
Incidentally detected large upper tract urothelial carcinoma presenting without hematuria and treated with thulium fiber laser—a case report
Highlight box
Key findings
• This is a rare presentation of a large upper tract urothelial carcinoma (UTUC), detected incidentally without hematuria.
What is known and what is new?
• It is known that UTUCs present with hematuria in 95% of cases. Small lesions can be incidentally detected and have been reported in the literature when found during endoscopic procedures done for other reasons.
• Further, this manuscript adds that it’s very unusual for a large 3 cm upper tract urothelial tumor to be asymptomatic and be detected incidentally on computed tomography scan.
What is the implication, and what should change now?
• Standard treatment for these tumors is radical nephroureterectomy. Rarely, large volume low-grade UTUC can be treated by ureteroscopic laser fulguration. Thulium fiber laser is an upcoming promising laser and can adequately fulgurate such lesions because of better hemostatic qualities.
Introduction
Upper tract urothelial carcinoma (UTUC), with an estimated incidence of 1–2 cases per 100,000, is a rare diagnosis that makes up 5% of urothelial cancer and less than 10% of renal tumors (1). These cancers develop in the lining of the urinary system, extending from the renal pelvis to the ureter. Gross hematuria is the most common symptom reported in 70–80% of patients (1,2). Other infrequent symptoms include flank pain (20%) and flank mass (10%). It is very unusual for a large UTUC to be asymptomatic. The typical work-up requires urinalysis, cystoscopy, urinary cytology, and upper tract imaging with computed tomography (CT) or magnetic resonance (MR) urogram. We present a case report in which gastrointestinal symptoms led to a workup and an eventual CT that revealed a large UTUC. We present this case in accordance with the CARE reporting checklist (available at https://ales.amegroups.com/article/view/10.21037/ales-22-71/rc).
Case presentation
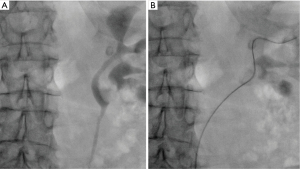
An 86-year-old female with a past medical history of hypertension, asthma, and gallbladder disease presented with dyspepsia and vague upper abdominal pain for lasting over 6 months. She denied having any severe flank pain, gross hematuria, or other urinary tract infection (UTI)-related symptoms. She underwent additional testing, such as an upper gastrointestinal endoscopy, an abdominal ultrasound, and a contrast-enhanced CT scan of the abdomen, while receiving treatment for duodenitis and gallstones. CT abdomen revealed mild left hydronephrosis, urothelial thickening, and soft tissue nodularity in the renal pelvis measuring, concerning urothelial carcinoma, therefore, the patient was referred to a urologist for further management (Figure 1A). Her urine microscopy didn’t reveal microscopic hematuria and her urine cytology was negative for high-grade urothelial carcinoma. CT urogram was done which reveals a large 3 cm poorly enhancing mass lesion occupying the left renal pelvis and left lower calyx (Figure 1B,1C). To further confirm we performed a left retrograde pyelogram, as well as a left flexible ureteroscopy. The retrograde pyelogram revealed a bifid left pelvicalyceal system, with both the left lower moiety’s lower calyx and renal pelvis having significant filling defects (Figure 2A,2B). A fiberoptic flexible ureteroscope (P-6, Olympus®, Tokyo, Japan) was used to perform a left flexible ureteroscopy where we discovered a large papillary urothelial growth in the renal pelvis, lower calyx, and lower calyceal infundibulum of the left lower moiety. Several biopsies were taken with a 2.4 Fr stainless steel basket. Selective urine cytology was also sent from the left kidney. Biopsy revealed low-grade papillary urothelial carcinoma with no lamina propria invasion seen. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.

Using a thulium fiber laser (TFL; FiberDust, Quanta®, Samarate, Italy) and 200 µm laser fiber, we performed a complete tumor fulguration and resection of the tumor in the renal pelvis, inferior calyx, and inferior calyx infundibulum. 1 J × 20 Hz was used for fulguration. A thorough nephroscopy revealed no evidence of a tumor in the remaining pelvicalyceal system. Hemostasis was achieved adequately. She was given the option of undergoing a radical nephroureterectomy, receiving adjuvant intrarenal mitomycin gel (Jelmyto, UroGen Pharma, Inc., Princeton, NJ, USA), or simply having routine surveillance ureteroscopies with no adjuvant therapy. Given her advanced age, underlying comorbidities, and asymptomatic presentation, the patient refused radical nephroureterectomy or intrarenal Jelmyto instillation and was placed on a 3-month ureteroscopy surveillance schedule. After 3 months of low-grade UTUC treatment, surveillance ureteroscopy found no signs of any residual tumor, with the exception of scarring and a mild fibrotic narrowing of the left inferior calyx. With a flexible ureteroscope and TFL we performed a lower calyx laser infundibulotomy to widen the lower calx. The patient is doing well and is being followed up with ureteroscopies every 3 months and an annual CT urogram. No recurrence was seen upto 9 months after laser fulguration of UTUC.
Discussion
In this case report, we present a large UTUC that was discovered incidentally and manifested as generalized, non-specific gastrointestinal symptoms. UTUC are histologically similar to bladder carcinoma and are frequently classified as urothelial carcinomas; however, their incidence is much lower than that of bladder carcinoma (1-3). They account for 5% of all urothelial carcinomas and are twice as common in men as in women. Hematuria, flank pain, a palpable flank mass, and clot colic are the most common symptoms. Around 15% of cases will have vague gastrointestinal or systemic symptoms, but this is usually due to advanced disease with local infiltration (4). Most asymptomatic UTUCs are very small and are discovered during a flexible ureteroscopy for another pathology, such as renal stones (5,6). A large UTUC occupying the entire lower calyx and a portion of the renal pelvis that presents asymptomatically is rare and unusual and has never been reported before.
According to recent literature, CT urography is the gold-standard imaging modality for diagnosing UTUC with the highest accuracy (7). On CT urogram, UTUC appears as low density and poorly enhancing soft tissue masses and can be granular, linear, or punctate. Furthermore, as seen in this patient, these tumors can cause urinary tract filling defects or luminal narrowing (8). Research has shown that the presence of hydronephrosis is associated with a worse prognosis as it is associated with advanced-stage disease (9). Cytology should be used in conjunction with biopsies, as positive cytology is suggestive of high-grade disease invariably needing radical nephroureterectomy. Flexible ureteroscopy is a highly effective method of diagnosis because it allows for direct visualization of UTUCs and ureteroscopic biopsy is the confirmatory test for UTUC and serves as the foundation for the definitive treatment of UTUC.
The gold standard treatment for UTUC is radical nephroureterectomy with ipsilateral bladder cuff excision. This method of treatment is associated with high morbidity and mortality particularly in elderly patients. Even though it can be performed using minimally invasive techniques such as a robotic or laparoscopic approach, there is an increased risk of postoperative chronic kidney disease as renal function is reduced after radical nephroureterectomy.
Improvements in instrumentation and available energy sources have facilitated retrograde ureteroscopic therapies. Low-grade UTUC can be treated with minimally invasive endoscopic procedures such as ureteroscopic laser fulguration. With more experience, indications for ureteroscopic therapy have expanded to include larger volume and multifocal low-grade lesions (10).
Cutress et al. demonstrated that endoscopic treatment of UTUC does provide effective oncological control in selected patients (11). Similarly, Shvero et al. demonstrated that patients with large, low-grade UTUC (>2 cm) can be effectively treated with ureteroscopic treatment when monitored closely. In this study, a cancer-specific survival rate of 84% was found with a recurrence rate of 51.2% (12). Following endoscopic control of low-grade, low-volume UTUC, intrarenal mitomycin (Jelmyto) treatment, which gels at body temperature and remains in the renal pelvicalyceal system, acting on the urothelium can result in better recurrence-free survival (13).
While holmium:yttrium-aluminum-garnet (Ho:YAG) and neodymium:YAG (Nd:YAG) have been widely used for UTUC over the last two decades, there has been a surge of interest in TFLs in recent years. TFL’s high water absorption coefficient results in improved hemostatic quality, which is especially useful when fulgurating UTUC.
Conclusions
It is very unusual for large UTUC to present incidentally without causing any hematuria. Such tumors can be managed by ureteroscopic laser fulguration using TFL. Better hemostasis with TFL maintains good visibility during ureteroscopy allowing successful treatment of UTUC.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://ales.amegroups.com/article/view/10.21037/ales-22-71/rc
Peer Review File: Available at https://ales.amegroups.com/article/view/10.21037/ales-22-71/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://ales.amegroups.com/article/view/10.21037/ales-22-71/coif). N.S. serves as an unpaid editorial board member of Annals of Laparoscopic and Endoscopic Surgery from November 2022 to October 2024. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277-300. [Crossref] [PubMed]
- Rouprêt M, Zigeuner R, Palou J, et al. European guidelines for the diagnosis and management of upper urinary tract urothelial cell carcinomas: 2011 update. Eur Urol 2011;59:584-94. [Crossref] [PubMed]
- Stewart GD, Bariol SV, Grigor KM, et al. A comparison of the pathology of transitional cell carcinoma of the bladder and upper urinary tract. BJU Int 2005;95:791-3. [Crossref] [PubMed]
- Andersen K, Burroughs S, Munis A, et al. Gastric Outlet Obstruction as the Initial Presentation of Upper Tract Urothelial Carcinoma. Case Rep Gastrointest Med 2020;2020:8850062. [Crossref] [PubMed]
- Resseguie LJ, Nobrega FT, Farrow GM, et al. Epidemiology of renal and ureteral cancer in Rochester, Minnesota, 1950-1974, with special reference to clinical and pathologic features. Mayo Clin Proc 1978;53:503-10. [PubMed]
- Tseng CW, Chen WNJ, Juang GD, et al. Staghorn calculi and xanthogranulomatous pyelonephritis associated with transitional cell carcinoma. Urol Sci 2015;26:69-71. [Crossref]
- Cowan NC, Turney BW, Taylor NJ, et al. Multidetector computed tomography urography for diagnosing upper urinary tract urothelial tumour. BJU Int 2007;99:1363-70. [Crossref] [PubMed]
- Martingano P, Cavallaro MFM, Bozzato AM, et al. CT Urography Findings of Upper Urinary Tract Carcinoma and Its Mimickers: A Pictorial Review. Medicina (Kaunas) 2020;56:705. [Crossref] [PubMed]
- Messer JC, Terrell JD, Herman MP, et al. Multi-institutional validation of the ability of preoperative hydronephrosis to predict advanced pathologic tumor stage in upper-tract urothelial carcinoma. Urol Oncol 2013;31:904-8. [Crossref] [PubMed]
- Wen J, Ji ZG, Li HZ. Treatment of upper tract urothelial carcinoma with ureteroscopy and thulium laser: a retrospective single center study. BMC Cancer 2018;18:196. [Crossref] [PubMed]
- Cutress ML, Stewart GD, Wells-Cole S, et al. Long-term endoscopic management of upper tract urothelial carcinoma: 20-year single-centre experience. BJU Int 2012;110:1608-17. [Crossref] [PubMed]
- Shvero A, Hubosky SG. Management of Upper Tract Urothelial Carcinoma. Curr Oncol Rep 2022;24:611-9. [Crossref] [PubMed]
- Kim SH, Lerner SP. Drug instillation in the management of urinary tract urothelial carcinoma. Curr Opin Urol 2022;32:531-5. [Crossref] [PubMed]
Cite this article as: Sharma N, Sengupta P, Shelmire LF. Incidentally detected large upper tract urothelial carcinoma presenting without hematuria and treated with thulium fiber laser—a case report. Ann Laparosc Endosc Surg 2024;9:9.


