
Second-stage treatment strategy for severely obese patient with primary aldosteronism—laparoscopic adrenalectomy after laparoscopic sleeve gastrectomy: a case report
Highlight box
Key findings
• We report the case of a severely obese patient with primary aldosteronism (PA) who was successfully treated with laparoscopic adrenalectomy 6 months after laparoscopic sleeve gastrectomy (LSG).
What is known and what is new?
• Obesity is related to perioperative complications and also affects therapeutic efficacy after adrenalectomy.
• Metabolic surgery, such as LSG, are powerful surgical procedures for weight loss in severely obese patients.
• Here, we report the usefulness of LSG before adrenalectomy in severely obese patients with PA.
What is the implication, and what should change now?
• Performing LSG prior to adrenalectomy in severely obese patients with PA should be considered a rational treatment strategy to reduce the risk of perioperative complications and improve therapeutic efficacy after adrenal surgery.
Introduction
Primary aldosteronism (PA) accounts for approximately 10% of patients with hypertension who visit primary care centers, and is a major cause of curable hypertension (1). If a unilateral aldosterone-producing tumor can be confirmed by a detailed examination such as computed tomography (CT) or adrenal venous sampling (AVS), adrenalectomy is usually selected as a radical treatment (1). However, obesity can affect the PA treatment after adrenalectomy (2,3), and when treating obese patients with PA, it is prudent to consider weight loss.
Worldwide, the proportion of patients suffering from severe obesity has increased due to various factors including Westernized diets and a lack of exercise (4). Obesity not only contributes to the difficulty of surgical procedures, but also increases the risk of complications such as postoperative respiratory failure and deep vein thrombosis (5). In Japan, laparoscopic sleeve gastrectomy (LSG) for patients suffering from severe obesity has been covered by insurance since 2014; subsequently the number of LSGs has increased (6). Performing the procedure prior to radical surgery for benign or slow-growth diseases under the management of skilled obesity treatment teams is a rational treatment strategy to reduce the risks associated with obesity described above. We have also encountered cases of LSG prior to radical surgery for pancreatic neuroendocrine neoplasm and abdominal aortic aneurysm, and reported on its efficacy and safety (5,7,8).
In the present study, we report the case of a severely obese patient with PA who was successfully treated with LSG and laparoscopic adrenalectomy in two phases. We present this case in accordance with the CARE reporting checklist (available at https://ales.amegroups.com/article/view/10.21037/ales-23-50/rc).
Case presentation
A 36-year-old man treated for hypertension over the previous 9 years developed hypokalemia (2.9 mmol/L). Blood tests revealed a high plasma aldosterone concentration (PAC) to plasma renin activity (PRA) ratio, and enhanced CT revealed a 15-mm low-density tumor in the left adrenal gland (Figure 1). The test results suggested PA; therefore, the patient was referred to our hospital for further examination.
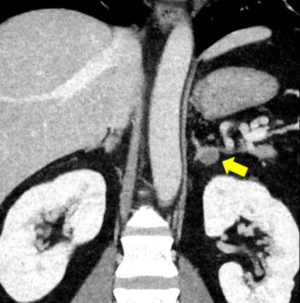
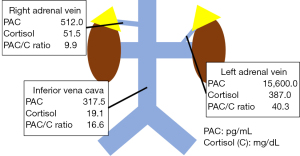
The patient was severely obese, with a body weight of 136.0 kg and a body mass index (BMI) of 45.9 kg/m2. He also had several obesity-related health issues including type 2 diabetes (T2D), obstructive sleep apnea, and hyperuricemia. He was being treated with 6 different antihypertensive drugs, but his systolic and diastolic blood pressures still measured 167 and 112 mmHg, respectively. Blood tests showed a high PAC (192 pg/mL) and PAC/PRA ratio [480]. The patient’s hemoglobin A1C (HbA1c) level was also high (6.4%). Both the furosemide upright and captopril challenge tests were positive, but the dexamethasone suppression test was negative. AVS performed after adrenocorticotropic hormone administration showed unilateral PAC elevation in the left adrenal vein (Figure 2). Based on these results, the patient was diagnosed with PA caused by an adrenal adenoma localized in the left adrenal gland.
Laparoscopic adrenalectomy was considered, but we were concerned about the surgical procedure’s difficulty and perioperative complications associated with severe obesity. To address these worries, we chose to perform LSG before laparoscopic adrenalectomy according to a surgical method we previously reported (9) (Figure 3). During the procedure, we used a liquid antiadhesive agent (AdSpray; Terumo Corporation, Tokyo, Japan) before closing the incision to prevent intra-abdominal adhesions.
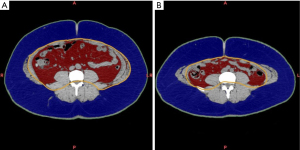
Six months after the LSG, the patient’s body weight and BMI decreased to 101.0 kg and 34.1 kg/m2, respectively, and the number of antihypertensive drugs used decreased to four. In addition, the T2D symptoms resolved dramatically, with an HbA1c level of 5.4%, and improved insulin resistance. CT volumetry showed that the subcutaneous and visceral fat areas decreased significantly after LSG (Figure 4). As a result, we performed a laparoscopic adrenalectomy once substantial weight loss and metabolic improvement had been achieved.
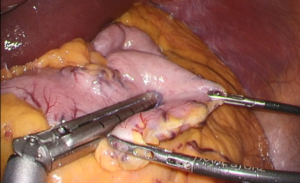
Under general anesthesia, the patient was placed in the right lateral decubitus position, and three trocars were inserted. Laparoscopic adrenalectomy was performed via the abdominal cavity, and only a small number of intra-abdominal adhesions were observed (Figure 5). After mobilizing the descending colon and spleen, the left adrenal glands and surrounding tissue were identified and dissected. The central vein of the left adrenal gland was clipped and divided. Finally, the resected left adrenal gland was placed in a plastic bag and extracted via a 12-mm trocar wound on the left upper abdomen. Macroscopic findings revealed a 15-mm yellow tumor in the adrenal gland (Figure 6A), while, histopathological examination showed clear cell growth and no evidence of malignancy (Figure 6B). Therefore, adrenal adenoma, compatible with an aldosterone-producing tumor, was finally diagnosed. The patient’s postoperative course was uneventful, and he was discharged on postoperative day 10. Twelve months after LSG (6 months after laparoscopic adrenalectomy), the patient’s blood pressure was stabilized with only one antihypertensive drug, and further weight loss and improvement in insulin resistance were achieved. Table 1 summarizes the treatment course.
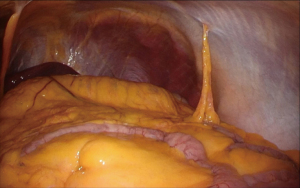
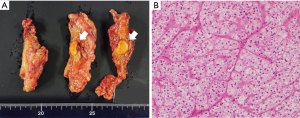
Table 1
| Variables | Before LSG | 6 months after LSG | 12 months after LSG |
|---|---|---|---|
| Body weight (kg) | 136.0 | 101.0 | 97.8 |
| BMI (kg/m2) | 45.9 | 34.1 | 32.7 |
| SBP (mmHg) | 167 | 128 | 130 |
| DBP (mmHg) | 112 | 80 | 93 |
| Antihypertensive drug (mg/day) | |||
| Azilsartan | 40 | 20 | – |
| Amlodipine | 10 | 10 | 10 |
| Carvedilol | 20 | 10 | – |
| Doxazosin | 4 | – | – |
| Trichlormethiazide | 2 | – | – |
| Methyldopa | 500 | – | – |
| Spironolactone | – | 50 | – |
| PAC (pg/mL) | 192 | 200 | 41.4 |
| PRA (ng/mL/h) | 0.4 | 0.3 | 0.6 |
| BS (mg/dL) | 142 | 97 | 101 |
| HbA1c (%) | 6.4 | 5.4 | 5.0 |
| HOMA-IR (no unit) | 5.0 | 3.2 | 2.4 |
| Insulinogenic index (no unit) | 0.5 | 1.0 | 1.0 |
| Matsuda index (no unit) | 1.8 | 2.4 | 3.4 |
| Disposition index (no unit) | 0.9 | 2.4 | 3.2 |
LSG, laparoscopic sleeve gastrectomy; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; PAC, plasma aldosterone concentration; PRA, plasma renin activity; BS, blood sugar; HbA1c, hemoglobin A1C; HOMA-IR, homeostatic model assessment for insulin resistance.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
In the present study, we report a rational two-stage surgical strategy for patients suffering from severe obesity complicated by PA. Shinoda et al. reported the efficacy of LSG before laparoscopic adrenalectomy in patients with severe obesity complicated by PA (10) and Table 2 summarizes the comparison with our case. All three patients were male, and their BMIs before LSG were between 35.9 and 45.9 kg/m2. Subsequently, all cases underwent laparoscopic adrenalectomy within 1 year after LSG, and the BMIs at laparoscopic adrenalectomy were between 27.7 and 34.1 kg/m2. Furthermore, treatment with antihypertensive drugs was reduced, and T2D symptoms improved after LSG, which contributed dramatically to the reduction in perioperative risk in severely obese patients with PA awaiting laparoscopic adrenalectomy.
Table 2
| Case | First author | Age (year) | Gender | LSG | Interval between LSG and LA (months) | LA | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| BMI (kg/m2) | Number of AD | HbA1c (%) | BMI (kg/m2) | Number of AD | HbA1c (%) | |||||
| 1 | Shinoda M (10) | 43 | Male | 35.9 | – | 6.6 | 8 | 27.7 | 0 | 5.4 |
| 2 | Shinoda M (10) | 51 | Male | 39.0 | – | 8.9 | 11 | 32.1 | – | 6.0 |
| 3 | Kumagai H | 36 | Male | 45.9 | 6 | 6.4 | 6 | 34.1 | 4 | 5.4 |
BMI, body mass index; LSG, laparoscopic sleeve gastrectomy; AD, antihypertensive drug; HbA1c, hemoglobin A1C; LA, laparoscopic adrenalectomy.
In PA caused by a unilateral aldosterone-producing tumor, adrenalectomy is usually performed as a radical treatment (1). However, obesity affects the therapeutic efficacy of adrenalectomy (2,3). BiLiGe et al. reported that the odds ratio in patients with BMIs from 25 to 29.9 kg/m2 in uncured hypertension after adrenalectomy was 2.97, and 6.42 in patients with a BMI ≥30 kg/m2 (3). Thus, we should recognize the importance of surgical procedures and weight loss in treating obese patients with PA. In addition, these retrospective observational studies reported that the number of antihypertensive drugs administered before adrenalectomy affected the postoperative course of PA treatment (2,3). In our case, and that reported by Shinoda et al. (10), a decrease in antihypertensive drugs was achieved after LSG. For severely obese patients with PA, performing LSG before adrenalectomy is considered an effective treatment strategy to optimize therapeutic efficacy after adrenal surgery.
Patients suffering from obesity are at risk of experiencing difficulty during surgical procedures due to the increase in visceral fat associated with excess weight. Additionally, obesity can increase postoperative complications such as respiratory failure, deep vein thrombosis, and surgical site infection (5). Therefore, weight reduction before radical surgery for the primary disease is important for reducing perioperative complications. LSG is an effective treatment option for these complications. We have previously reported the weight loss and metabolic improvement effects of LSG in severely obese patients (4,9,11,12). Weight loss occurred rapidly from 6 to 12 months after LSG (excess weight loss: 51.4% at 6 months, and 55.8% at 12 months) (11). The T2D remission rate was 70% to 80% in severely obese patients within 1 year after surgery (11,12), and obstructive sleep apnea also improved dramatically within 1 year after surgery (apnea-hypopnea index: 51.1 events/h at baseline, 25.4 events/h at 6 months, and 23.0 events/h at 12 months, respectively) (11). In addition, we encountered severely obese patients who underwent LSG before radical surgery for pancreatic neuroendocrine neoplasm and abdominal aortic aneurysm, and reported its efficacy (5,7,8). In our previously reported cases, radical surgery for the primary disease was performed 6 to 15 months after LSG, and the postoperative course was uneventful. These results suggest that the appropriate time for radical surgery for the primary disease is 6 months after LSG. However, strict perioperative blood pressure management is essential before LSG in patients suffering from severe obesity complicated by PA. In our hospital, a multidisciplinary obesity treatment team, including surgeons, endocrinologists, diabetologists, registered dietitians, and physical therapists treats severely obese patients. In this study, the obesity treatment team chose, after repeated discussions, to perform LSG before adrenalectomy. Under the management of a skilled obesity treatment team, a two-stage treatment strategy that indicates LSG before adrenalectomy may be a safe treatment option for severely obese patients with PA.
Laparoscopic minimally invasive surgery is the recognized standard of care for unilateral aldosterone-producing tumors. We have previously reported on the technical safety, cosmetic benefits, and potential for postoperative pain reduction of laparoscopic single-site surgery for unilateral adrenal tumors (13,14). In this case series, an additional 5 mm port was required in two patients with BMIs >27 kg/m2; thus, we chose conventional multiport laparoscopic adrenalectomy for the present patient. Recently, the safety and efficacy of partial adrenalectomy and robotic-assisted surgery for unilateral aldosterone-producing tumors have been reported (15-17). Compared to total adrenalectomy, partial adrenalectomy, which can be expected to preserve adrenal function, has been suggested to shorten the operative time and length of hospital stay and reduce the overall postoperative complication rate (15,16). Prospective validation by case series or randomized control studies is needed in the future.
In summary, performing LSG before adrenalectomy is a rational strategy for severely obese patients with PA to reduce the risk of perioperative complications and improve therapeutic efficacy after adrenal surgery. However, strict blood pressure control is required during the LSG-to-adrenalectomy perioperative period. In addition, it is clear that patients with severe obesity typically suffer from many obesity-related diseases, and that comprehensive disease management by skilled obesity treatment teams, including surgeons and endocrinologists, is essential.
Conclusions
A two-stage treatment strategy that indicates LSG before adrenalectomy can be a rational treatment strategy for severely obese patients with PA. This approach may reduce the risk of perioperative complications and enhance therapeutic efficacy after adrenalectomy. Severely obese patients typically have hypertension associated with PA, as well as other obesity-related diseases, and comprehensive disease management by a skilled obesity treatment team is essential for achieving treatment success.
Acknowledgments
We thank Dr. Kazuhiro Ito, Dr. Noriyuki Uesugi, and Dr. Tamotsu Sugai for their histopathological advice. We also thank Editage (www.editage.jp) for the English language editing.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://ales.amegroups.com/article/view/10.21037/ales-23-50/rc
Peer Review File: Available at https://ales.amegroups.com/article/view/10.21037/ales-23-50/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://ales.amegroups.com/article/view/10.21037/ales-23-50/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Naruse M, Katabami T, Shibata H, et al. Japan Endocrine Society clinical practice guideline for the diagnosis and management of primary aldosteronism 2021. Endocr J 2022;69:327-59. [Crossref] [PubMed]
- Worth PJ, Kunio NR, Siegfried I, et al. Characteristics predicting clinical improvement and cure following laparoscopic adrenalectomy for primary aldosteronism in a large cohort. Am J Surg 2015;210:702-9. [Crossref] [PubMed]
- BiLiGe W. Predicting factors related with uncured hypertension after retroperitoneal laparoscopic adrenalectomy for unilateral primary aldosteronism. Medicine (Baltimore) 2019;98:e16611. [Crossref] [PubMed]
- Umemura A, Sasaki A, Nikai H, et al. Improvements of lung volumes and respiratory symptoms after weight loss through laparoscopic sleeve gastrectomy. Langenbecks Arch Surg 2022;407:2747-54. [Crossref] [PubMed]
- Shioi Y, Sasaki A, Nitta H, et al. Two-stage surgery to repair a dissecting abdominal aortic aneurysm in a severely obese patient: Open bifurcated graft replacement after laparoscopic sleeve gastrectomy. Asian J Endosc Surg 2016;9:149-51. [Crossref] [PubMed]
- Sasaki A, Yokote K, Naitoh T, et al. Metabolic surgery in treatment of obese Japanese patients with type 2 diabetes: a joint consensus statement from the Japanese Society for Treatment of Obesity, the Japan Diabetes Society, and the Japan Society for the Study of Obesity. Diabetol Int 2022;13:1-30. [Crossref] [PubMed]
- Hasegawa Y, Sasaki A, Nitta H, et al. Two-stage surgery in a morbidly obese patient: laparoscopic pylorus-preserving pancreaticoduodenectomy after laparoscopic sleeve gastrectomy. Surg Obes Relat Dis 2013;9:e101-4. [Crossref] [PubMed]
- Umemura A, Sasaki A, Nitta H, et al. A novel second-stage surgical strategy for severely obese patient with pancreatic neuroendocrine tumor: a case report. Surg Case Rep 2022;8:125. [Crossref] [PubMed]
- Umemura A, Sasaki A, Nitta H, et al. Effects of changes in adipocyte hormones and visceral adipose tissue and the reduction of obesity-related comorbidities after laparoscopic sleeve gastrectomy in Japanese patients with severe obesity. Endocr J 2014;61:381-91. [Crossref] [PubMed]
- Shinoda M, Saito S, Sasanuma H, et al. Laparoscopic sleeve gastrectomy followed by laparoscopic adrenalectomy for patients with obesity and primary aldosteronism: Report of two patients. Int J Surg Case Rep 2023;109:108540. [Crossref] [PubMed]
- Yanari S, Sasaki A, Umemura A, et al. Therapeutic effect of laparoscopic sleeve gastrectomy on obstructive sleep apnea and relationship of type 2 diabetes in Japanese patients with severe obesity. J Diabetes Investig 2022;13:1073-85. [Crossref] [PubMed]
- Umemura A, Sasaki A, Nitta H, et al. Prognostic factors and a new preliminary scoring system for remission of type 2 diabetes mellitus after laparoscopic sleeve gastrectomy. Surg Today 2020;50:1056-64. [Crossref] [PubMed]
- Shimabuku M, Sasaki A, Higa M, et al. Single-incision laparoscopic adrenalectomy for primary aldosteronism: report of a case. Surg Today 2011;41:1306-9. [Crossref] [PubMed]
- Sasaki A, Nitta H, Otsuka K, et al. Laparoendoscopic single site adrenalectomy: initial results of cosmetic satisfaction and the potential for postoperative pain reduction. BMC Urol 2013;13:21. [Crossref] [PubMed]
- Simone G, Anceschi U, Tuderti G, et al. Robot-assisted Partial Adrenalectomy for the Treatment of Conn's Syndrome: Surgical Technique, and Perioperative and Functional Outcomes. Eur Urol 2019;75:811-6. [Crossref] [PubMed]
- Flammia RS, Anceschi U, Tufano A, et al. Minimally Invasive Partial vs. Total Adrenalectomy for the Treatment of Unilateral Primary Aldosteronism: A Systematic Review and Meta-Analysis. J Clin Med 2022;11:1263. [Crossref] [PubMed]
- Anceschi U, Mormando M, Flammia RS, et al. The Therapeutic Intensity Score as Predictor of Clinical Outcomes after Total and Partial Adrenalectomy for Unilateral Primary Aldosteronism: Results of a Multicentric Series. J Clin Med 2023;12:997. [Crossref] [PubMed]
Cite this article as: Kumagai H, Sasaki A, Umemura A, Tanahashi Y, Iwasaki T, Katagiri H, Nitta H. Second-stage treatment strategy for severely obese patient with primary aldosteronism—laparoscopic adrenalectomy after laparoscopic sleeve gastrectomy: a case report. Ann Laparosc Endosc Surg 2024;9:18.


