Laparoscopic treatment for perforated gastroduodenal ulcer: direct repair surgical technique
Highlight box
Surgical highlights
• Diagnostic laparoscopy by open approach.
• Trocar placement according to suspected site of perforation.
• Peritoneal fluid sample for microbiological analyses.
• Laparoscopic suture technique.
• Safe trocar extraction under direct visualization.
What is conventional and what is novel/modified?
• Perforated gastroduodenal ulcer repair can be performed using either running or interrupted suture, in either single- or double-layered. The use of a pedicled omental flap to patch the suture can be a choice.
• We generally perform a single-layered, running suture with a knotless barbed absorbable thread sealed with non-absorbable polymer clips.
What is the implication, and what should change now?
• Even if the surgical approach is still debated, whenever feasible laparoscopy seems to guarantee significant benefits compared to open surgery.
• In the emergency setting, we suggest performing the simplest closure technique the surgeon is most confident with. Complex repairs require time and high technical skills and are not always feasible in the acutely ill patient.
Introduction
Background
Peptic ulcer disease (PUD) affects about 4 million people worldwide, with an incidence of 1.5–3.0% (1-3). Perforated peptic ulcer (PPU) is a life-threatening complication of PUD, with a lifetime prevalence of 5% and a mortality rate of up to 20–30% (1-5). Over the last decades, improvements of medical therapy, including the use of proton pump inhibitors and eradication of Helicobacter pylori infection, have determined a decrease in hospitalization rates for PUD (1). However, the incidence of PPU has remained constant at about 2–4% of peptic ulcers. Nowadays, PPU represents one of the most common causes of hollow viscus perforation and is responsible for about 5% of all abdominal surgical emergencies (1-5).
Rationale
Source control is the mainstay of treatment in PPU patients and surgical delay is a critical determinant of survival in these patients. As demonstrated by Buck et al., every hour of delay to surgery is associated with a 2.4% decreased probability of survival (6). Prompt diagnosis is therefore essential for a better outcome. Several scoring systems have been proposed for prediction of morbidity and mortality of PPU patients, including the American Society of Anesthesiologists (ASA) score, the Boey score (7), the Peptic Ulcer Perforation (PULP) score (8), and the Mannheim Peritonitis Index (MPI). In particular, the PULP score has been shown to accurately predict 30-day mortality in patients operated for PPU. Moreover, the evaluated prognostic factors can be readily identified prior to surgery, thus allowing for an accurate and early identification of high-risk patients (8).
Historically, PPU has been managed by means of an open approach, i.e., midline laparotomy and direct closure of the perforation site with interrupted sutures, with or without placement of an omental plug. However, several other techniques have been described over the decades and minimally invasive approaches have progressively emerged as a viable and feasible option (4,5,9-13). Although no consensus exists as to how to perform the repair of the perforation site, it is generally agreed that laparoscopy is nowadays the gold-standard approach for PPU treatment. However, patient selection is crucial for guaranteeing the best outcomes. In general, patients with shock at admission, late presentation (>24 hours after the onset of symptoms), older age (>65–70 years), American Society of Anesthesiologists (ASA) score III or IV, high Boey score (≥2) or high PULP score (≥8), should be considered high-risk patients and not appropriate candidates for minimally invasive surgery. A laparoscopic-first approach, i.e., commencing the procedure laparoscopically, may be selectively adopted in hemodynamically stable patients as current evidence does not show an increased postoperative morbidity or mortality versus initial laparotomy (3-5,10-13). Anyhow, according to the latest guidelines published by World Society of Emergency Surgery (WSES) (3), a minimally invasive direct repair is not recommended in case of large defects (>2.0 cm) and in the absence of appropriate laparoscopic skills and equipment.
Objective
The aim of this article and its related video is to show a simple and direct laparoscopic technique for closure of PPU in a step-by-step and self-explanatory manner, which will help the reader to visualize the key steps of the procedure. We believe that direct closure of the defect is a basic, straightforward surgical technique that can brilliantly resolve an acute, potentially life-threatening situation and every general surgeon should be able to perform it. We present this manuscript in accordance with the SUPER (Surgical techniqUe rePorting chEcklist and standaRds) reporting checklist (available at https://ales.amegroups.com/article/view/10.21037/ales-23-17/rc).
All procedures performed in this study were in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for the publication of this article and accompanying images/video. A copy of the written consent is available for review by the editorial office of this journal.
Preoperative preparations and requirements
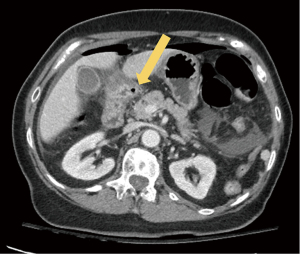
According to international guidelines, all patients with suspected PPU undergo a thorough clinical and physical evaluation, including routine laboratory studies and arterial blood gas analysis, as well as an assessment by the anesthesiologist, to evaluate the severity of the disease and the appropriateness of the indication for minimally invasive surgery (i.e., hemodynamically stable patients). Rapid resuscitation and adjustment of physiological parameters are performed according to the patient’s clinical presentation. A preoperative radiological evaluation by means of a computed tomography (CT) scan is required in stable patients to optimize the laparoscopic surgical approach according to the suspected site of perforation (Figure 1).
In the perioperative setting, the patient is started on early empiric broad-spectrum antibiotic therapy. Subsequent adjustments may be required according to the results of microbiological analyses. Additional antifungal therapy does not seem to have a significant effect on mortality and its routine use is not supported by current evidence (3-5).
Step-by-step description
All the materials needed for surgery are listed in Table 1.
Table 1
| Trocars: two 12-mm trocars, one 5-mm trocar, one extra 5-mm trocar (optional) |
| Camera: 30-degree, 10-mm, laparoscope |
| 5-mm laparoscopic graspers |
| 5-mm monopolar hook |
| 5-mm laparoscopic scissors |
| 5-mm laparoscopic suction irrigation system |
| 5-mm laparoscopic needle holder |
| 5-/10-mm laparoscopic clip applier |
| 5-mm laparoscopic retractor (to be available if required) |
| Sutures: barbed absorbable sutures for ulcer repair; absorbable sutures for fascial closures; absorbable or non-absorbable sutures for skin closure (according to surgeon’s preference) |
There is no agreement on how to perform the laparoscopic repair of PPU as it depends on individual surgeon’s preference. The key-steps of the procedure are illustrated in the Video 1.
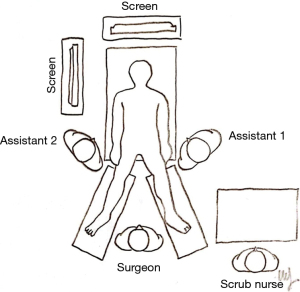
The patient is generally placed supine, in a reverse Trendelenburg position at about 20–30°, with both arms out (Figure 2). The operating surgeon usually stands between the legs of the patient, but the patient’s side is an acceptable alternative according to the surgeon’s preference. The number, size, and location of the trocars may differ according to the habits and experience of the operating surgeon and the characteristics of the patient. There are several variations in the positioning of trocars. A key concept to keep in mind is to preserve both the ergonomics and the triangulation between the optic and operating trocars for an adequate exposure of the surgical field.
In our current practice, an open technique is used to get into the abdomen, although a Verres needle is an acceptable alternative. The first trocar is a 12-mm optic trocar which is placed in the umbilical area (between or immediately over the navel) using an open technique. After insufflation of the pneumoperitoneum (generally at a maintenance pressure of maximum 10–12 mmHg), the whole abdominal cavity is inspected using a 30° camera.
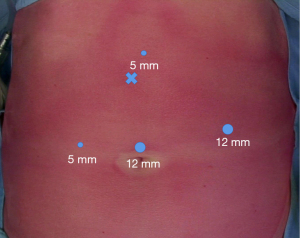
The other trocars (usually in number of 2) are then placed under direct visual control. A 12-mm trocar is positioned in the left upper quadrant, on the mid-clavicular line, according to the suspected site of perforation. Another 5-mm trocar is placed diametrically opposite on the right side, maintaining the triangulation with the optic and operating trocars. An optional third 5-mm trocar can be placed in the epigastrium, slightly on the left of the midline, for liver traction if necessary (Figure 3).
Generally, peptic ulcers are most often located in the first part of the duodenum or in the pre-pyloric region of the stomach (the antrum) (1-3). Therefore, the pyloro-duodenal region must be thoroughly searched for the perforation. If the epiploon is attached to the suspected site of perforation, the surgeon gently pulls it away by blunt dissection to assess the underlying pathology. Instrumental compression of the antrum of the stomach and the first part of the duodenum can help identify the ulcer by inducing a small escape of fluids and bubbles from the perforation site. Intraoperative identification of the perforation site can also be aided by either methylene blue injection or air insufflation through the gastric tube.
Once the perforation is identified, its closure represents the key moment of the surgical procedure and several methods of closure have been described, depending on the characteristics of the ulcer and the surgeon’s preference. Independent of the site of perforation, we do not usually perform biopsy of the ulcer as all patients will undergo postoperative endoscopic examination. Moreover, the yield of intraoperative biopsy is low and may unnecessarily increase the size of the ulcer, thus potentially increasing the risk of postoperative leak (3-5,14).
In our practice, we perform primary suture of the perforation with a knotless barbed absorbable thread (i.e., 2-0 V-LocTM, Medtronic Inc., Minneapolis, MN, USA). Both running and interrupted sutures are accepted, with either single- or double-layer closure. The ulcer edges are approximated by taking a good bite of full-thickness healthy tissue longitudinally, across the perforation, avoiding passing the needle through the posterior wall. Once the perforation site is closed, the suture is usually fixed with non-adsorbable polymer clips (e.g., Lapra-Ty®, Ethicon Endo-Surgery, Cincinnati, OH, USA) to ensure better anchoring.
Although some authors cover the suture with a pedicled flap of omentum, which is sutured to the healthy gastric or duodenal walls by means of interrupted stitches (1,4,5), in our current practice an omental graft is not routinely performed. When the ulcer edges appeared to be friable and difficult to mobilize, an omental patch can be used to achieve the closure of the lesion. In this case, we prefer the Cellan-Jones repair, in which a strand of viable omentum is drawn over the perforation site and held in place by full thickness sutures placed on either side of the perforation.
Leak test using methylene blue is routinely performed after closure of the defect. Thereafter, the abdomen is washed with warm normal saline solution until the abdominal cavity is clean. A drain is usually left in the proximity of the perforation site. A second drain can be possibly placed in the pelvis in case of severe intra-abdominal contamination, to help better drainage after thorough irrigation of the abdomen.
After resolution of the pneumoperitoneum, the 12-mm midline port fascia is closed by means of interrupted, continuous, or figure-of-eight sutures, using fast-absorbable Polyglactin (e.g., Vicryl, Ethicon Inc.; Johnson & Johnson, Somerville, NJ, USA) or Slow-absorbable Polydioxanone (e.g., PDS II, Ethicon Inc.; Johnson & Johnson, Somerville, NJ, USA) threads. Skin closure can be performed by either absorbable or non-absorbable stitches.
Postoperative considerations and tasks
The postoperative management depends on the patient’s physiology and frailty and may vary considerably among surgeons (5,15). In our practice, patients developing severe sepsis and associated organ failure are initially treated in the intensive care unit, whereas those with no or limited systemic insult are hospitalized in the surgical department.
A gastric tube is generally left in place and removed on first postoperative day, in the absence of contraindications. Based on enhanced recovery after surgery (ERAS) protocols, early oral feeding is resumed as soon as possible to promote better postoperative outcomes. Drainages are monitored daily for quality and usually removed on second postoperative day, if no signs of leakage occur. Antibiotics are discontinued according to the reduction of inflammatory markers (e.g., white blood cell count, C-reactive protein, procalcitonin). In the absence of contraindications, early mobilization and ambulation is promoted in order to optimize the patient’s ability to quickly return to baseline functioning.
Tips, pearls, and pitfalls
- Good planning of the operation includes accurate evaluation of preoperative CT scan for identification of the possible perforation site, allowing for optimal positioning of laparoscopic operating trocars.
- Self-locking sutures are equipped with a surgical needle at one end and a small loop at the other. This terminal ring allows for tissue approximation without the need for making knots, but it is devoid of the one-way barbs that are located circumferentially on the surface of the thread to ensure a tight seal. Therefore, when using self-locking sutures, the first passage should be placed on healthy tissue as the first stich is generally loose.
- If you anticipate difficulties at reaching the most distal part of the defect, place the first suture proximally and use it to traction the distal margin as you go forward.
- When approximating the ulcer edges, attention must be paid not to pass the needle through either the posterior wall or the gastric tube.
Discussion
Surgical highlights
The preoperative conditions of the patient can affect the decision about which type of surgery to perform. In general, open surgery should be reserved for hemodynamically unstable patients, patients with severe cardiac and/or pulmonary conditions contraindicating pneumoperitoneum, and patients with expected hostile abdomen. As a rule, always consider patient’s conditions and local resources before attempting any kind of surgical treatment.
Strengths and limitations
Current evidence suggests that laparoscopic surgery is the gold-standard approach for PPU patients (1-5). The advantages of minimally invasive approaches are well known and include less postoperative pain and decreased in-hospital stay compared to open surgery. Although traditionally associated with longer operative times, insufficient lavage, and possible repair site leakage, these concerns are not supported by high-quality data and seems to be on the decline as laparoscopic experience is progressively increasing among younger generations of surgeons (3-5,10-14). In our current practice, the operating team is always composed by an experienced senior consultant and a junior surgeon (i.e., residents or early career specialty surgeons). Although personal laparoscopic experience may differ among surgeons, mean operative time of laparoscopic direct repair of a PPU is 30–60 minutes, depending on the degree of abdominal contamination. The learning curve required to perform the procedure is not steep and abide by standard laparoscopic surgical experience.
Comparison with other surgical techniques and researches
Non-operative management and endoscopic techniques for PPU may be taken into account in hemodynamically stable patients with early presentation (<24 hours) and small (<1 cm) or contained defects. However, no definitive consensus has been achieved on this topic as current evidence is quite limited and of poor quality (3).
According to literature data, the choice of the perforation closure technique depends on the characteristics of the lesion. In general, when the ulcer margins can be easily approximated without tension, direct suturing can be sufficient. In our local practice, the ulcer defect is closed using barbed absorbable sutures. However, literature data on use of barbed sutures in this context are sparse and inconclusive; further research may be undertaken to define the best type of suture to utilize in these patients. Although an omental graft seems to provide a stimulus for fibrin formation, there are currently no high-quality recommendations supporting the use of omental patches in these cases. However, when the ulcer appears to have edematous, friable, and/or difficult to mobilize edges, surgical repair can be performed by means of an omental patch, with or without the use of one or more sealant devices (1-5).
Implications and actions recommended
The most commonly reported reasons for intraoperative conversion include: the size of the perforation, difficulty at localizing the perforation, peritoneal adhesions from previous surgeries, ulcers with friable edges, and generalized peritonitis. In general, according to the latest European Association of Endoscopic Surgery (EAES) guidelines, predictive factors of conversion are shock at the time of admission and longer free interval between the beginning of the perforation and the diagnosis (3,4,11).
Peritoneal irrigation should reduce the incidence of postoperative intra-abdominal collections, which may be responsible for re-operation. Although it is reasonable to irrigate a contaminated cavity, the quantity of saline solution to be used is not agreed upon. Most surgeons irrigate with 2–6 liters, but greater quantities of up to 30 liters have been described (3-5). In general, we believe that a thorough washing and aspiration of the peritoneal cavity are paramount to avoid possible postoperative collections.
Special consideration must be given to the “difficult” situations. In this context, it is important to remember that PPU represents a surgical emergency and, as such, surgeons should focus on a damage control approach rather than definitive therapy.
In case of big holes (>2.0–2.5 cm), with extensive tissue loss and/or friable margins, it may be appropriate to plug the defect with an omental graft forgoing any attempt at its direct suturing. Complete exposure of the surgical field is of primary importance for the success of the operation. In case of large defects involving the duodenum, adequate exposure must be achieved by performing a Kocher’s maneuver or, in some cases, a Cattell-Braasch maneuver.
Emergency gastrectomy has been frequently advocated for large/giant ulcers and/or suspected malignancy, but in the emergency setting the procedure is associated with increased morbidity and mortality. In this perspective, the results of a recent review performed by Chan et al. are particularly interesting as they have demonstrated that an omental patch repair confers similar perioperative outcomes to gastrectomy in patients with large (>2 cm) PPU (16).
Sometimes, PPU is associated with bleeding. Bleeding gastric ulcers are generally best treated by surgical excision and repair of the resulting gastric defect. Wedge resection can be easily achieved for ulcers along the greater curvature, antrum, or body of the stomach without determining a significant deformation in the gastric anatomy. In contrast, ulcers along the lesser curvature are more challenging, as a wedge resection can be difficult and can result in either luminal obstruction or volvulus. For lesser curve ulcers located in the area of the incisura angularis, a distal gastrectomy with a Billroth I or II reconstruction is often the treatment of choice. When the lesser curve ulcer is located proximal to the gastro-esophageal junction, the best approach can be problematic, and it may require a distal gastrectomy extended to the lesser curvature and a Roux-en-Y esophagogastrojejunostomy (Csendes procedure).
When the bleeding perforated ulcer affects the duodenum, the standard approach is to perform an anterior longitudinal duodenotomy, extending across the pylorus to the distal stomach. The bleeding vessel, almost always represented by the gastroduodenal artery, is ligated by placing a figure-of-eight suture at the top and at the bottom of the ulcer crater, in order to control the artery both proximally and distally. The duodenal incision is then closed vertically (Heineke-Mikulicz pyloroplasty). In this context, the suture line may need to be protected by duodenal decompression. This can be easily achieved by performing a duodenostomy distally to the Treitz ligament but remember to place a feeding jejunostomy as well.
When a distal gastrectomy (antrectomy) is performed, leakage from the duodenal stump represents one of the most feared complications. To reduce this risk, a Bancroft’s procedure can be performed to close the duodenal stump. This technique is characterized by transection of the stomach proximal to the pylorus, followed by dissection and removal of the mucosal layer of the stomach and duodenum. The duodenal closure is then reinforced by closing the seromuscular layer over the duodenal stump.
Conclusions
The gold-standard treatment for PPU is direct repair of the defect. Whenever feasible and in the presence of adequate surgical expertise, a laparoscopic procedure should be preferred. Independent of the type of technique applied to repair the defect, surgery should be integrated in the setting of a multimodality treatment.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Abe Fingerhut) for the series “The Expert’s Technical Corner: How I Do It” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Reporting Checklist: The authors have completed the SUPER reporting checklist. Available at https://ales.amegroups.com/article/view/10.21037/ales-23-17/rc
Peer Review File: Available at https://ales.amegroups.com/article/view/10.21037/ales-23-17/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://ales.amegroups.com/article/view/10.21037/ales-23-17/coif). The series “The Expert’s Technical Corner: How I Do It” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for the publication of this article and accompanying images/video. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pansa A, Kurihara H, Memon MA. Updates in laparoscopic surgery for perforated peptic ulcer disease: state of the art and future perspectives. Ann Laparosc Endosc Surg 2020;5:5. [Crossref]
- Chung KT, Shelat VG. Perforated peptic ulcer - an update. World J Gastrointest Surg 2017;9:1-12. [Crossref] [PubMed]
- Tarasconi A, Coccolini F, Biffl WL, et al. Perforated and bleeding peptic ulcer: WSES guidelines. World J Emerg Surg 2020;15:3. [Crossref] [PubMed]
- Mirabella A, Lupo M, Agresta F, et al. Perforated gastroduodenal ulcer. In: Agresta F, Campanile F, Anania G, et al. editors. Emergency Laparoscopy. Springer, Cham; 2016.
- Søreide K, Thorsen K, Harrison EM, et al. Perforated peptic ulcer. Lancet 2015;386:1288-98. [Crossref] [PubMed]
- Buck DL, Vester-Andersen M, Møller MH, et al. Surgical delay is a critical determinant of survival in perforated peptic ulcer. Br J Surg 2013;100:1045-9. [Crossref] [PubMed]
- Boey J, Choi SK, Poon A, et al. Risk stratification in perforated duodenal ulcers. A prospective validation of predictive factors. Ann Surg 1987;205:22-6. [Crossref] [PubMed]
- Møller MH, Engebjerg MC, Adamsen S, et al. The Peptic Ulcer Perforation (PULP) score: a predictor of mortality following peptic ulcer perforation. A cohort study. Acta Anaesthesiol Scand 2012;56:655-62. [Crossref] [PubMed]
- Lee CW, Sarosi GA Jr. Emergency ulcer surgery. Surg Clin North Am 2011;91:1001-13. [Crossref] [PubMed]
- Sanabria A, Villegas MI, Morales Uribe CH. Laparoscopic repair for perforated peptic ulcer disease. Cochrane Database Syst Rev 2013;CD004778. [Crossref] [PubMed]
- Agresta F, Ansaloni L, Baiocchi GL, et al. Laparoscopic approach to acute abdomen from the Consensus Development Conference of the Società Italiana di Chirurgia Endoscopica e nuove tecnologie (SICE), Associazione Chirurghi Ospedalieri Italiani (ACOI), Società Italiana di Chirurgia (SIC), Società Italiana di Chirurgia d'Urgenza e del Trauma (SICUT), Società Italiana di Chirurgia nell'Ospedalità Privata (SICOP), and the European Association for Endoscopic Surgery (EAES). Surg Endosc 2012;26:2134-64. [Crossref] [PubMed]
- Quah GS, Eslick GD, Cox MR. Laparoscopic Repair for Perforated Peptic Ulcer Disease Has Better Outcomes Than Open Repair. J Gastrointest Surg 2019;23:618-25. [Crossref] [PubMed]
- Kim CW, Kim JW, Yoon SN, et al. Laparoscopic repair of perforated peptic ulcer: a multicenter, propensity score matching analysis. BMC Surg 2022;22:230. [Crossref] [PubMed]
- Anbalakan K, Chua D, Pandya GJ, et al. Five year experience in management of perforated peptic ulcer and validation of common mortality risk prediction models - are existing models sufficient? A retrospective cohort study. Int J Surg 2015;14:38-44. [Crossref] [PubMed]
- Gormsen J, Brunchmann A, Henriksen NA, et al. Perioperative clinical management in relation to emergency surgery for perforated peptic ulcer: A nationwide questionnaire survey. Clin Nutr ESPEN 2022;47:299-305. [Crossref] [PubMed]
- Chan KS, Wang YL, Chan XW, et al. Outcomes of omental patch repair in large or giant perforated peptic ulcer are comparable to gastrectomy. Eur J Trauma Emerg Surg 2021;47:1745-52. [Crossref] [PubMed]
Cite this article as: Biloslavo A, Mastronardi M, Sandano M, Gabrieli A, Troian M. Laparoscopic treatment for perforated gastroduodenal ulcer: direct repair surgical technique. Ann Laparosc Endosc Surg 2023;8:22.