 2016;95:e3986 [Crossref] [PubMed]
- Hiki Y. Endoscopic mucosal resection (EMR) for early gastric cancer. Nippon Geka Gakkai Zasshi 1996;97:273-8. [PubMed]
- Facciorusso A, Antonino M, Di Maso M, et al. Endoscopic submucosal dissection vs endoscopic mucosal resection for early gastric cancer: A meta-analysis. World J Gastrointest Endosc 2014;6:555-63. [Crossref] [PubMed]
- Ono H, Yao K, Fujishiro M, et al. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer. Dig Endosc 2016;28:3-15. [Crossref] [PubMed]
- Kato M, Kaise M, Yonezawa J, et al. Trimodal imaging endoscopy may improve diagnostic accuracy of early gastric neoplasia: a feasibility study. Gastrointest Endosc 2009;70:899-906. [Crossref] [PubMed]
- Zhu L, Qin J, Wang J, et al. Early Gastric Cancer: Current Advances of Endoscopic Diagnosis and Treatment. Gastroenterol Res Pract 2016;2016:9638041.
- Lee BE, Kim GH, Park DY, et al. Acetic acid-indigo carmine chromoendoscopy for delineating early gastric cancers: its usefulness according to histological type. BMC Gastroenterol 2010;10:97. [Crossref] [PubMed]
- Choi J, Kim SG, Im JP, et al. Endoscopic prediction of tumor invasion depth in early gastric cancer. Gastrointest Endosc 2011;73:917-27. [Crossref] [PubMed]
- Pei Q, Wang L, Pan J, et al. Endoscopic ultrasonography for staging depth of invasion in early gastric cancer: A meta-analysis. J Gastroenterol Hepatol 2015;30:1566-73. [Crossref] [PubMed]
- Choi J, Kim SG, Im JP, et al. Comparison of endoscopic ultrasonography and conventional endoscopy for prediction of depth of tumor invasion in early gastric cancer. Endoscopy 2010;42:705-13. [Crossref] [PubMed]
- Mocellin S, Pasquali S. Diagnostic accuracy of endoscopic ultrasonography (EUS) for the preoperative locoregional staging of primary gastric cancer. Cochrane Database Syst Rev 2015;CD009944 [PubMed]
- Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2015;47:829-54. [Crossref] [PubMed]
- Kim SY, Chung JW, Park DK, et al. Efficacy of carbon dioxide insufflation during gastric endoscopic submucosal dissection: a randomized, double-blind, controlled, prospective study. Gastrointest Endosc 2015;82:1018-24. [Crossref] [PubMed]
- ASGE Technology Committee. Endoscopic submucosal dissection. Gastrointest Endosc 2015;81:1311-25. [Crossref] [PubMed]
- Oda I, Odagaki T, Suzuki H, et al. Learning curve for endoscopic submucosal dissection of early gastric cancer based on trainee experience. Dig Endosc 2012;24:129-32. [Crossref] [PubMed]
- Kato M, Gromski M, Jung Y, et al. The learning curve for endoscopic submucosal dissection in an established experimental setting. Surg Endosc 2013;27:154-61. [Crossref] [PubMed]
- Herreros de Tejada A. ESD training: A challenging path to excellence. World J Gastrointest Endosc 2014;6:112-20. [Crossref] [PubMed]
- Nakamoto S, Sakai Y, Kasanuki J, et al. Indications for the use of endoscopic mucosal resection for early gastric cancer in Japan: a comparative study with endoscopic submucosal dissection. Endoscopy 2009;41:746-50. [Crossref] [PubMed]
- Pyo JH, Lee H, Min BH, et al. Long-Term Outcome of Endoscopic Resection vs. Surgery for Early Gastric Cancer: A Non-inferiority-Matched Cohort Study. Am J Gastroenterol 2016;111:240-9. [Crossref] [PubMed]
- Shin DW, Hwang HY, Jeon SW. Comparison of Endoscopic Submucosal Dissection and Surgery for Differentiated Type Early Gastric Cancer within the Expanded Criteria. Clin Endosc 2016; [Epub ahead of print]. [Crossref] [PubMed]
- Fukunaga S, Nagami Y, Shiba M, et al. Long-term prognosis of expanded-indication differentiated-type early gastric cancer treated with endoscopic submucosal dissection or surgery using propensity score analysis. Gastrointest Endosc 2017;85:143-52. [Crossref] [PubMed]
- Ryu SJ, Kim B-W, Kim BG, et al. Endoscopic submucosal dissection versus surgical resection for early gastric cancer: a retrospective multicenter study on immediate and long-term outcome over 5 years. Surg Endosc 2016;30:5283-9. [Crossref] [PubMed]
- Gotoda T, Iwasaki M, Kusano C, et al. Endoscopic resection of early gastric cancer treated by guideline and expanded National Cancer Centre criteria. Br J Surg 2010;97:868-71. [Crossref] [PubMed]
- Nakamura K, Honda K, Akahoshi K, et al. Suitability of the expanded indication criteria for the treatment of early gastric cancer by endoscopic submucosal dissection: Japanese multicenter large-scale retrospective analysis of short- and long-term outcomes. Scand J Gastroenterol 2015;50:413-22. [Crossref] [PubMed]
- Park CH, Shin S, Park JC, et al. Long-term outcome of early gastric cancer after endoscopic submucosal dissection: expanded indication is comparable to absolute indication. Dig Liver Dis 2013;45:651-6. [Crossref] [PubMed]
- Ahn JY, Jung HY, Choi KD, et al. Endoscopic and oncologic outcomes after endoscopic resection for early gastric cancer: 1370 cases of absolute and extended indications. Gastrointest Endosc 2011;74:485-93. [Crossref] [PubMed]
- Lee H, Yun WK, Min BH, et al. A feasibility study on the expanded indication for endoscopic submucosal dissection of early gastric cancer. Surg Endosc 2011;25:1985-93. [Crossref] [PubMed]
- Choi KK, Bae JM, Kim SM, et al. The risk of lymph node metastases in 3951 surgically resected mucosal gastric cancers: implications for endoscopic resection. Gastrointest Endosc 2016;83:896-901. [Crossref] [PubMed]
- Kwee RM, Kwee TC. Predicting lymph node status in early gastric cancer. Gastric Cancer 2008;11:134-48. [Crossref] [PubMed]
- Gotoda T, Sasako M, Ono H, et al. Evaluation of the necessity for gastrectomy with lymph node dissection for patients with submucosal invasive gastric cancer. Br J Surg 2001;88:444-9. [Crossref] [PubMed]
- Gotoda T, Yanagisawa A, Sasako M, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer 2000;3:219-25. [Crossref] [PubMed]
- Oh SY, Lee KG, Suh YS, et al. Lymph Node Metastasis in Mucosal Gastric Cancer: Reappraisal of Expanded Indication of Endoscopic Submucosal Dissection. Ann Surg 2017;265:137-42. [Crossref] [PubMed]
- Cho JH, Cha SW, Kim HG, et al. Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: a comparison study to surgery using propensity score-matched analysis. Surg Endosc 2016;30:3762-73. [Crossref] [PubMed]
- Kim YI, Kim YW, Choi IJ, et al. Long-term survival after endoscopic resection versus surgery in early gastric cancers. Endoscopy 2015;47:293-301. [Crossref] [PubMed]
- Choi IJ, Lee JH, Kim YI, et al. Long-term outcome comparison of endoscopic resection and surgery in early gastric cancer meeting the absolute indication for endoscopic resection. Gastrointest Endosc 2015;81:333-41.e1. [Crossref] [PubMed]
- Horiki N, Omata F, Uemura M, et al. Risk for local recurrence of early gastric cancer treated with piecemeal endoscopic mucosal resection during a 10-year follow-up period. Surg Endosc 2012;26:72-8. [Crossref] [PubMed]
- Probst A, Pommer B, Golger D, et al. Endoscopic submucosal dissection in gastric neoplasia - experience from a European center. Endoscopy 2010;42:1037-44. [Crossref] [PubMed]
- Fu QY, Cui Y, Li XB, et al. Relevant risk factors for positive lateral margin after en bloc endoscopic submucosal dissection for early gastric adenocarcinoma. J Dig Dis 2016;17:244-51. [Crossref] [PubMed]
- Lee JY, Cho KB, Kim ES, et al. Risk factors for local recurrence after en bloc endoscopic submucosal dissection for early gastric cancer. World J Gastrointest Endosc 2016;8:330-7. [Crossref] [PubMed]
- Park CH, Kim EH, Kang JH, et al. Low Incidence of Synchronous or Metachronous Tumors after Endoscopic Submucosal Dissection for Early Gastric Cancer with Undifferentiated Histology. PLoS One 2016;11:e0147874 [Crossref] [PubMed]
- Najmeh S, Cools-Lartigue J, Mueller C, et al. Comparing Laparoscopic to Endoscopic Resections for Early Gastric Cancer in a High Volume North American Center. J Gastrointest Surg 2016;20:1547-53. [Crossref] [PubMed]
- Kim YI, Kim HS, Kook MC, et al. Discrepancy between Clinical and Final Pathological Evaluation Findings in Early Gastric Cancer Patients Treated with Endoscopic Submucosal Dissection. J Gastric Cancer 2016;16:34-42. [Crossref] [PubMed]
- Toyokawa T, Ohira M, Tanaka H, et al. Optimal management for patients not meeting the inclusion criteria after endoscopic submucosal dissection for gastric cancer. Surg Endosc 2016;30:2404-14. [Crossref] [PubMed]
- Hatta W, Gotoda T, Oyama T, et al. Is radical surgery necessary in all patients who do not meet the curative criteria for endoscopic submucosal dissection in early gastric cancer? A multi-center retrospective study in Japan. J Gastroenterol 2017;52:175-84. [Crossref] [PubMed]
- Sunagawa H, Kinoshita T, Kaito A, et al. Additional surgery for non-curative resection after endoscopic submucosal dissection for gastric cancer: a retrospective analysis of 200 cases. Surg Today 2017;47:202-9. [Crossref] [PubMed]
- Min BH, Kim ER, Kim KM, et al. Surveillance strategy based on the incidence and patterns of recurrence after curative endoscopic submucosal dissection for early gastric cancer. Endoscopy 2015;47:784-93. [Crossref] [PubMed]
- Kato M, Nishida T, Yamamoto K, et al. Scheduled endoscopic surveillance controls secondary cancer after curative endoscopic resection for early gastric cancer: a multicentre retrospective cohort study by Osaka University ESD study group. Gut 2013;62:1425-32. [Crossref] [PubMed]
- Choi KS, Kim SH, Kim SG, et al. Early Gastric Cancers: Is CT Surveillance Necessary after Curative Endoscopic Submucosal Resection for Cancers That Meet the Expanded Criteria? Radiology 2016;281:444-53. [Crossref] [PubMed]
- Libânio D, Costa MN, Pimentel-Nunes P, et al. Risk factors for bleeding after gastric endoscopic submucosal dissection: a systematic review and meta-analysis. Gastrointest Endosc 2016;84:572-86. [Crossref] [PubMed]
- Furuhata T, Kaise M, Hoteya S, et al. Postoperative bleeding after gastric endoscopic submucosal dissection in patients receiving antithrombotic therapy. Gastric Cancer 2017;20:207-14. [Crossref] [PubMed]
- Tan ES, Wang H, Lua GW, et al. Fibrin Glue Spray as a Simple and Promising Method to Prevent Bleeding after Gastric Endoscopic Submucosal Dissection. Dig Surg 2016;33:455-61. [Crossref] [PubMed]
- Matsumura T, Arai M, Maruoka D, et al. Risk factors for early and delayed post-operative bleeding after endoscopic submucosal dissection of gastric neoplasms, including patients with continued use of antithrombotic agents. BMC Gastroenterol 2014;14:172. [Crossref] [PubMed]
- Koh R, Hirasawa K, Yahara S, et al. Antithrombotic drugs are risk factors for delayed postoperative bleeding after endoscopic submucosal dissection for gastric neoplasms. Gastrointest Endosc 2013;78:476-83. [Crossref] [PubMed]
- Yoshio T, Nishida T, Kawai N, et al. Gastric ESD under Heparin Replacement at High-Risk Patients of Thromboembolism Is Technically Feasible but Has a High Risk of Delayed Bleeding: Osaka University ESD Study Group. Gastroenterol Res Pract 2013;2013:365830.
- Nishizawa T, Suzuki H, Akimoto T, et al. Effects of preoperative proton pump inhibitor administration on bleeding after gastric endoscopic submucosal dissection: A systematic review and meta-analysis. United European Gastroenterol J 2016;4:5-10. [Crossref] [PubMed]
- Mochizuki S, Uedo N, Oda I, et al. Scheduled second-look endoscopy is not recommended after endoscopic submucosal dissection for gastric neoplasms (the SAFE trial): a multicentre prospective randomised controlled non-inferiority trial. Gut 2015;64:397-405. [Crossref] [PubMed]
- Shin WG, Kim SJ, Choi MH, et al. Can rebamipide and proton pump inhibitor combination therapy promote the healing of endoscopic submucosal dissection-induced ulcers? A randomized, prospective, multicenter study. Gastrointest Endosc 2012;75:739-47. [Crossref] [PubMed]
- Kajiura S, Hosokawa A, Ueda A, et al. Effective healing of endoscopic submucosal dissection-induced ulcers by a single week of proton pump inhibitor treatment: a retrospective study. BMC Res Notes 2015;8:150. [Crossref] [PubMed]
- Toyokawa T, Inaba T, Omote S, et al. Risk factors for perforation and delayed bleeding associated with endoscopic submucosal dissection for early gastric neoplasms: analysis of 1123 lesions. J Gastroenterol Hepatol 2012;27:907-12. [Crossref] [PubMed]
- Suzuki H, Oda I, Sekiguchi M, et al. Management and associated factors of delayed perforation after gastric endoscopic submucosal dissection. World J Gastroenterol 2015;21:12635-43. [Crossref] [PubMed]
- Yoo JH, Shin SJ, Lee KM, et al. Risk factors for perforations associated with endoscopic submucosal dissection in gastric lesions: emphasis on perforation type. Surg Endosc 2012;26:2456-64. [Crossref] [PubMed]
- Chung IK, Lee JH, Lee SH, et al. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc 2009;69:1228-35. [Crossref] [PubMed]
- Kato M, Uraoka T, Wada M, et al. A large muscle-layer defect of the stomach caused by endoscopic submucosal dissection is closed by using the endoloop-clips technique. Gastrointest Endosc 2016;83:1282-3. [Crossref] [PubMed]
- Iizuka H, Kakizaki S, Sohara N, et al. Stricture after endoscopic submucosal dissection for early gastric cancers and adenomas. Dig Endosc 2010;22:282-8. [Crossref] [PubMed]
- Kim GH, Jee SR, Jang JY, et al. Stricture occurring after endoscopic submucosal dissection for esophageal and gastric tumors. Clin Endosc 2014;47:516-22. [Crossref] [PubMed]
- Tsunada S, Ogata S, Mannen K, et al. Case series of endoscopic balloon dilation to treat a stricture caused by circumferential resection of the gastric antrum by endoscopic submucosal dissection. Gastrointest Endosc 2008;67:979-83. [Crossref] [PubMed]
- Mori H, Kobara H, Fujihara S, et al. Recanalization of severe gastric antral stricture after large endoscopic submucosal dissection: mucosal incision and local steroid injection. J Gastrointestin Liver Dis 2012;21:435-7. [PubMed]
- Mukasa M, Takedatsu H, Matsuo K, et al. Clinical characteristics and management of gastric tube cancer with endoscopic submucosal dissection. World J Gastroenterol 2015;21:919-25. [Crossref] [PubMed]
- Lee JY, Min BH, Lee JG, et al. Endoscopic Submucosal Dissection for Early Gastric Neoplasia Occurring in the Remnant Stomach after Distal Gastrectomy. Clin Endosc 2016;49:182-6. [Crossref] [PubMed]
- Nishide N, Ono H, Kakushima N, et al. Clinical outcomes of endoscopic submucosal dissection for early gastric cancer in remnant stomach or gastric tube. Endoscopy 2012;44:577-83. [Crossref] [PubMed]

肖怀文
上海市浦东新区浦南医院普外科。从事普外科工作23年以来,先后在多所三甲医院及同济大学医学院不断学习普外科医疗及腹腔镜、内镜技术,师从上海多位高级专家,酷爱医学事业,熟练掌握普外医疗及科研技术。(更新时间:2021/8/12)
(本译文仅供学术交流,实际内容请以英文原文为准。)
Cite this article as: Gray KD, Moore MD, Elmously A, Zarnegar R. Endoscopic submucosal dissection for early gastric adenocarcinoma: a review of the literature. Ann Laparosc Endosc Surg 2017;2:17.