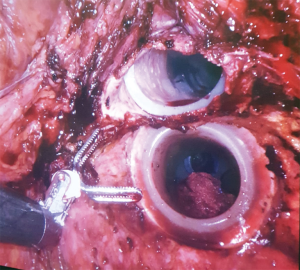
Advanced applications of transanal total mesorectal excision (taTME): beyond taTME planes (a cohort study)
Introduction
Total mesorectal excision (TME), the current standard in rectal cancer surgery, is a technique which involves removing the rectum and its surrounding mesentery en bloc. In more recent years this technique, which was first described by Heald in 1979 (1), has evolved to form the basis of the hybrid transabdominal-transanal TME (taTME) as an alternative surgical technique for rectal dissection (2). The first taTME performed on a human was published by Sylla and Lacy in 2010 who demonstrated that complete rectal and mesorectal dissection could be achieved transanally (3).
Since that time, at least three systematic reviews have been published which have demonstrated that the advantages of taTME include safe division of the low rectum, adequate visualisation of the distal margin of a rectal cancer and good views of the anterior extraperitoneal plane, particularly in patients with a narrow pelvis. Furthermore, they reported an excess of 85% complete TME rates, adequate lymph node harvest rates and circumferential resection margin (CRM) positivity rates of 2–11.8% (4-6). A pathway for the introduction of taTME into Australasia has been recently evaluated by Abbott et al. (2018) whereby the authors successfully initiated a pathway for training in this new minimally invasive technique (7). The study acknowledged that taTME has emerged as a technique with many advantages over the original approach, however has a learning curve and several technical factors that merit a structured training pathway for surgeons.
Early adopters of the technique have described false planes where urethral injury and nerve damage can be encountered (8). These same planes when purposefully adopted by trained individuals can facilitate more advanced applications of taTME. Abbott et al. (2018) successfully demonstrated the importance of teaching these technical aspects of the surgery in a safe and controlled manner.
The aim of the current study is to describe and highlight the advanced applications of taTME in locally advanced rectal cancer for improved pathological and oncological results as well as for restoration of intestinal continuity.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/ales-2019-tatme-10).
Methods
A retrospective cohort study was performed across two institutions between 2018 and 2019 (Peter MacCallum Cancer Centre, Melbourne, Australia and Epworth Healthcare, Melbourne, Australia). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Peter MacCallum Cancer Centre Ethics Committee (EC00235) and individual consent for this retrospective analysis was waived. Both the Peter MacCallum Cancer Centre and Epworth Healthcare are high-volume exenterative centers and the senior author is a high-volume exenterative surgeon. All patients were discussed at a preoperative multidisciplinary meeting. Patients were deemed suitable for the procedure at this meeting.
The operating team always utilised a two-team approach (Cecil approach). All patients who underwent the procedure received standard preoperative bowel preparation. General anaesthesia and antibiotics were given at the time of induction.
Definitions and outcomes of interest
Surgical risk was classified according to the American Society of Anesthesiologist (ASA) classification. Complications and unplanned readmissions were registered up to 30 days post-operatively. Ileus was defined as functional obstruction of the gastrointestinal tract, characterised by the absence of peristalsis, usually accompanied by abdominal pain, bloating, and sometimes nausea and vomiting, requiring nasogastric tube insertion for greater than 24 hours post-operatively. Anastomotic leak was defined as any clinical or radiological evidence of a defect of the intestinal wall at the anastomosis communicating the intra- and extra-luminal compartments. The histopathological staging was recorded according to the TNM classification (AJCC 8th Edition for Cancer Staging) (8).
Patients were divided into three distinct groups. The reported cases are consecutive cases.
- Posterior advanced rectal cancers with or without major vascular resection;
- Anterior advanced rectal cancers in males, allowing for selective removal of seminal vesicles;
- Anterior advanced rectal cancers in females requiring en bloc vaginectomy, and/or hysterectomy.
All patients considered for advanced applications for taTME had T4 cancers and required preoperative chemoradiotherapy according to institutional protocols.
All patients had preoperative magnetic resonance imaging (MRI) scans and computed tomography-positron emission tomography (CT-PET) imaging as part of their primary staging. Restaging scans were performed at week 4 post-treatment.
Two consultant surgeons were used for all cases (SK Warrier/P Smart), or (SK Warrier/J McCormick). SK Warrier and J McCormick are high-volume exenterative surgeons. SW and PS are robotic proctors and high-volume robotic colorectal surgeons. SW is a taTME proctor for Australia and one of the highest-volume taTME surgeons in Australia.
Technical approach
Transabdominal approach
The procedure was performed at least 8 weeks following chemoradiotherapy. All patients had undergone appropriate restaging. The abdominal phase involved medial to lateral mobilisation of the left colon with proximal ligation of the inferior mesenteric artery and vein in all cases. This was performed by robotic, laparoscopic and open approaches based on the case selection. In the majority of cases the ureters were identified and mobilized where appropriate, to follow a ureteric plane. Where a pelvic sidewall clearance was required, the ureter was either medialised to the vesico-ureteric junction or lateralised completely, pending surgeon preference, and then a complete sidewall clearance with preservation of obturator nerve performed.
The posterior plane for abdominal dissection was chosen depending on the pathology which included a normal TME (extra-fascial plane), or presacral fascial plane.
Transanal phase
The taTME was performed as a synchronous procedure. The patient was placed in the Trendelenburg position and buttock retracting sutures were used with aid of a Lonestar retractor (Cooper Surgical). This allowed for anal eversion. The Gelpoint Path (Applied Medical) was then placed into the anal canal and appropriate chlorhexidine cytocidal wash performed. A gauze was used selectively in the anus and then a purse-string suture placed transanally with 1.0 prolene suture on a 26-mm rounded needle. Pneumo-insufflation was established with an AirSeal System (CONMED), and the pressures increased from 5 to 12 mmHg as the transanal space increased.
The rectotomy from below was performed in the standard manner ensuring the mucosa, submucosa and muscularis layers were divided using an articulating single incision laparoscopic hook diathermy. The purse-string was reinforced following the rectotomy in many of these cases to prevent the possibility of intraluminal content spillage. Following this, select specific planes were utilised depending on the pathology.
With anterior based pathology both in the male and female patient, circumferential dissection up to the R1 risk point was performed. The posterior plane in these cases was in front of the endopelvic fascia in the extra-fascial TME plane. In some cases, the (surgical) tonsil was medialized anteriorly on purpose to allow entry into the lateral pelvic sidewall space. Abdominal visualisation was used to aid the sidewall clearance, but easy straight entry into the obturator space was possible from below.
In the female patient with a SIMS tube present in the vagina and, with the abdominal operator ready, the vagina was opened transanally and posterior vaginectomy performed with a bottom up approach. In the male patient, following clear division below and either with a partial prostatectomy or dissecting on the prostate, the seminal vesicle was dropped and removed, in select cases, from below.
When the pathology was posterior, the presacral plane was purposefully selected and in some cases, this involved lifting the endopelvic fascia onto bare muscles. The bony sacrum was stripped of fascia up to the point of the R1 risk point. Here, the abdominal operator could be guided back into the correct plane. In one case, an oscillating drill was used to allow for an anterior table sacrectomy to attain a clear margin. In one case, transanal intraoperative radiotherapy was used from below.
Statistical analysis
All characteristics were summarised using descriptive statistics, including counts and frequencies for categorical variables, and median and interquartile range for continuous variables. All statistical analyses were performed using Microsoft Excel 2010.
Results
In the initial series, a total of eight patients were deemed suitable for a combined (Cecil) advanced taTME approach.
Table 1 shows the type of abdominal approach, reconstruction used and inpatient stay for all cases.
Table 1
| Patient | Abdominal approach | En bloc | Conversion | R0 resection | Length of stay (days) | Complication | Reconstruction | Anastomosis |
|---|---|---|---|---|---|---|---|---|
| 1 | Laparoscopic | SV, right ureter | No | Yes | 10 | Urine leak | Colonic pouch | stapled |
| 2 | Open | SV, partial prostate | N/A | Yes | 5 | Nil | Colonic pouch | Stapled |
| 3 | Robotic | Cervix, uterus, posterior vagina | No | Yes | 3 | Nil | Colonic pouch | Handsewn |
| 4 | Robotic | Cervix, uterus, posterior vagina | No | Yes | 12 | SBO, intervention day 3 | Colonic pouch | Handsewn |
| 5 | Robotic | Cervix, vagina, sidewall, uterus | No | Yes | 10 | Ileus | Colonic pouch | Handsewn |
| 6 | Laparoscopic | Presacral fascia, sidewall clearance, IORT | No | Yes | 10 | Urinary retention | Colonic pouch | Stapled |
| 7 | Open | En bloc anterior table S2 | N/A | Yes | 6 | Nil | Colonic pouch | Stapled |
| 8 | Open | Left internal iliac artery and vein, S1,2 nerve root, piriformis muscle | N/A | Yes | 10 | Nil | Colonic pouch | Stapled |
SV, seminal vesicle; IORT, intraoperative radiation therapy; SBO, small bowel obstruction; N/A, not available; R0, clear margin; S1, S2, sacral level 1 and 2.
Anterior involvement in a male (n=2)
Two patients were included in this group (patients 1, 2). All patients had an R0 resection performed, with one undergoing an open abdominal approach, and one having a laparoscopic abdominal approach.
Patient 1
Patient 1 underwent a laparoscopic ultralow with taTME and en bloc resection of seminal vesicle. An en bloc ureterectomy was also performed. The seminal vesicle resection was primarily performed transanally in this patient. The patient’s recovery was complicated by a delayed urine leak relating to the ureteric anastomosis.
Patient 2
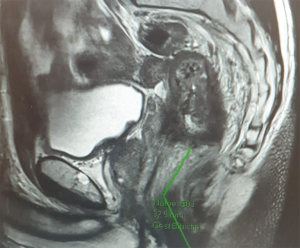
Patient 2 underwent an open abdominal approach with taTME dissection. Again, the seminal vesicle was removed as part of the surgery, primarily with the aid of the bottom up approach. The patient’s preoperative MRI pelvis is highlighted in Figure 1. This patient had an uneventful recovery with no immediate postoperative complications.
Anterior rectal cancer involvement with cervical and vaginal involvement (n=3)
Three patients were included in this group (patients 3,4,5). All patients had an R0 resection. All patients had a complete robotic abdominal approach and transanal resection. For all patients in this cohort, the taTME component was critical in assessing the at-risk margin (R1 risk point). This point was best visualised transanally and allowed for a controlled posterior vaginectomy to be performed. One patient underwent a concurrent pelvic sidewall dissection.
Patient 3
Patient 3, a 46-year-old female, underwent a robotic ultralow anterior resection (ULAR) and en bloc hysterectomy and posterior vaginectomy. This was our first patient undertaking a combined advanced robot ULAR and taTME. The patient had a perforated rectal cancer into the cervix, but there was concern about some posterior vaginal involvement. Intraoperative blood loss was minimal. The patient was discharged at day 3 with a drain tube.
Patient 4
Patient 4, a 51-year-old female, had a locally advanced rectal cancer with cervical involvement. Preoperative chemoradiation followed by a robotic abdominal hysterectomy and taTME was performed with a transanal vaginectomy. The patient had a small bowel obstruction requiring a laparoscopy at day 3 and was discharged home at day 12. No further complications were seen.
Patient 5
Patient 5, a 61-year-old female, had a very low bulky anterior rectal cancer with left pelvic sidewall disease. She underwent a preoperative long course of chemoradiation, restaging and then robotic ULAR with en bloc hysterectomy and transanal directed posterior vaginectomy with a robotic left pelvic sidewall clearance. Histopathology confirmed an R0 resection. Her surgery was complicated by an ileus and she was discharged on postoperative day 13. Figure 2 shows the synchronous transanal port and transvaginal port in situ.
Posterior advanced rectal cancers with or without major vascular resection (n=3)
Three patients had posterior based advanced tumors (patients 6,7,8). All tumours were resected with a clear margin, which included presacral stripping and one with removal of anterior table of sacrum. The addition of intraoperative radiotherapy was selectively used where applicable. No major intraoperative or postoperative complications were encountered.
Patient 6
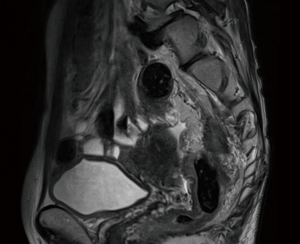
Patient 6, a 55-year-old female underwent a laparoscopic ULAR with taTME and stripping of presacral fascia for large presacral nodal deposit. Figure 3 shows the post-therapy MRI pelvis for the patient. A left pelvic sidewall clearance was performed transanally and intraoperative radiotherapy given through transanal means. The patient was discharged at day 10. She suffered from postoperative urinary retention as her left hypogastric nerve was sacrificed for oncological reasons.
Patient 7
Patient 7, a 64-year-old male with a locally advanced rectal cancer. He underwent an open ULAR with taTME. The taTME was used to facilitate presacral stripping and enable reconstruction which otherwise would not have been possible. In addition, with the aid of a neurosurgical drill, an anterior table S2 sacrectomy was performed. The patient was discharged day 6 postoperatively without complications.
Patient 8
Patient 8 had a locally advanced rectal cancer requiring an open ULAR, left internal iliac artery and vein resection with S1 and S2 nerve root and part of piriformis resection. The taTME component, as a Cecil approach, was utilised to ensure reconstruction. The transanal disconnect in this case ensured an R0 resection and was easily achievable given the bulk and fixity of the tumour.
Discussion
Our preliminary series shows that advanced transanal TME applications are feasible by utilising extramesorectal fascial planes in otherwise complex advanced oncological scenarios. Our series also demonstrates that good short-term outcomes are possible. Importantly, all patients received reconstructive procedures with an R0 resection. This highlights the importance of the introduction of surgical advances by specialist units, ensuring oncological principles and multidisciplinary care is adhered to. The rationale behind the approach stemmed most importantly from ensuring the R1 risk point is visualised and controlled utilising the taTME approach. The authors have previously highlighted this concept before (9-11).
There are three different scenarios where the taTME can be utilised for the oncological and reconstructive benefits of the patient in the advanced setting. These are anterior urogenital involvement in a male, anterior gynaecological involvement in a female and posterior sacral or lateral sidewall involvement. In the first scenario the urogenital structures, namely the seminal vesicles and prostate are at risk. A Cecil approach is required, and the transanal approach ensures the bottom part of Denonvilliers fascia can be crossed, a partial prostatectomy can be performed (if required), and the seminal vesicles can be taken. This is an easier approach than utilising a top down approach whereby, if there is bulk to the tumour, resecting the rectum or seminal vesicles in a controlled manner can be difficult. The second scenario is where a low bulky tumour abuts or invades the vagina, whereby safe reconstruction would not be possible. Utilising the taTME Cecil approach, clear demarcation of the lower border of the invasion (R1 risk point) can be determined. This allows for a synchronous, transanal, posterior vaginectomy and abdominal hysterectomy (if required). Such an approach is novel and has been published by our group (9). The third scenario is where the posterior structures are involved. The main role of a taTME approach here is to facilitate reconstruction, and strip presacral fascia on the bone ensuring that taTME false planes are followed. The transanal approach can also be utilised in this group to perform IORT or to perform and aid in a difficult pelvic sidewall dissection. Our group reported on the first transanal IORT in the world (10).
The safety profile of taTME is currently being evaluated in light of the Norwegian moratorium and studies from the Dutch indicating an inability to pick up the technique safely in low volume centres (12). Having formally reviewed their data, the authors believe the oncological issues encountered by these studies relate to a lack of multidisciplinary selection (as many patients with recurrences did not have chemoradiation and should have, by any modern selection criteria), inadequate purse-strings (and therefore shedding of cells—although difficult to prove), and inappropriate selection of patients for the transanal TME surgery whilst on a learning curve. The current results, while not presenting long-term oncological data, show that taTME can be offered in complex scenarios provided the surgeons are adequately-trained in complex surgery. The senior author (SK Warrier) is a taTME course facilitator for Australasia and one of the highest volume surgeons for taTME in Australasia who operates live on taTME courses. He along with J McCormick/P Smart have been active in the introduction of new technology safely into Australasia. The authors do not advocate these approaches for all taTME competent surgeons, as surgeons should be familiar with beyond TME planes and setups, and have regular experience in such operating.
The current paper is limited by its retrospective nature and, being a cohort study, is reporting on outcomes in a novel application of taTME. Despite this, the clinicopathological outcomes are promising.
These techniques will continue to evolve, and patients with locally advanced rectal cancers will continue to be offered novel techniques to ensure that an R0 resection can be performed and that reconstruction remains possible in such challenging patients. There may be a role for interunit/country multidisciplinary meetings in the future for challenging patients who otherwise provide a conundrum to reconstruct. Such case sharing has been already tried by the Pelvic Exenteration Collaborative. In the future, improvements in MIS platforms, including robotics, may make delivery of advanced taTME applications more easily disseminated (13).
Conclusions
In an exenterative unit with appropriately trained surgeons and following multidisciplinary principles, taTME can be used judiciously in the treatment of locally advanced T4 rectal cancers. Such an approach allows for en bloc adjacent organ resection with universal reconstruction and the ability to visualise the R1 risk point. Further oncological data is required to validate these techniques.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Satish Kumar Warrier and Alexander Heriot) for the series “taTME” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/ales-2019-tatme-10
Peer Review File: Available at http://dx.doi.org/10.21037/ales-2019-tatme-10
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales-2019-tatme-10). The series “taTME” was commissioned by the editorial office without any funding or sponsorship. Dr. SKW served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Annals of Laparoscopic and Endoscopic Surgery from Jul 2019 to Aug 2021. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Peter MacCallum Cancer Centre Ethics Committee (EC00235) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Heald RJ, Husband EM, Ryall RD. The mesorectum in rectal cancer surgery--the clue to pelvic recurrence? Br J Surg 1982;69:613-6. [Crossref] [PubMed]
- Lacy AM, Tasende MM, Delgado S, et al. Transanal Total Mesorectal Excision for Rectal Cancer: Outcomes after 140 Patients. J Am Coll Surg 2015;221:415-23. [Crossref] [PubMed]
- Sylla P, Rattner DW, Delgado S, et al. NOTES transanal rectal cancer resection using transanal endoscopic microsurgery and laparoscopic assistance. Surg Endosc 2010;24:1205-10. [Crossref] [PubMed]
- Simillis C, Hompes R, Penna M, et al. A systematic review of transanal total mesorectal excision: is this the future of rectal cancer surgery? Colorectal Dis 2016;18:19-36. [Crossref] [PubMed]
- Arunachalam L, O'Grady H, Hunter IA, et al. A Systematic Review of Outcomes After Transanal Mesorectal Resection for Rectal Cancer. Dis Colon Rectum 2016;59:340-50. [Crossref] [PubMed]
- Araujo SE, Crawshaw B, Mendes CR, et al. Transanal total mesorectal excision: a systematic review of the experimental and clinical evidence. Tech Coloproctol 2015;19:69-82. [Crossref] [PubMed]
- Abbott SC, Stevenson ARL, Bell SW, et al. An assessment of an Australasian pathway for the introduction of transanal total mesorectal excision (taTME). Colorectal Dis 2018;20:O1-6. [Crossref] [PubMed]
- Penna M, Cunningham C, Hompes R. Transanal Total Mesorectal Excision: Why, When, and How. Clin Colon Rectal Surg 2017;30:339-45. [Crossref] [PubMed]
- Teoh B, Waters PS, Peacock O, et al. Utilising taTME and robotics to reduce R1 risk in locally advanced rectal cancer with rectovaginal and cervical involvement. Tech Coloproctol 2019;23:387-90. [Crossref] [PubMed]
- Waters PS, Peacock O, Larach T, et al. Utilization of a Transanal TME Platform to Enable a Distal TME Dissection En Bloc with Presacral Fascia and Pelvic Sidewall with Intraoperative Radiotherapy Delivery in a Locally Advanced Rectal Cancer: Advanced Application of taTME. J Laparoendosc Adv Surg Tech A 2020;30:53-7. [Crossref] [PubMed]
- Baker EJ, Waters PS, Peacock O, et al. Advanced Application of TaTME Platform For a T4 Anterior Rectal Tumor. Surg Laparosc Endosc Percutan Tech 2019;29:e45-9. [Crossref] [PubMed]
- Larsen SG, Pfeffer F, Kørner H, et al. Norwegian moratorium on transanal total mesorectal excision. Br J Surg 2019;106:1120-1. [Crossref] [PubMed]
- Nikolic A, Waters PS, Peacock O, et al. Hybrid abdominal robotic approach with conventional transanal total mesorectal excision (TaTME) for rectal cancer: feasibility and outcomes from a single institution. J Robot Surg 2020;14:633-41. [Crossref] [PubMed]
Cite this article as: Commins I, McCormick J, Smart P, Warrier SK. Advanced applications of transanal total mesorectal excision (taTME): beyond taTME planes (a cohort study). Ann Laparosc Endosc Surg 2020;5:34.