
Narrative review of flaps and grafts in robotic reconstructive urologic surgery
Introduction
Background
Robotic reconstruction of the urinary tract is intended to restore anatomy and function to improve quality of life. Tissue substitution, in the form of flaps and grafts is a common strategy to replace damaged or absent tissue and fill or obliterate dead space. Flaps are tissues that remain attached to their original blood supply, which have the advantage of increased perfusion, resulting in improved take and reduced infections. However, flaps can be difficult to harvest, carry significant morbidity, or may not reach the desired location for reconstructive use. Grafts are detached from their original blood supply and then translocated to the desired location, which have the advantage of being able to reach and being easy to harvest, resulting in reduced morbidity. Grafts rely on blood and nutrient supply at the host site via imbibition (passive diffusion of nutrients) and inosculation (ingrowth of new vasculature). When these processes are insufficient to support the graft, graft failure and atrophy occur. Minimally invasive approaches using the robot have the advantage of smaller incisions, better visualization, and shorter recovery than open approaches.
Rationale and knowledge gap
Flaps and grafts are broadly employed in reconstructive urology. However, there are few reviews discussing and comparing the properties of these various grafts and flaps and their applicability to reconstructive urology employing a robotic approach.
Objective
In this review we will discuss flaps and grafts used in robotic reconstructive urologic surgery, including their potential roles, and considerations for harvest and placement during robotic surgery. The authors of this review will not take a position as to whether tissue interposition is necessary for various reconstructive applications but rather discuss options for flaps and grafts that are amenable to placement via a robotic approach when tissue interposition is desired. We present this article in accordance with the Narrative Review reporting checklist (available at https://ales.amegroups.com/article/view/10.21037/ales-23-36/rc).
Methods
A thorough literature search was conducted using PubMed to search for the terms listed in Table 1 between June 5th and 6th, 2023 by KMD. Literature was then reviewed for relevance by KMD. Literature review was evaluated for completeness by DA.
Table 1
| Items | Specification |
|---|---|
| Date of search | 6/5/2023, 6/6/2023 |
| Databases and other sources searched | PubMed |
| Search items used | Robotic AND omental flap; VRAM and urology; VRAM AND robotic; vertical rectus abdominis myocutaneous AND robotic, vertical rectus abdominis, robotic AND peritoneal flap; epiploic flap; robotic AND gracilis flap; robotic AND boari flap; appendiceal interposition; robotic AND ileal interposition; robotic AND ileal diversion; TAMIS AND urology; robotic AND alloderm; buccal graft AND robotic; fascia lata AND urology |
| Timeframe | 2000–2023 |
| Inclusion and exclusion criteria | Inclusion: related to robotic urologic surgery and/or graft use; original research or systematic reviews. Exclusion: duplicate, non-English language, full text unavailable, describing non-urologic procedures, article commentaries or narrative review articles, non-human studies |
| Selection process | Selection was performed by KMD and evaluated by DA |
Findings
General considerations
Reconstructive procedures in urology are employed to restore form and function in the setting of injuries or congenital disruptions of typical passage of urine from the kidneys out of the body. Robotic surgery is uniquely suited for urologic reconstruction owing to the familiarity of many urologists with robotic surgery and the ability of the robot to reach structures within the pelvis (1). Additionally, it has been increasingly established that various flaps and grafts used in urologic reconstruction can be harvested and utilized via a robotic approach (2-4). In selecting graft or flaps, it is important to consider desired tissue properties as well as anatomic location of both donor and recipient site (Table 2). Assessing health of flaps is tantamount to reconstructive success and the robotic approach has the advantage of allowing indocyanine green to be employed to evaluate flap perfusion (5).
Table 2
| Tissue type | Flap or graft | Blood supply | Donor site | Common uses |
|---|---|---|---|---|
| Ileum | Flap | Ileocolic artery, branch of the superior mesenteric artery | Ileum | Urinary diversion; bladder augmentation; ureteroplasty |
| Visceral peritoneum | Flap | Arterial: superior and inferior mesenteric arteries; venous: portal vein | Intraabdominal organs | Covering defects in the pelvis |
| Parietal peritoneum | Flap | Arterial: iliac, lumbar, and epigastric arteries; venous: inferior vena cava | Abdominal wall | Covering defects in the pelvis |
| Omentum | Flap | Left and right gastroepiploic artery and vein | Transverse colon | Wrapping ureter; occupying intrabdominal or pelvic space |
| Sigmoid epiploica | Flap | Contain 2 arterioles and 1 venule | Sigmoid colon | Covering defects in the pelvis |
| Gracilis | Flap | Deep femoral artery | Medial thigh | Covering defects in the pelvis; gender affirming genitoplasty |
| VRAM | Flap | Deep inferior epigastric artery and vein | Abdominal wall | Occupying intrabdominal or pelvic space |
| Boari | Flap | Superior vesical artery | Superior bladder | Ureteroplasty |
| Appendiceal | Flap | Appendiceal artery, branch of the ileocolic artery | Mobilized from cecum | Ureteroplasty; catheterizable channel |
| Rectal | Flap or graft | Rectal arteries and veins | Rectum | Urethroplasty; vaginal reconstruction |
| Oral mucosa | Graft | Imbibition/inosculation | Cheek, lip, or tongue | Urethroplasty; ureteroplasty |
| Fascia lata | Graft | Imbibition/inosculation | Medial thigh | Sacrocolpopexy; sacrohysteropexy |
VRAM, ventral rectus abdominis musculocutaneous.
Obliterating dead space/interposition
Omental flaps
The omentum is a hammock of fat, connective tissue, and lymphatics that is attached at the greater curvature of the stomach and the transverse colon. Omental harvest is performed by dissecting it off the transverse colon and then rotating this flap to the desired location, such as the pelvis. The omental flap is dependent on the right and left gastroepiploic artery.
Surgical approach
Following preparation of the desired location of the omental flap, the omentum can often be stretched or pulled and sutured in place with minimal dissection of the omentum itself (Figure 1). It is important, however that the omentum is able to reach without tension to facilitate adequate repair. In some individuals, the omentum is easily able to reach the pelvis with minimal dissection. If it is unable to reach the desired location, it can safely be divided along either the right or left gastroepiploic artery and will be perfused by its remaining attachment. This maneuver allows for a great length of well-vascularized tissue. This tissue can then be fixed into its desired location with suture.
Common uses and advantages
Advantages of this flap include that it is large and well-vascularized even following the division of one arm of its blood supply. It can be used to occupy space, such as allowing for exclusion of the small bowel from the pelvis or filling an intrabdominal defect.
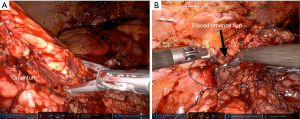
Omental flaps are excellent for wrapping the ureter after ureteral reconstruction or ureterolysis, which may disrupt the ureteral blood supply (6). A well-vascularized omentum can supply needed nutrients to support ureteral health following ureteral surgery (6). An omental wrap can also be used to augment grafting for ureteral strictures, providing additional blood supply and support (7). This strategy can work for native tissue or for grafts, for example in wrapping a buccal graft ureteroplasty (8,9). Additionally, omental flaps, which can reach the pelvis, can be used as an interposing flap to cover defects or injuries when performing reconstructive surgery on the pelvic organs. One such use is when the rectum is injured during a prostatectomy, during which omental coverage of a primary rectal closure can provide extra tissue to prevent breakdown of the rectal repair and subsequent fistula formation (10,11). Additionally, repair of fistulae, such as rectovesical, rectourethral, and vesicovaginal fistula, can be successfully augmented with omental flaps (1,12-15).
Disadvantages and complications
Port placement allowing for access to the omental blood supply as well as the desired landing area must be carefully planned prior to employment of omental flaps during robotic surgery. The omentum can shrink substantially in the setting of abdominal radiation or malnutrition, leading to small size or low flap quality. Thus, when planning to use this flap when either of these conditions are present, it is important to have a backup plan. Additionally, when patients have had extensive prior abdominal surgeries, the omentum is unlikely to be of adequate quality to be used (16). Use of omentum can be complicated by delayed return of bowel function, ileus, small bowel obstruction (SBO), and bowel injury during mobilization. Fortunately, the improved visualization with the robot aids in recognition and management of bowel injuries during such cases.
Peritoneal flaps
The peritoneum is the tissue overlying the abdominal wall and visceral organs, which can be easily mobilized to cover other structures in the abdomen. The visceral peritoneum is supplied by the superior and inferior mesenteric arteries. Use of a peritoneal flap for robotic-assisted laparoscopic vesicovaginal fistula repair was first reported in 2005 by Melamud et al. (17). Since then it has been used extensively in robotic reconstruction.
Surgical approach
To harvest the graft robotically, the peritoneal tissue is incised at the desired location using electrocautery. It is then sharply and bluntly dissected from the extraperitoneal fat. Once mobilized from the fatty tissue, it can be rotated into the pelvis for an additional layer of closure.
Uses and advantages
Peritoneal flaps can be used for management of fistulae, including vesicovaginal fistulas and prostatosymphyseal fistulas (16-18). Additionally, the peritoneum is conveniently located to serve as a flap when rectal injuries occur in the course of the posterior dissection of a prostatectomy, which may reduce the risk of subsequent fistula formation (11). Gender affirming feminizing genitoplasty also frequently utilizes peritoneal flaps in the creation of the apex of the neovagina (19). Use of peritoneum in this setting enhances vaginal length (20). Recently, there has been significant interest in the use of peritoneal flaps during robotic prostatectomy to prevent lymphoceles. Multiple randomized clinical trials have been conducted to demonstrate reduction in lymphoceles following employment of this simple technique (21-23) (Figure 2).
Disadvantages and complications
Peritoneal flaps are associated with few complications even in the setting of complex surgeries (18,20). However, one study did report a fistula between the urethra and the neovagina following feminizing genitoplasty, without clearly implicating the peritoneal flap harvest as a contributing factor (24). Peritoneal flaps following prostatectomy do not completely eliminate lymphocele risk, so it is important to remain vigilant for symptomatic lymphoceles even with employment of this technique (25). Additionally, the peritoneum can be affected by the disease process that caused the need for reconstruction, such as radiation damage, which can cause it to be unhealthy and unusable.
Vascular rectus abdominis musculocutaneous (VRAM) flaps
The rectus abdominis attaches at the 5th–7th costal cartilage and the pubic symphysis, and is enclosed by the rectus sheath. This muscle is supplied by the deep inferior epigastric artery and vein, from the external iliac vessels, which enter the muscle laterally (4).
Surgical approach
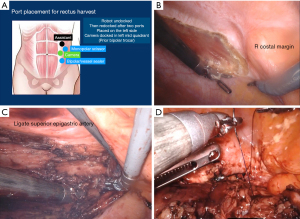
VRAM flaps are easily harvested robotically via three trocars placed on the contralateral side of the abdomen (4) (Figure 3A). The flap can be mobilized robotically by incising the anterior peritoneum vertically to the costal margin cranially and the arcuate line caudally (Figure 3B). The rectus muscle is then sharply and bluntly dissected off the anterior rectus sheath. The superior epigastric artery is ligated and the muscle is transected, allowing the cranial aspect of the flap to be rotated into the pelvis for reconstruction (Figure 3C) (4). The flap can then be transposed into the desired location (Figure 3D).
Uses and advantages
These flaps are large and have a robust blood supply, making them an excellent space occupying flap (4). VRAM flaps are commonly employed in urologic oncology for reconstruction following pelvic exenteration or for coverage of groin wounds following removal of inguinal lymph nodes (26-28). It is easily rotated from its donor site into the inguinal region to cover large defects (27). VRAM flaps also provide excellent coverage for fistulas, especially in the setting of an inability to use omental flaps due to atrophic or radiated omentum (29).
Disadvantages and complications
To employ a VRAM flap via a robotic approach, surgeons must be familiar with both the flap and robotic surgery. Thus, adoption of this flap may be reduced by a lack of robotically trained plastic surgeons or urologic surgeons familiar with the harvest of VRAM flaps. Furthermore, the trocar positioning that is optimal for VRAM flap harvest is different than that of most pelvic surgeries, and thus may require extra port placement for this portion of a case or suboptimal port placement for both parts of the procedure (4). Use of a VRAM flap can result in a defect/bulge at the donor site as the rectus abdominis muscle provides significant support for the abdominal wall (30). This complication is especially common in individuals with larger abdominal girth or history of prior abdominal surgery. Mesh can be placed into the abdominal wall at the time of flap harvest to reduce the risk of this complication (30). Other donor site complications include dehiscence and infections (28). Finally, while devitalization and failure of this flap is uncommon, it does occur, which may result in failure of a reconstructive surgery and need for additional procedures to further restore structure and function (28).
Sigmoid epiploica
The colon is studded with pedunculated fat or epiploica, which can both occupy space and provide support for structures in reconstructive urology (31).
Surgical approach
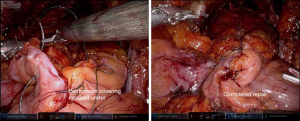
To use this flap, the sigmoid colon is mobilized and relocated near the desired recipient site and sutured in place (1,16) (Figure 4). The epiploic fat remains attached to the colon, allowing for a rich nutrient supply.
Uses and advantages
Multiple groups have described using sigmoid epiploic fat as an interposition flap in to facilitate repair of vesicovaginal fistulae (1,16,31). The sigmoid colon is already within the pelvis, making this flap a convenient interposition flap for fistula repair without extensive mobilization (16). The sigmoid colon is suitable for repair of fistulae to the bladder, even down to the level of the trigone (31). Use of this tissue is well tolerated and associated with few complications (16,31).
Disadvantages and complications
The epiploic appendix can experience ischemia or torsion if care is not taken to place the epiploic fat in its anatomic position (31). Additionally, the requirement for bowel mobilization can result in large bowel obstruction or injury, which can require additional procedures to manage or extend the length of hospital stay.
Gracilis flaps
The gracilis muscle is found in the medial thigh and can be relocated as a flap without causing significant deficits in most patients. They can be harvested from the leg and then passed through into the pelvis for use in robotic reconstruction. The gracilis muscle is supplied by the medial femoral circumflex system.
Surgical approach
In some instances, its mobilization will be done by plastic surgeons working alongside the urologist. Mobilization begins with identification of the adductor longus; approximately 2–3 cm below this the surgeon identifies the gracilis muscle. A longitudinal incision on the medial thigh is made with care to protect the greater saphenous vein. The gracilis is dissected from the adductor longus, protecting the pedicle. The muscle is released distally from the tendon after measuring the desired length, and then can be rotated proximally into the perineum for use (32-37).
Uses and advantages
Gracilis flaps are used for perineal and pelvic reconstruction owing to the proximity of the donor site to the pelvis and the high-quality, well vascularized flap that results (28,32). Gracilis flaps can be used as an interposition flap during repair of pelvic fistulas, such as complex vesicovaginal fistulas and post-prostatectomy rectourethral fistulas (33-35). Gracilis is extensively used to reconstruct defects following pelvic exenteration procedures for urinary tract and bowel cancers (28). Gracilis flaps are also used to provide blood supply in the setting of complex urethroplasties to prevent necrosis at the bulbar segment, although to our knowledge this has not been performed via a robotic approach (36). The use of the gracilis muscle has been described for gender affirming masculinizing surgery (32,37). During this procedure, a vaginectomy is performed with use of vaginal mucosa and labial tissue to form the pars fixa (37). The pars fixa has a high stricture rate owing to the risk for ischemia of this tissue following mobilization (37). The gracilis muscle can serve as support and is a well vascularized flap, which provide much needed blood supply to this area (32,37). Gracilis muscle harvest takes approximately 20 minutes and thus does not add significant duration to a surgery (37). Additionally, when the vaginectomy is being performed robotically, the gracilis flap harvest can be performed simultaneously, which is an advantage of the robotic employment of this flap (32). This procedure is well-tolerated and all patients were able to ambulate in one series on post-operative day 1 (37).
Disadvantages and complications
In one series of subjects undergoing robotic masculinizing surgery with gracilis flaps, one subject developed a seroma and one developed an infection (37). Other donor site complications include wound dehiscence (28). Gracilis flaps, like any flaps, can fail, which may require additional reconstructive surgeries for management (28). Gracilis flap harvest is associated with a mild deficit in hamstring strength, which improves with time (38). Thus, use of this flap is generally well-tolerated.
AllodermTM
AllodermTM is a commercially available extracellular matrix depleted of cells which can be implanted as a space occupying material. It is available in various sizes and thicknesses. Following implantation, the matrix is then populated by ingrowth of cells from the surrounding tissue.
Surgical approach
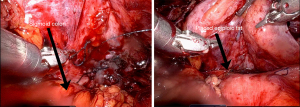
After selecting the AllodermTM graft that is most suitable for the desired tissue location, the AllodermTM graft is soaked in sterile normal saline for approximately 2 minutes. It can then be placed in the desired location and stitched into place with absorbable sutures (Figure 5).
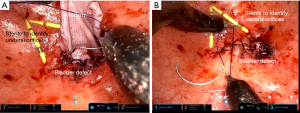
Uses and advantages
AllodermTM has been successfully used to repair a rectourethral fistula via a transrectal approach (39). AllodermTM has also been used during revision vaginoplasty for gender affirmation with a high degree of success (40). Because AllodermTM is an exogenous product, it has the advantage of avoiding the morbidity of graft or flap harvest. There is also an ongoing clinical trial concerning the use of AllodermTM to close the donor site following buccal graft harvest (41).
Disadvantages and complications
Complications with AllodermTM are fairly uncommon. One study noted that 2/9 subjects undergoing vaginoplasty with alloderm had an area of excess AllodermTM that failed to epithelialize (40). Because it is not a flap and does not have a blood supply it may fail, and does not provide any nutritional support to surrounding tissues following placement. Other complications observed in this study were likely not attributable to the use of AllodermTM. Thus, its use is fairly well-tolerated.
Ureteral substitution
Boari flaps
A Boari flap is a tubularized strip of bladder tissue mobilized from the superior bladder (42,43). Boari flaps can be quite long and are able to bridge substantial gaps (8–15 cm) in the urinary system (43,44). They are dependent on the blood supply of the bladder and, in harvesting these flaps, it is important to maintain a broad base to facilitate good blood supply to the entire segment (42,45).
Surgical approach
The ureteral remnant is spatulated to facilitate a patent repair. A full thickness flap of a desired length is raised from the anterior bladder wall with an ideal length to width ratio of 2:1–5:1 (46). This is then sutured to the spatulated ureter and this is tunneled submucosally into the bladder and sutured in place. The flap is then tubularized, often with placement of a double-J stent, and the bladder opening is closed in 2 layers.
Uses and advantages
Boari flaps are especially useful for ureteral reconstruction with a long defect because they can cover an extended distance to form a tension free anastomosis between the remaining ureter and the bladder (42). Such situations include long distal ureteral strictures, iatrogenic ureteral injuries or avulsions, and resection of distal ureteral segments for management of tumors (42,44,45,47). One group demonstrated the harvest of a long Boari flap to perform a calycovesicostomy for a refractory ureteropelvic junction obstruction (48). Thus, this flap is able to create a long, tubularized segment with a high degree of success for bridging gaps between the ureter and the bladder (43). Additionally, if a Boari flap results in insufficient length, it can be augmented with other procedures such as mobilization of the kidney or the use of grafts (49).
Disadvantages and complications
The use of a Boari flap can be complicated by urine leak, which may require drainage (43). Other structures can be injured during dissection of the flap and, in one study, a rectovesical fistula was noted following Boari flap creation in 1 patient of a cohort of 721, indicating that this is a rare scenario (43). Additionally, if the proximal portion of the flap is too narrow, the segment can become ischemic, resulting in stricture formation (42). Cystoscopy should be performed prior to the utilization of a Boari flap in the setting of urothelial cancer to verify that no bladder tumors are present. Additionally, prior to use of a Boari flap, it is important to evaluate the baseline bladder capacity and function with either uroflowmetry or formal urodynamics studies to confirm that an individual will tolerate the removal of a portion of their bladder.
Oral mucosa grafts
The oral mucosa, including the buccal, lingual, and labial tissues, is highly amenable to grafting in the urinary tract.
Surgical approach
Prior to harvest of an oral mucosal graft, surgeons must communicate with the anesthesiology team to ensure positioning of the endotracheal tube does not interfere with graft procurement. Oral graft harvest begins with measurement of the recipient site and marking out an appropriate area of the donor site to ensure adequate graft length and width, with the knowledge that grafts shrink 8–10% following harvest (50). Injection of lidocaine with epinephrine along the submucosal plane is used to hydrodissect and reduce bleeding. The graft is then cut along its outline with sharp dissection, removed from its underlying attachments, defatted, and then placed in the desired location. When harvesting a buccal mucosal graft, it is important to avoid Stensen’s duct to prevent problems with salivary secretions (50).
Uses and advantages
The tissue quality of oral mucosa makes it especially useful in replacement of urinary tissues. It is well-vascularized with a dense plexus of vessels and the mucosa is non-keratinized and self-lubricating (8). Buccal mucosa has been described as suitable robotic ureteral replacement tissue by multiple groups via multiple different approaches (8,51,52). Robotic ureteroplasty has also been performed successfully with lingual mucosa (7,49). Additionally, buccal ureteroplasty with the single port robot has been described (53). Oral tissue can be used to augment the ureter anywhere along its length, including at the ureteropelvic junction (9,54,55). One study demonstrated pyeloplasty with buccal grafting improved diuretic renography findings in 2 subjects, indicating the success of this procedure (54). The use of oral mucosa for ureteral reconstruction is suitable for both pediatric and adult patients (8,9). Compared to an open approach for ureteral reconstruction with oral mucosal grafting, the robotic approach has the advantages of improved visualization for delicate placement and suturing of the graft (51). Oral tissue has also been successfully used in ureteral reconstruction even in the setting of revisions (8,9). Bladder neck contracture, which is a known complication of prostatic surgery, has been successfully managed with buccal grafting via a robotic approach without recurrence or complication (56).
Disadvantages and complications
Longer strictures are associated with an increased rate of failure of buccal ureteroplasty (52). At a median 2-year follow up, one small study demonstrated an approximately 10% failure rate of buccal ureteroplasty with both failures occurring in the subjects with a history of prior ureteral surgery (8). Additionally, the revision setting was associated with both increased blood loss and longer operative time in one study of robotic pyeloplasty with oral mucosa (55). In one series of buccal ureteroplasty cases, the complication rate was approximately 20%; however, none of the complications were clearly related to the buccal graft or ureteroplasty portions of the surgery (8). The oral mucosal donor site can be a source of irritation for the patient during healing, and the use of lingual mucosa can be associated with tongue numbness during the recovery period (7,49).
Rectal mucosa
There has been recent interest in the harvest of rectal mucosa for repair of urologic structures or management of fistulas to the urinary tract (39). These can be harvested via the robotic transanal minimally invasive surgery (R-TAMIS) approach (39).
Surgical approach
Harvest can be performed both via a multi-port robot with a transanal access port or a single port robot (3,57,58). The robot is docked transanally with the patient in the dorsal lithotomy position. The pressure is set between 12–15 mm of mercury to ensure adequate working space without collapse (3). The desired area of flap placement is measured to determine the flap length and injection of lidocaine with epinephrine is used to hydrodissect the flap and reduce bleeding. The flap is elevated, removed, and translocated to its desired site of placement. The defect is left to heal by second intention.
Uses and advantages
Rectal mucosa has been used to close rectourethral and rectovesical fistulas by numerous surgeons, with a high degree of success (39,59,60). Rectal mucosa can also be harvested in a similar manner to oral mucosa and used as a graft for replacement of other structures for substitution urethroplasties or complex vaginal reconstruction (3,57,58). One advantage of rectal mucosa over buccal mucosa is that the graft that can be harvested can be quite large, allowing for management of very long strictures (3,57).
Disadvantages and complications
This procedure is well-tolerated with minimal morbidity (57). Given the involvement of bowel work and the need for insufflation of the rectum, there is a potential for rectal injury; however, with the use of a rectal access port, careful dissection, and pressures of 12–15 mm of mercury, the risk of rectal injury is low (39,57,61).
Ileum
Following separation of a segment of the ileum and the bowel reanastamosis, with its blood supply intact, can be relocated to the desired portion of the ureter, tailored, tubularized and used as graft to the ureter (Figure 6) (62).
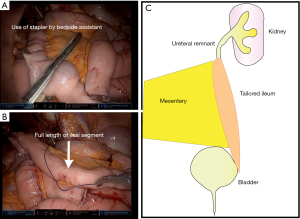
Surgical approach
A segment of ileum the desired length to cover the defect is measured and taken approximately 15 centimeters from the ileocecal junction. Care must be taken not to disrupt the ileocecal valve, which plays a role in preventing bacterial overgrowth and reducing malabsorption (63). It can then be divided on its antimesenteric side and tailored to a segment of suitable width.
Uses and advantages
The ileum is amenable to ureteral reconstruction, including as a ureteral replacement or as an inlay or onlay flap into the ureter. The robotic approach is very suitable to an intracorporeal formation of an ileal ureteral replacement (62,64,65). In one series, of 36 subjects, 3 of whom underwent a robotic ileal ureter reconstruction, 83% remained patent after 4-year follow-up (64).
Disadvantages and complications
One series, which included both robotic and open ileal ureteral replacements, demonstrated a 23.9% complication rate, including wound infections, urine leak, recurrent ureteral obstruction, and abdominal bowel related complications such as ileus (64).
Appendiceal interposition flaps
The appendix can be removed without significant consequences to a patient and can serve as an excellent flap. The appendiceal artery serves as its blood supply.
Surgical approach
The appendix is identified and divided from its attachment to the cecum with care taken to leave its blood supply intact. This can then be interposed between a spatulated end of the ureter and the bladder. It can also be divided on its anti-mesenteric border and be inlaid into a ureter that is incompletely disrupted.
Uses and advantages
The appendix is a tubular structure meaning that it is very suitable for providing a flap for reconstruction of a tubular structure such as a ureter (66-69). The appendix also has a rich blood supply from the appendiceal artery, which helps maintain its vitality. Additionally, it is a well-described clinical principle that grafts and flaps should not be fully tubularized at their time of placement due to the risk of stenosis; however, a structure that is already tubular avoids this risk (67,70). The appendix can also be de-tubularized and inlaid or onlaid into a ureter that is not completely disrupted (2,68). The appendix has been utilized in the performance of robotic pyeloplasty to add additional tissue to the stenotic segment (71). The appendix can more readily be used to replace the distal right ureter than other structures due to the proximity of the right distal ureter to the native location of the appendix (67). When a ureteral stricture is radiation-induced, the appendix may have been excluded from the field of radiation, allowing for replacement of the ureter with tissue that is non-irradiated and thus healthier (72). While many urologic surgeons would place a stent to maintain patency following robotic ureteral reconstruction with the appendix, recent reports of stentless appendiceal ureteroplasty have also yielded promising results (66,67,73).
Disadvantages and complications
The size of the appendix varies greatly, and a short appendix can limit the length of ureter which can be replaced during an appendiceal ureteroplasty (66). Appendiceal flaps may stenose leading to recurrent ureteral obstruction and thus should be monitored for symptoms and via ultrasounds (68). Additionally, despite ureteral replacement, some patients will not have improvement of their kidney function, so careful patient evaluation and selection to avoid the morbidity of a surgical intervention that will not work should be employed (69). Because of the use of a structure on the bowel, bowel injury or bowel obstruction can occur (69). The use of the appendix for replacement of the left ureter requires more extensive mobilization, which can be technically challenging (69). Finally, radiation that damages the ureter may also damage the appendix, so an alternative plan must be in place prior to approaching repair of radiation-induced ureteral strictures with the hope of using the appendix (72).
Mesh alternatives
Fascia lata grafts
Fascia lata is the deep fascia of the thigh, which can be harvested with minimal deficit. Use of this tissue can yield up to a 5 by 18 cm graft (74).
Surgical approach
To harvest fascia lata, a mark is made from the anterior iliac crest to the lateral knee to delineate the approximate location of the fascia. A small incision on the lateral knee is made and the subcutaneous fat is separated from the fascia. A cut is then made through the fascia lata and the posterior aspect is separated bluntly from the underlying tissue. An incision at the proximal extent of the graft is then made. The fascia lata is then cut along its lateral aspects to the desired length, removed from its distal attachment and passed through the proximal incision where it is separated from its proximal attachment.
Uses and advantages
Fascia lata has been successfully used for robotic sacrocolpopexy to treat pelvic organ prolapse, and is an option for mesh-averse patients, although with poorer long term success rates than sacrocolpopexy with mesh (74-76). Fascia lata can also be utilized for sacrohysteropexy if retention of the uterus is desired (77). Autologous fascia for pelvic organ suspension is preferred over synthetic mesh slings if future pregnancy is desired (77). Another application of fascia lata is autologous fascial urethral slings for urethral suspension in the setting of stress incontinence (74).
Disadvantages and complications
The use of fascia lata can be associated with donor site complications, such as a thigh bulge, paresthesia, or seroma formation (74). However, this procedure is typically well-tolerated without significant gait deficits or complications (74,75,77).
Conclusions
A variety of flaps and grafts are available for robotic reconstruction of the genitourinary tract. Surgeons must be thoughtful about the desired tissue quality and anatomic considerations in flap and graft selections. Each graft or flap has advantages and disadvantages. Future research will aim to improve patient and situation selection for each type of reconstructive technique.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Nitin Sharma) for the series “Laparoscopic Urology” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://ales.amegroups.com/article/view/10.21037/ales-23-36/rc
Peer Review File: Available at https://ales.amegroups.com/article/view/10.21037/ales-23-36/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://ales.amegroups.com/article/view/10.21037/ales-23-36/coif). The series “Laparoscopic Urology” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent for publication of this study was obtained from the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Antonelli A, Veccia A, Morena T, et al. Robot-assisted vesico-vaginal fistula repair: technical nuances. Int Braz J Urol 2021;47:684-5. [Crossref] [PubMed]
- Gabrielson A, Li O, Cohen AJ. Robotic-Assisted Augmented Roof Ureteroplasty With Appendiceal Onlay Flap. Urology 2023;176:243-5. [Crossref] [PubMed]
- Howard KN, Zhao LC, Weinberg AC, et al. A novel surgery: robotic transanal rectal mucosal harvest. Tech Coloproctol 2019;23:691. [Crossref] [PubMed]
- Pedersen J, Song DH, Selber JC. Robotic, intraperitoneal harvest of the rectus abdominis muscle. Plast Reconstr Surg 2014;134:1057-63. [Crossref] [PubMed]
- Lee Z, Moore B, Giusto L, et al. Use of indocyanine green during robot-assisted ureteral reconstructions. Eur Urol 2015;67:291-8. [Crossref] [PubMed]
- Neulander EZ, Rivera I, Kaneti J, et al. Ureterolysis with ureterotomy and omental sleeve wrap in patients with radiation induced pelvic retroperitoneal fibrosis. Cent European J Urol 2019;72:307-11. [PubMed]
- Wang J, Zhang B, Fan J, et al. The application of the "omental wrapping" technique with autologous onlay flap/graft ureteroplasty for the management of long ureteral strictures. Transl Androl Urol 2021;10:2871-8. [Crossref] [PubMed]
- Zhao LC, Weinberg AC, Lee Z, et al. Robotic Ureteral Reconstruction Using Buccal Mucosa Grafts: A Multi-institutional Experience. Eur Urol 2018;73:419-26. [Crossref] [PubMed]
- Ahn JJ, Shapiro ME, Ellison JS, et al. Pediatric Robot-assisted Redo Pyeloplasty With Buccal Mucosa Graft: A Novel Technique. Urology 2017;101:56-9. [Crossref] [PubMed]
- Yee DS, Ornstein DK. Repair of rectal injury during robotic-assisted laparoscopic prostatectomy. Urology 2008;72:428-31. [Crossref] [PubMed]
- Roberts WB, Tseng K, Walsh PC, et al. Critical appraisal of management of rectal injury during radical prostatectomy. Urology 2010;76:1088-91. [Crossref] [PubMed]
- Sotelo R, de Andrade R, Carmona O, et al. Robotic repair of rectovesical fistula resulting from open radical prostatectomy. Urology 2008;72:1344-6. [Crossref] [PubMed]
- Medina LG, Cacciamani GE, Hernandez A, et al. Robotic Management of Rectourethral Fistulas After Focal Treatment for Prostate Cancer. Urology 2018;118:241. [Crossref] [PubMed]
- Sayegh AS, La Riva A, Perez LC, et al. Robotic Simultaneous Repair of Rectovesical Fistula With Vesicourethral Anastomotic Stricture after Radical Prostatectomy: Step-by-Step Technique and Outcomes. Urology 2023;175:107-13. [Crossref] [PubMed]
- Bragayrac LA, Azhar RA, Fernandez G, et al. Robotic repair of vesicovaginal fistulae with the transperitoneal-transvaginal approach: a case series. Int Braz J Urol 2014;40:810-5. [Crossref] [PubMed]
- Agrawal V, Kucherov V, Bendana E, et al. Robot-assisted Laparoscopic Repair of Vesicovaginal Fistula: A Single-center Experience. Urology 2015;86:276-81. [Crossref] [PubMed]
- Melamud O, Eichel L, Turbow B, et al. Laparoscopic vesicovaginal fistula repair with robotic reconstruction. Urology 2005;65:163-6. [Crossref] [PubMed]
- Lundy SD, Hauser N, Wood H, et al. Management of prostatosymphyseal fistula following photoselective vaporization of the prostate: Case series and systematic review of the literature. Curr Urol 2022;16:88-93. [Crossref] [PubMed]
- Jun MS, Gonzalez E, Zhao LC, et al. Penile Inversion Vaginoplasty with Robotically Assisted Peritoneal Flaps. Plast Reconstr Surg 2021;148:439-42. [Crossref] [PubMed]
- Jacoby A, Maliha S, Granieri MA, et al. Robotic Davydov Peritoneal Flap Vaginoplasty for Augmentation of Vaginal Depth in Feminizing Vaginoplasty. J Urol 2019;201:1171-6. [Crossref] [PubMed]
- Student V Jr, Tudos Z, Studentova Z, et al. Effect of Peritoneal Fixation (PerFix) on Lymphocele Formation in Robot-assisted Radical Prostatectomy with Pelvic Lymphadenectomy: Results of a Randomized Prospective Trial. Eur Urol 2023;83:154-62. [Crossref] [PubMed]
- Wagner J, McLaughlin T, Pinto K, et al. The Effect of a Peritoneal Iliac Flap on Lymphocele Formation After Robotic Radical Prostatectomy: Results From the PLUS Trial. Urology 2023;173:104-10. [Crossref] [PubMed]
- Gloger S, Ubrig B, Boy A, et al. Bilateral Peritoneal Flaps Reduce Incidence and Complications of Lymphoceles after Robotic Radical Prostatectomy with Pelvic Lymph Node Dissection-Results of the Prospective Randomized Multicenter Trial ProLy. J Urol 2022;208:333-40. [Crossref] [PubMed]
- Salibian AA, Schechter LS, Kuzon WM, et al. Vaginal Canal Reconstruction in Penile Inversion Vaginoplasty with Flaps, Peritoneum, or Skin Grafts: Where Is the Evidence? Plast Reconstr Surg 2021;147:634e-43e. [Crossref] [PubMed]
- Deutsch S, Hadaschik B, Lebentrau S, et al. Clinical Importance of a Peritoneal Interposition Flap to Prevent Symptomatic Lymphoceles after Robot-Assisted Radical Prostatectomy and Pelvic Lymph Node Dissection: A Systematic Review and Meta-Analysis. Urol Int 2022;106:28-34. [Crossref] [PubMed]
- Chokshi RJ, Kuhrt MP, Arrese D, et al. Reconstruction of total pelvic exenteration defects with rectus abdominus myocutaneous flaps versus primary closure. Am J Surg 2013;205:64-70. [Crossref] [PubMed]
- Alnajjar HM, MacAskill F, Christodoulidou M, et al. Long-term outcomes for penile cancer patients presenting with advanced N3 disease requiring a myocutaneous flap reconstruction or primary closure-a retrospective single centre study. Transl Androl Urol 2019;8:S13-21. [Crossref] [PubMed]
- Eseme EA, Scampa M, Viscardi JA, et al. Surgical Outcomes of VRAM vs. Gracilis Flaps in Vulvo-Perineal Reconstruction Following Oncologic Resection: A Proportional Meta-Analysis. Cancers (Basel) 2022;14:4300. [Crossref] [PubMed]
- Tasdemir N, Abali R, Celik C, et al. The use of an anterior abdominal wall peritoneal flap in the laparoscopic repair of vesicouterine fistula. Int Surg 2014;99:673-6. [Crossref] [PubMed]
- Davila AA, Goldman J, Kleban S, et al. Reducing Complications and Expanding Use of Robotic Rectus Abdominis Muscle Harvest for Pelvic Reconstruction. Plast Reconstr Surg 2022;150:190-5. [Crossref] [PubMed]
- Sanderson DJ, Rutkowski J, Attuwaybi B, et al. Robotic Repair of Supratrigonal Vesicovaginal Fistula with Sigmoid Epiploica Interposition. JSLS 2018;22:e2018.00055.
- Jun MS, Shakir NA, Blasdel G, et al. Robotic-assisted Vaginectomy During Staged Gender-affirming Penile Reconstruction Surgery: Technique and Outcomes. Urology 2021;152:74-8. [Crossref] [PubMed]
- Takeda T, Shonaka T, Tani C, et al. Gracilis muscle flap combined with a laparoscopic transabdominal approach is effective in the treatment of post-prostatectomy rectourethral fistula: A case report. Int J Surg Case Rep 2022;92:106856. [Crossref] [PubMed]
- Zmora O, Potenti FM, Wexner SD, et al. Gracilis muscle transposition for iatrogenic rectourethral fistula. Ann Surg 2003;237:483-7. [Crossref] [PubMed]
- Roth T, Rodney MG, Blythe J, et al. Suprapubic approach for repair of a massive vesicovaginal fistula utilizing a myocutaneous gracilis muscle flap. Journal of Pelvic Medicine and Surgery 2003;9:19-22. [Crossref]
- Rozanski AT, Vanni AJ. Ventral Buccal Mucosa Graft Urethroplasty with Gracilis Muscle Flap for High Risk, Long Segment Urethral Strictures: A 20-Year Experience. Urology 2020;140:178-80. [Crossref] [PubMed]
- Cohen O, Stranix JT, Zhao L, et al. Use of a Split Pedicled Gracilis Muscle Flap in Robotically Assisted Vaginectomy and Urethral Lengthening for Phalloplasty: A Novel Technique for Female-to-Male Genital Reconstruction. Plast Reconstr Surg 2020;145:1512-5. [Crossref] [PubMed]
- Burks RT, Crim J, Fink BP, et al. The effects of semitendinosus and gracilis harvest in anterior cruciate ligament reconstruction. Arthroscopy 2005;21:1177-85. [Crossref] [PubMed]
- Hebert KJ, Naik N, Allawi A, et al. Rectourethral Fistula Repair Using Robotic Transanal Minimally Invasive Surgery (TAMIS) Approach. Urology 2021;154:338. [Crossref] [PubMed]
- Parker A, Brydges H, Blasdel G, et al. Mending the Gap: AlloDerm as a Safe and Effective Option for Vaginal Canal Lining in Revision Robotic Assisted Gender Affirming Peritoneal Flap Vaginoplasty. Urology 2023;173:204-8. [Crossref] [PubMed]
- clinicaltrials.gov: Buccal Mucosa Healing Trial With Tissue Matrix Placement. Washington DC: National Institutes of Health; c2019 [cited 2023 Sept 24. NCT02768467.
- Sagalovich D, Garisto J, Bertolo R, et al. Minimally Invasive Management of Ureteral Distal Strictures: Robotic Ureteroneocystostomy With a Bilateral Boari Flap. Urology 2018;120:268. [Crossref] [PubMed]
- Corse TD, Dayan L, Cheng N, et al. A Multi-Institutional Experience Utilizing Boari Flap in Robotic Urinary Reconstruction. J Endourol 2023;37:775-80. [Crossref] [PubMed]
- Ficarra V, Rossanese M, Crestani A, et al. A Contemporary Case Series of Complex Surgical Repair of Surgical/Endoscopic Injuries to the Abdominal Ureter. Eur Urol Focus 2021;7:1476-84. [Crossref] [PubMed]
- Allaparthi S, Ramanathan R, Balaji KC. Robotic distal ureterectomy with boari flap reconstruction for distal ureteral urothelial cancers: a single institutional pilot experience. J Laparoendosc Adv Surg Tech A 2010;20:165-71. [Crossref] [PubMed]
- Bansal A, Sinha RJ, Jhanwar A, et al. Laparoscopic ureteral reimplantation with Boari flap for the management of long- segment ureteral defect: A case series with review of the literature. Turk J Urol 2017;43:313-8. [Crossref] [PubMed]
- Kumar S, Modi P, Mishra A, et al. Robot-assisted laparoscopic repair of injuries to bladder and ureter following gynecological surgery and obstetric injury: A single-center experience. Urol Ann 2021;13:405-11. [Crossref] [PubMed]
- Kumar S, Chandna A, Aggarwal D, et al. Robot-assisted boari flap calycovesicostomy for failed uretero-pelvic junction obstruction: a novel approach to a complex problem. J Robot Surg 2019;13:345-9. [Crossref] [PubMed]
- Li B, Zhou Y, Chai S, et al. Robotic lingual mucosal graft ureteroplasty combined with Boari flap ureteroneocystostomy for one-stage repair of complex ureteral strictures: initial experience. Int Urol Nephrol 2023;55:893-5. [Crossref] [PubMed]
- BhalaguruIyyan A. Evaluation of the Extent of Primary Buccal Mucosal Graft Contracture in Augmentation Urethroplasty for Stricture Urethra: A Prospective Observational Study at a Tertiary Healthcare Centre. Adv Urol 2021;2021:9913452. [PubMed]
- Zhao LC, Yamaguchi Y, Bryk DJ, et al. Robot-Assisted Ureteral Reconstruction Using Buccal Mucosa. Urology 2015;86:634-8. [Crossref] [PubMed]
- Lee Z, Lee M, Koster H, et al. A Multi-Institutional Experience With Robotic Ureteroplasty With Buccal Mucosa Graft: An Updated Analysis of Intermediate-Term Outcomes. Urology 2021;147:306-10. [Crossref] [PubMed]
- Liu W, Shakir N, Zhao LC. Single-Port Robotic Posterior Urethroplasty Using Buccal Mucosa Grafts: Technique and Outcomes. Urology 2022;159:214-21. [Crossref] [PubMed]
- Zampini AM, Nelson R, Zhang JJH, et al. Robotic Salvage Pyeloplasty With Buccal Mucosal Onlay Graft: Video Demonstration of Technique and Outcomes. Urology 2017;110:253-6. [Crossref] [PubMed]
- Lee M, Lee Z, Strauss D, et al. Multi-institutional Experience Comparing Outcomes of Adult Patients Undergoing Secondary Versus Primary Robotic Pyeloplasty. Urology 2020;145:275-80. [Crossref] [PubMed]
- Avallone MA, Quach A, Warncke J, et al. Robotic-assisted Laparoscopic Subtrigonal Inlay of Buccal Mucosal Graft for Treatment of Refractory Bladder Neck Contracture. Urology 2019;130:209. [Crossref] [PubMed]
- Howard KN, Zhao LC, Weinberg AC, et al. Robotic transanal minimally invasive rectal mucosa harvest. Surg Endosc 2019;33:3478-83. [Crossref] [PubMed]
- Ozgur I, Justiniano CF, Wood HM, et al. Single-Port Endorobotic Rectal Mucosa Harvest For Urethral Reconstruction. Dis Colon Rectum 2023;66:e54-7. [Crossref] [PubMed]
- Tseng SI, Huang CW, Huang TY. Robotic-assisted transanal repair of rectourethral fistula. Endoscopy 2019;51:E96-7. [Crossref] [PubMed]
- Gan T, Naik ND, Hebert KJ, et al. Robotic Transanal Minimally Invasive Surgery: Rectourethral Fistula Closure. Dis Colon Rectum 2023;66:e120. [Crossref]
- Nicita G, Villari D, Caroassai Grisanti S, et al. Minimally Invasive Transanal Repair of Rectourethral Fistulas. Eur Urol 2017;71:133-8. [Crossref] [PubMed]
- Brandao LF, Autorino R, Zargar H, et al. Robotic ileal ureter: a completely intracorporeal technique. Urology 2014;83:951-4. [Crossref] [PubMed]
- Cosnes J, Gendre JP, Le Quintrec Y. Role of the ileocecal valve and site of intestinal resection in malabsorption after extensive small bowel resection. Digestion 1978;18:329-36. [Crossref] [PubMed]
- Launer BM, Redger KD, Koslov DS, et al. Long-term Follow Up of Ileal Ureteral Replacement for Complex Ureteral Strictures: Single Institution Study. Urology 2021;157:257-62. [Crossref] [PubMed]
- Sim A, Todenhöfer T, Mischinger J, et al. Intracorporeal ileal ureter replacement using laparoscopy and robotics. Cent European J Urol 2014;67:420-3. [Crossref] [PubMed]
- Gn M, Lee Z, Strauss D, et al. Robotic Appendiceal Interposition With Right Lower Pole Calycostomy, Downward Nephropexy, and Psoas Hitch for the Management of an Iatrogenic Near-complete Ureteral Avulsion. Urology 2018;113:e9-e10. [Crossref] [PubMed]
- Yarlagadda VK, Nix JW, Benson DG, et al. Feasibility of Intracorporeal Robotic-Assisted Laparoscopic Appendiceal Interposition for Ureteral Stricture Disease A Case Report. Urology 2017;109:201-5. [PubMed]
- Jun MS, Stair S, Xu A, et al. A Multi-Institutional Experience With Robotic Appendiceal Ureteroplasty. Urology 2020;145:287-91. [Crossref] [PubMed]
- Burns ZR, Sawyer KN, Selph JP. Appendiceal Interposition for Ureteral Stricture Disease: Technique and Surgical Outcomes. Urology 2020;146:248-52. [Crossref] [PubMed]
- Wessells H, Angermeier KW, Elliott S, et al. Male Urethral Stricture: American Urological Association Guideline. J Urol 2017;197:182-90. [Crossref] [PubMed]
- Cao H, Zhou H, Yang F, et al. Laparoscopic appendiceal interposition pyeloplasty for long ureteric strictures in children. J Pediatr Urol 2018;14:551.e1-5. [Crossref] [PubMed]
- Lee M, Lee Z, Metro MJ, et al. Robotic Ureteral Bypass Surgery with Appendiceal Graft for Management of Long-Segment Radiation-Induced Distal Ureteral Strictures: A Case Series. J Endourol Case Rep 2020;6:305-9. [Crossref] [PubMed]
- Avci E, Atıcı SD, Uylas U, et al. Stentless repair of left urethral defect with appendiceal interposition: A case report. Int J Surg Case Rep 2022;91:106805. [Crossref] [PubMed]
- Patel S, Chaus FM, Funk JT, et al. Total Autologous Fascia Lata Sacrocolpopexy for Treatment of Pelvic Organ Prolapse: Experience in Thirty-Four Patients. Urology 2022;170:73-7. [Crossref] [PubMed]
- Damiani GR, Villa M, Falcicchio G, et al. Robotic Sacrocolpopexy with Autologous Fascia Lata: A Case Series. Gynecol Minim Invasive Ther 2023;12:10-4. [Crossref] [PubMed]
- Tate SB, Blackwell L, Lorenz DJ, et al. Randomized trial of fascia lata and polypropylene mesh for abdominal sacrocolpopexy: 5-year follow-up. Int Urogynecol J 2011;22:137-43. [Crossref] [PubMed]
- Shoureshi PS, Dubinskaya A, Magner D, et al. Robotic Sacrohysteropexy With Concurrent Rectopexy using Fascia Lata Graft. Urology 2023;173:228. [Crossref] [PubMed]
Cite this article as: Doersch KM, Hines L, Ajay D. Narrative review of flaps and grafts in robotic reconstructive urologic surgery. Ann Laparosc Endosc Surg 2024;9:5.


