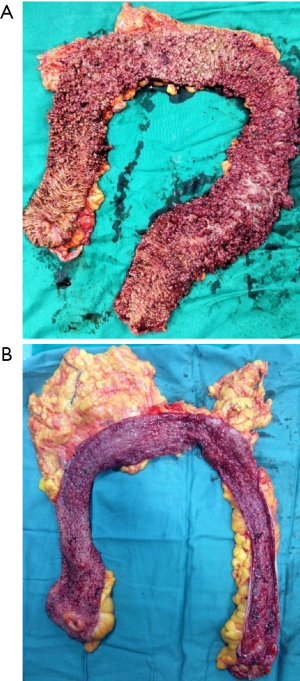
Technique of laparoscopic-assisted total proctocolectomy and ileal pouch anal anastomosis
Introduction
Total proctocolectomy (TPC) with ileal pouch anal anastomosis (IPAA) was first described in 1978 (1). Since then, this procedure has been applied to patients with ulcerative colitis (UC) and familial adenomatous polyposis (FAP) as a first choice. Laparoscopic-assisted TPC with IPAA has been applied since the early 2000s (2). Several reports have demonstrated that laparoscopic operations are feasible and safe, with decreased postoperative time in hospital, better cosmetic result, fewer intra-abdominal adhesions, and less wound complications (3-5).
Laparoscopic-assisted TPC with IPAA has been gradually gaining acceptance. Stapled ileal J pouch-anal anastomosis has been the preferred technique for its efficiency and good long-term functional outcome (6). We have employed laparoscopic-assisted TPC with IPAA for the treatment of UC and FAP since 2010. Most of our patients underwent a two-stage procedure while patients with acute severe UC underwent a three-stage procedure. Here we present our current technique and experience of laparoscopic-assisted TPC with IPAA followed at our centre.
Patient selection and workup
Laparoscopic-assisted TPC with IPAA has become a first choice surgical procedure for UC patients who require surgery and FAP. The surgical strategy for UC patients depends on whether they are elective cases or emergency (7).
IPAA can be performed as a one, two or three stage procedure. In elective cases the procedure is performed either as a two or one stage (pouch construction without a diverting ileostomy) procedure. In our practice, we prefer a two-stage approach as the standard procedure. A restorative proctocolectomy with an IPAA and diverting loop ileostomy is the first stage while reversal of loop ileostomy is the second stage operation.
Patients with acute severe colitis who are in poor general conditions and/or experiencing the side effects of corticosteroids or immunomodulators should led to a three stage procedure. A total colectomy with end ileostomy will precede the pouch procedure as the first stage in the three stage approach. A restorative proctectomy approximately 3–6 months later with completion proctectomy, IPAA and diverting loop ileostomy is the second stage followed by reversal of the ileostomy as the final stage.
Pre-operative preparation
A multidisciplinary approach between the gastroenterologists and surgeons is essential if the patient has a diagnosis of UC.
The patient’s medical condition should be optimized prior to surgery, by correcting anemia, coagulopathy, hypovolemia, electrolyte or acid-base imbalances, and any nutritional deficiencies. Corticosteroids should be weaned if possible. Failure to wean from prednisolone 20 mg daily or equivalent for more than six weeks prior to surgery, should postpone pouch construction to a second stage (7).
Patient’s tolerance of prolonged general anaesthesia should be assessed. All patients received oral mechanical bowel preparation. The patients were managed with a single dose of broad-spectrum intravenous antibiotics preoperatively.
Equipment preference card
Commonly used laparoscopic instruments:
- Video camera unit;
- Light source;
- CO2 insufflator;
- 30-degree laparoscope (5 or 10 mm);
- Suction/irrigator;
- Veress needle;
- Scissors with cautery attachment;
- Babcock graspers;
- Atraumatic bowel handling graspers;
- Circular stapler;
- Laparoscopic linear stapler;
- Hem-O-Lock;
- LigaSure (optional);
- 5-mm harmonic scalpel.
Procedure
Patient position and room setup
Patient is positioned in Trendelenburg with low lithotomy, both arms by side of patient and tucked in, legs in Allen stirrups such that the thighs are level with the abdomen. During the operation, wide position swings and steep inclinations of the operating table are required to assist in achieving proper exposure of the operative field. Take care of the legs position to avoid injury to the peroneal nerves and other pressure-related injury.
Two monitors are best placed on both sides of patient. The position of the surgeon should be ergonomically altered depending on the dissection of individual segment of the colon. The key to the appropriate position of the surgeon is to maintain a parallel view with the laparoscope, working instruments, and the monitors.
Port placement
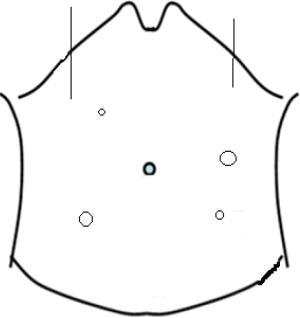
Pneumoperitoneum is established with Veress needle and 10-mm port is inserted through supraumbilical after induction of anaesthesia. The 30-degree laparoscope is introduced through the 10-mm trocar. The 12-mm working port in through the right lower quadrant rectus abdominis muscle (the site for the ileostomy, identified prior to insufflations). The 5-mm ports are inserted in right upper quadrant, left upper quadrant and left lower quadrant at mid clavicular line (Figure 1). The intra-abdominal pressure is set at 12 mmHg and flow at 10 L/min.
Operative steps
There are five main phases in the operation. First phase is consisted of mobilization of the rectum, sigmoid colon and transection of the rectum as far as the pelvic floor. Second phase consisted of mobilization of the descending colon, splenic flexure and left half of the transverse colon. Third phase consisted of mobilization of terminal ileum, cecum, ascending colon, hepatic flexure, and the right half of transverse colon and lengthening of root of mesentery. Fourth phase is consisted of specimen extraction and creation of ileal J-pouch outside abdomen, return to laparoscopy and ileal pouch-anal anastomosis. Fifth phase is construction of ileostomy.
Mobilization and division of the sigmoid colon and rectum
The primary operating surgeon stands on the right side of the patient, with the camera surgeon and assistant surgeon standing on the patient’s left side. In steep Trendelenburg position small intestinal loops are gravitational migrated to the upper abdomen and sacral promontory is identified. In female patients, the uterus may be suspended to the lower anterior abdominal wall by a sling suture passing through the body of the uterus and brought out over the abdominal wall, just above the symphysis pubis.
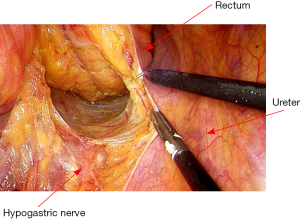
With the retraction of the sigmoid colon cephalad and medially, the peritoneal incision is then extended distally to the midrectum entering the presacral space. The presacral space is developed by the division of the fine adhesions and care should be taken to protect hypogastric nerves and both ureters. Then mobilization is made in the avascular plane behind the mesenteric vessels. The superior hemorrhoidal and sigmoid vessels are isolated and divided between Hemolock clips (Figure 2). As disease is not malignant, there is no need to clear the root of inferior mesenteric artery, which may damage super hypogastric nerve (8).
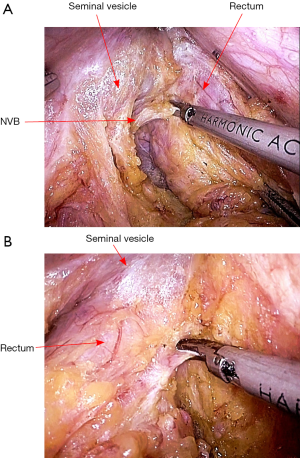
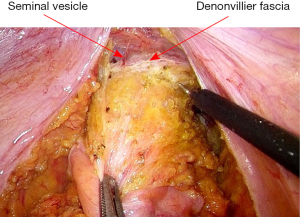
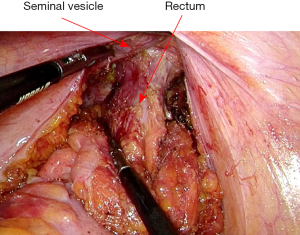
After vascular ligation, dissection continues down the presacral space in this avascular plane toward the pelvic floor, which is similar to total mesorectal excision (TME) (Figure 3). The dissection continues laterally on either side until it meets posteriorly developing a presacral plane (Figures 4,5). During posterior and lateral dissection, care must be taken to avoid injury the inferior hypogastric nerves on sidewall of pelvis. Anterior dissection is commenced posterior to Denonvilliers’ fascia (Figure 6) (9). Take care to avoid injury to seminal vesicles and neurovascular bundle (NVB).
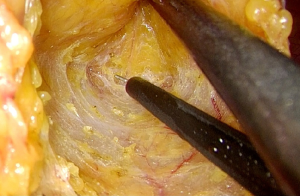
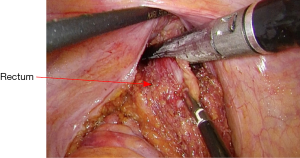
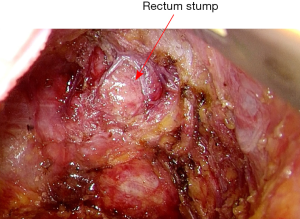
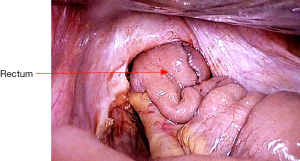
The mobilization continues down to the pelvic floor (Figure 7). The rectum is then divided at the pelvic floor using a reticulating endoscopic 60 mm linear cutting stapler (Figure 8). An adequate distal margin approximately 2 cm above the dentate line is confirmed by digital examination, which is performed to confirm the location of the distal staple line after placement of the stapler and before division of the bowel (Figure 9).
Mobilization of descending colon, splenic flexure and left half of transverse colon
After mobilization of the sigmoid colon and rectum, surgeon starts left colectomy standing on right side of patient. Camera surgeon stands on left of surgeon and assistant surgeon between the legs of the patient. The patient is rotated with the left side up, and the small bowel loops are swept to the right side of the abdominal cavity using the atraumatic bowel graspers.
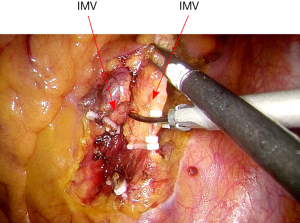
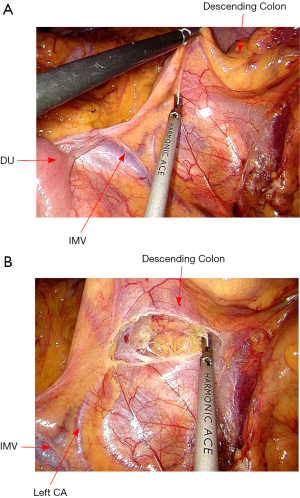
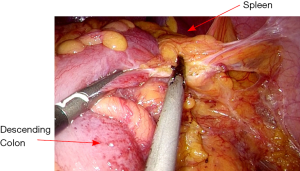
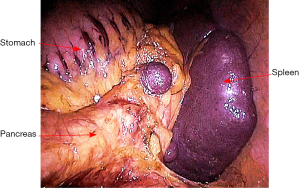
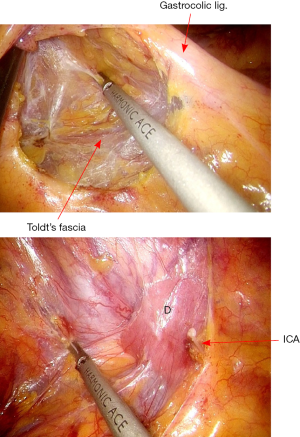
The rectosigmoid mesentery is stretched up toward the left lower quadrant, the descending colon is stretched up toward the left upper quadrant. The descending colon mesentery is incised lateral to the inferior mesenteric vein (IMV) (Figure 10). The left colonic vessels are isolated and divided between Hemolock clips. Expose the plane between mesocolon and left Gerota’s fascia to mobilize the left colon mesentery. Mobilize the left colon up along the Toldt’s fascia until the inferior margin of pancreas and then uncover the body and tail of pancreas (Figure 11). During the phase, dissection is carried out from medial to lateral and down to up. Take care to protect the gonadal vessels and left ureter. Lateral attachments of descending colon are divided with ultrasonic shears, until splenic flexure is mobilized (Figure 12).
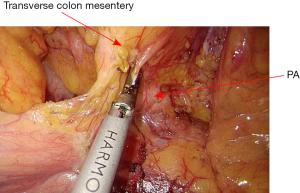
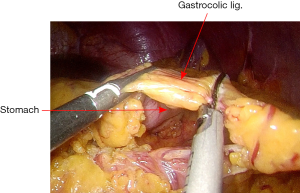
The assistant grasps the greater omentum superior to the distal transverse colon and retracts upward toward left. The surgeon dissects the omentum with the countertraction and enters the greater sac. The dissection is then advanced toward the splenic flexure (Figure 13). The left half of transverse colon is mobilized, taking the greater omentum and protecting the gastroepiploic arcade.
Mobilization of the right colon and terminal ileum
After splenic flexure and left half of transverse colon were mobilized (Figure 14), the surgeon moved to the patient’s left side, camera surgeon between the patient’s legs and an assistant surgeon on the right side of the patient. The operation table is tilted right side up and head low. The small bowel loops are swept to the left side of the abdominal cavity.
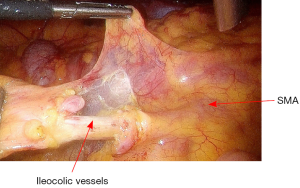
The dissection commences with the opening the peritoneum of right mesocolon. From medial to lateral direction, creates a plane between mesocolon and right Gerota’s fascia. As the ileocolic pedicle is mobilized, Toldt’s fascia is carefully protected on the retroperitoneum, thereby protecting the ureter, the duodenum, and the retroperitoneal structures (Figure 15). Dissection is carried out using ultracision. The ileocolic vessels are isolated and divided between Hemolock clips (Figure 16).
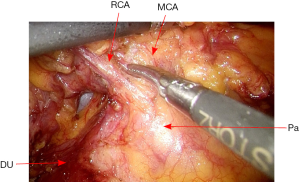
Then the peritoneal incision is extended superiorly toward the transverse colon, and then the dissection continues along the transverse colon inferiorly. The middle colic vessels and right colic vessels are then carefully mobilized and divided (Figure 17). The root of small bowel mesentery is mobilized adequately, so that the distal ileum loop will reach to the pelvic floor with no tension.
After the colon is freed from the peritoneal attachments on the medial side, the dissection continues along the white line of Toldt, starting from the cecum to the hepatic flexure and then right half of transverse colon.
Specimen extraction, creation of ileal pouch and pouch anal anastomosis
After mobilization of entire colorectum, in this phase, we prefer a 5-cm ventral midline incision into the abdominal cavity. Insert a plastic sheath in abdominal incision to protect the wound. Then, the TPC specimen is extracted, and sufficient length of distal ileum is brought out.
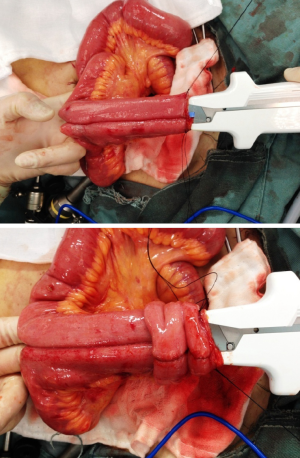
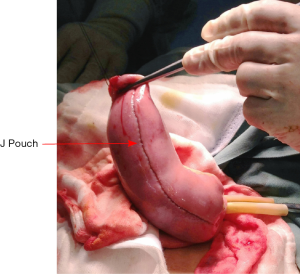
The pouch designs used for IPAA include J, S, or W type. The J-pouch is the most commonly used, for its good functional outcomes and easier to construct (7). A J-pouch is constructed from the terminal 40 to 50 cm of small intestine in a standard fashion. The distal ileum segment is folded into two segments. At the pouch apex, a 1 cm enterotomy is made longitudinally. A side-to-side anastomosis of the two segments of the ileum is done by firing two linear GIA 100 mm staplers (Figure 18). The blind loop of the J-pouch is closed by another linear stapler and then oversewn with 3/0 absorbable sutures.
The inside of pouch is inspected and cautery or sutures at stapler line are used to achieve complete hemostasis. Insufflation using saline is performed to confirm the integrity of the pouch (Figure 19). The anvil of 29 mm circular stapler is inserted in the pouch apex, fixed with a purse-string suture. Put the pouch into the pelvic floor to judge the pouch tension. A tension-free anastomosis is very important to IPAA operation. Ileocolic vessels ligation at the origin of SMA and adequate mobilization of small bowel mesentery are useful to decrease pouch tension. Small peritoneal incisions over the superior mesenteric vessels border can be done as an additional pouch lengthening maneuver. After all these maneuvers, if there is still obviously tension, permanent end ileostomy is inevitable.
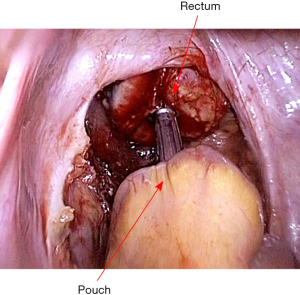
The pouch is then returned to the abdomen. The small bowel should be correctly oriented to prevent torsion of the mesentery. Insert the EEA stapler gun per anum, and fix the anvil to its head (Figure 20). An ileal pouch-anal anastomosis is then fashioned without torsion or tension (Figure 21). An air test is performed to ensure there is no leak. After confirming hemostasis and instrument count, a pelvic drain is placed.
Construction of loop ileostomy
Finally, take out the 12-mm trocar at right lower quadrant (previously marked ileostomy site), and expand the incision to about 3 cm. Loop of ileum (about 40 cm proximal to the anastomosis) is brought out. A temporary diverting ileostomy is constructed. Midline abdomen incision and ports are then closed in layers. Specimen is send to pathological examination (Figure 22).
Post-operative management
We do not recommend broad-spectrum intravenous antibiotics postoperatively. Clear liquid diet is started when the bowel function resumed. Bladder catheter is removed on fifth post-operative day. Drainage tube is removed when bowel sounds are present and output is low, usually on postoperative day 4–5. The patient is discharged from the hospital once he or she is mobile, and the ileostomy is well functioning. Sutures are removed on seventh post-operative day if no wound infection is present.
The patients with peristomal skin problems should be followed up by an ostomy nurse specialist. Ileostomy reversal is performed after confirming pouch integrity after approximately 12 weeks. The patients will be followed up in our out-patient clinic at 2 week, 1 month, 3 months and yearly.
Tips, tricks and pitfalls
- Care should be taken during handling of bowel in patients particularly in UC patients due to increased fragility of the tissues. Atraumatic graspers should be preferred to avoid direct grasping of the colon.
- In benign conditions, lymph nodes near the root of inferior mesenteric artery need not to be dissected, which may damage superior hypogastric nerve.
- As disease is benign, in order to avoid any nerve injury, we prefer to use similar TME dissection.
- The rectum should be divided at the pelvic floor and the staple line less than 2 cm above the dentate line.
- J-pouch with double stapling technique has been the preferred technique, because it is easier to create and get good functional outcomes.
- A tension-free anastomosis is very important to IPAA operation. Ileocolic vessels ligation at the origin of SMA and adequate mobilization of small bowel mesentery can provide an anastomosis with no tension.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2016.09.07). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Parks AG, Nicholls RJ. Proctocolectomy without ileostomy for ulcerative colitis. Br Med J 1978;2:85-8. [Crossref] [PubMed]
- Larson DW, Cima RR, Dozois EJ, et al. Safety, feasibility, and short-term outcomes of laparoscopic ileal-pouch-anal anastomosis: a single institutional case-matched experience. Ann Surg 2006;243:667-70; discussion 670-2. [Crossref] [PubMed]
- Baek SJ, Dozois EJ, Mathis KL, et al. Safety, feasibility, and short-term outcomes in 588 patients undergoing minimally invasive ileal pouch-anal anastomosis: a single-institution experience. Tech Coloproctol 2016;20:369-74. [Crossref] [PubMed]
- Delaney CP, Chang E, Senagore AJ, et al. Clinical outcomes and resource utilization associated with laparoscopic and open colectomy using a large national database. Ann Surg 2008;247:819-24. [Crossref] [PubMed]
- Tilney HS, Lovegrove RE, Heriot AG, et al. Comparison of short-term outcomes of laparoscopic vs open approaches to ileal pouch surgery. Int J Colorectal Dis 2007;22:531-42. [Crossref] [PubMed]
- Lovegrove RE, Constantinides VA, Heriot AG, et al. A comparison of hand-sewn versus stapled ileal pouch anal anastomosis (IPAA) following proctocolectomy: a meta-analysis of 4183 patients. Ann Surg 2006;244:18-26. [Crossref] [PubMed]
- Øresland T, Bemelman WA, Sampietro GM, et al. European evidence based consensus on surgery for ulcerative colitis. J Crohns Colitis 2015;9:4-25. [PubMed]
- Jani K, Shah A. Laparoscopic total proctocolectomy with ileal pouch-anal anastomosis for ulcerative colitis. J Minim Access Surg 2015;11:177-83. [Crossref] [PubMed]
- Madnani MA, Mistry JH, Soni HN, et al. Laparoscopic restorative proctocolectomy ileal pouch anal anastomosis: How I do it? J Minim Access Surg 2015;11:218-22. [Crossref] [PubMed]
Cite this article as: Wu B, Zhong ME. Technique of laparoscopic-assisted total proctocolectomy and ileal pouch anal anastomosis. Ann Laparosc Endosc Surg 2016;1:13.