
Advantages of robotic right colectomy over laparoscopic right colectomy beyond the learning curve: a systematic review and meta-analysis
Introduction
Over the last two decades robotic surgery has gained a solid place for colorectal procedures. The first tele-robotic-assisted laparoscopic colorectal surgeries were described in 2002 by Weber et al. (1) when he performed a right hemicolectomy and sigmoid colon resection for diverticulitis. Since 2007, studies comparing robotic and laparoscopic approaches have been reporting encouraging data on the safety and feasibility of robotic right colectomy (RRC). However, the application of robotic surgery is still associated with several drawbacks (mainly cost-related) in routine clinical practice. Recently, the introduction of the latest of robotic platforms, such as the da Vinci Xi robotic system, enhanced the interest towards this technique. Indeed, the new generation of surgical robot has been designed to overcome the previous limitations and it is now adapted for multi-quadrant surgeries (2,3). Since 2015, the publication rate on RRC has multiplied rapidly with 2 publications in 2005, 6 in 2010, 20 in 2015, 26 in 2018 and 36 in 2019. We found it essential to reevaluate the existing literature and analyze the reported outcomes by performing an up-to-date meta-analysis comparing RRC and LRC. More than 10 new studies have been published since the latest meta-analyses on the topic (4,5).
Therefore, the aim of the present article was to perform a systematic review and meta-analysis of randomized and non-randomized studies comparing the operative and postoperative outcomes of RRC vs. laparoscopic right colectomy (LRC). We present the following article in accordance with the PRISMA reporting checklist (available at http://dx.doi.org/10.21037/ales-20-36).
Methods
A systematic review was performed following the Cochrane collaboration specific protocol (6) and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist (7). Studies comparing operative, postoperative, and oncological outcomes (for cancer patients) between laparoscopic and robotic right hemicolectomies were searched in the following databases without date restrictions: Medline (through PubMed), Scopus, Embase, Cochrane Oral Health Group Specialized Register and Google Scholar. A specific research equation was used for each database using the following keywords and Mesh Terms: right colectomy, robotic, robotics, laparoscopy, laparoscopic. The most commonly used search equation was: ((right[All Fields] AND (“colectomy”[MeSH Terms] OR “colectomy”[All Fields])) AND (“robotics”[MeSH Terms] OR “robotics”[All Fields] OR “robotic”[All Fields])) AND (“laparoscopy”[MeSH Terms] OR “laparoscopy”[All Fields] OR “laparoscopic”[All Fields]).
Literature review was completed by an extensive search using the “related articles” function in PubMed. Moreover, the reference lists of the eligible records and of pertinent review articles, not included in this study, were double-checked in order to identify potential additional articles. Results were limited to human research, with review articles and case report being excluded. Articles were selected and reviewed if written in English or French. Literature search and selection were performed independently and blindly by two reviewers (LL and NB). Records were removed from the selection if both reviewers excluded the articles at the title/abstract screening levels. Disagreement was resolved with a discussion with a third reviewer (NdeA). Overall, the concordance rate between the two reviewers was 95%.
Eligibility criteria
According to the PICOS schema, the following criteria were used for literature search and selection. P: population: adult patients who underwent surgical treatment for pathology of right colon (right colectomy); I: intervention: robotic (full or laparoscopy assisted) right colectomy with intracorporeal or extracorporeal anastomosis; C: comparisons: LRC with intracorporeal or extracorporeal anastomosis; O: outcomes: operative variables (operative time, conversion rate, estimated blood loss, number of retrieved lymph nodes), postoperative variables (morbidity, mortality, readmission, reoperation 30 days after surgery, time to first flatus, length of hospital stay), oncological outcomes (3 and 5 years overall survival and disease-free survival), costs (total, surgery related, hospital stay related); S: study design: randomized (RCT) and non-randomized controlled trials (NRCT).
Patients with both oncological and non-oncological pathologies were included. Studies with hybrid procedures involving laparoscopic and robotic procedural steps were not eligible.
Data extraction
Two independent reviewers (LL and NB) performed the full-text analysis and data extraction by filling in an electronic database. Extracted data included first author name, year of publication, study design and type, number of included patients, study period, demographic variables (age, sex, BMI, ASA score, previous abdominal surgery), anastomosis type (intracorporeal or extracorporeal), operative and postoperative variables, oncological outcomes (see “outcomes” in eligibility criteria) and cost.
Study quality assessment and risk of bias
Two independent reviewers (LL and NB) carried out the study risk of bias evaluation of the selected articles. RCTs were assessed using the Revised Cochrane risk-of-bias tool for randomized trials (RoB 2) (8). NRCTs were assessed using the Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) tool (9). Both tools were used as recommended in Cochrane Handbook for Systematic Reviews of Interventions 2nd edition (6).
Statistical analysis
Data from the included studies were processed for the qualitative and quantitative analyses. For dichotomous outcome data, the risk ratio (RR) and 95% confidence interval (CI) were estimated using the Mantel-Haenszel method. For continuous data, the mean differences (MD) and 95% CI were estimated using inverse variance weighting. Whenever available, outcome measures [mean (SD) and median (interquartile range) or (range) values] were extracted for each surgical approach. If necessary and possible, outcome variables were calculated based on data available in the individual selected studies. In studies in which the mean or SD was not reported, these values were estimated from the median, range or interquartile range (6,10). Heterogeneity was assessed by the I2 statistic and values of 25%, 50%, and 75% were considered low, moderate, and high, respectively (11). The pooled estimates of the mean differences were calculated using random-effects models to take into account potential interstudy heterogeneity and to adopt a more conservative approach. The pooled effect was considered significant if P<0.05. The meta-analysis was performed using RevMan software (version 5.3; Cochrane Collaboration).
Results
Literature search and selection
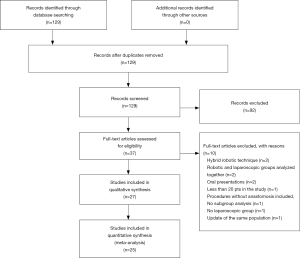
The initial search identified 129 articles that were screened for eligibility based on title and abstract evaluation. Ninety-two articles were rejected and the remaining 37 underwent full-text evaluation. No additional studies were identified through manual search, or cross-check of reference lists.
After full-text evaluation, 27 were selected for qualitative synthesis. Studies selection process and reasons for exclusion are shown in the PRISMA flow chart (Figure 1). Finally, only 25 studies were included in quantitative synthesis.
Studies characteristics
The selected 27 studies were published between October 2007 and October 2019 with a total number of 20,478 patients included. They included 2,314 patients undergoing RRC and 17,791 patients undergoing LRC between 2002 and 2019. There was only 1 RCT, whereas the remaining 26 studies were NRCTs. All NRCTs had a retrospective design. General characteristics of included studies are reported in Table 1.
Table 1
| Authors, year | Study period | Study design | No. of patients (total) | No. of patients (RRC) | No of patients (LRC) | Pathology |
|---|---|---|---|---|---|---|
| Gerbaud et al., 2019 | 2013 to 2019 | NRCT, Mono | 101 | 42 | 59 | Cancer, adenomas |
| Merola et al., 2019 | 2012 to 2017 | NRCT, Multi | 188 | 94 | 94 | Cancer |
| Yozgatli et al., 2019 | 2015 to 2017 | NRCT, Multi | 96 | 35 | 61 | Cancer |
| Blumberg, 2019 | 2013 to 2018 | NRCT, Mono | 122 | 21 | 101 | Cancers, polyps |
| Park et al., 2019 | 2010 to 2011 | RCT, Mono | 70 | 35 | 35 | Cancer |
| Megevand et al., 2019 | 2010 to 2015 | NRCT, Mono | 100 | 50 | 50 | Cancer |
| Khorgami et al., 2019 | 2012 to 2014 | NRCT, Multi | 7,685 | 442 | 7,243 | All |
| Solaini et al., 2019 | 2007 to 2017 | NRCT, Multi | 389 | 305 | 84 | Cancer and benign lesions |
| Spinoglio et al., 2018 | 2005 to 2015 | NRCT, Mono | 200 | 100 | 100 | Cancer |
| Haskins et al., 2018 | 2012 to 2014 | NRCT, Multi | 2,494 | 89 | 2,405 | All |
| Nolan et al., 2018 | 2011 to 2016 | NRCT, Mono | 106 | 10 | 96 | All |
| Ngu et al., 2018 | 2015 to 2017 | NRCT, Mono | 32 | 16 | 16 | Cancer, sealed perforations |
| Widmar et al., 2017 | 2012 to 2014 | NRCT, Mono | 282 | 119 | 163 | Cancer |
| Lujan et al., 2018 | 2009 to 2015 | NRCT, Mono | 224 | 89 | 135 | All |
| Kang et al., 2016 | 2007 to 2011 | NRCT, Mono | 63 | 20 | 43 | Cancer |
| Dolejs et al., 2017 | 2002 to 2004 | NRCT, Multi | 6,780 | 259 | 6,251 | All |
| Widmar et al., 2016 | 2009 to 2014 | NRCT, Mono | 276 | 69 | 207 | All |
| de'Angelis et al., 2016 | 2012 to 2015 | NRCT, Mono | 80 | 30 | 50 | Cancer |
| Ferrara et al., 2016 | 2008 to 2014 | NRCT, Mono | 28 | 13 | 15 | Cancer |
| Guerrieri et al., 2015 | 2013 to 2014 | NRCT, Mono | 29 | 18 | 11 | Cancer |
| Trastulli et al., 2015 | 2005 to 2014 | NRCT, Multi | 238 | 102 | 136 | Cancer, adenomas |
| Davis et al., 2014 | 2009 to 2011 | NRCT, Multi | 414 | 207 | 207 | All |
| Casillas et al., 2014 | 2005 to 2012 | NRCT, Mono | 162 | 52 | 110 | Neoplasms |
| Lujan et al., 2013 | 2008 to 2011 | NRCT, Mono | 47 | 22 | 25 | All |
| Deutsch et al., 2012 | 2004 to 2009 | NRCT, Mono | 65 | 18 | 47 | All |
| deSouza et al., 2010 | 2005 to 2009 | NRCT, Mono | 175 | 40 | 135 | All |
| Rawlings et al., 2007 | 2002 to 2005 | NRCT, Mono | 32 | 17 | 15 | All |
RRC, right robotic colectomy; LRC, laparoscopic right colectomy; NRCT, non-randomized control trial; Mono, monocentric; Multi, multicentric; RCT, randomized control trial.
The mean age (SD) in the RRC group was 68.43 (6.95) years, with 52% of the patients being males. In the LRC group, the mean age was 68.47 (3.25) years with 51.27% of male patients.
There was no significant difference between groups regarding age (MD −0.24; 95% CI: −2.11, 1.63; P=0.80), gender (RR 1.04; 95% CI: 0.97, 1.11; P=0.32), previous abdominal surgeries (RR 0.80; 95% CI: 0.59, 1.08; P=0.15), BMI (MD 0.21; 95% CI: −0.37, 0.79; P=0.48), and ASA score (RR 1.11; 95% CI: 0.97, 1.26; P=0.12).
Nine studies (12-20) included patients who underwent robotic and laparoscopic surgeries for right, left or rectal pathologies. Only data from subgroup analysis comparing RRC and LRC were extracted and used for this qualitative synthesis. Three studies (21-23) compared open, laparoscopic and robotic right colectomies. Subgroup analysis of laparoscopic and robotic approach was used for this synthesis.
One study by Trastulli et al. (24) described a subgroup of patients that received full RRC, whereas in the remaining studies laparoscopically assisted technique was performed (meaning the use of an assistant laparoscopic port).
Three studies (12,14,21), with the largest patient number (RRC n=790, LRC n=15,899) were performed as a retrospective data collection from national registries. While there was no information about the type of anastomosis in each group, these studies were selected for further qualitative synthesis. Similarly, three monocentric studies (13,22,25) did not specify the anastomosis type but were included in the analysis. Five studies (26-30) included patients only with intracorporeal anastomosis and six studies (15,18,19,23,31,32) only with extracorporeal; in the rest of the studies both types of anastomosis were performed.
In the study of Trastulli et al. (24) patients in LRC group received both—intracorporeal and extracorporeal—anastomosis, only patients with intracorporeal anastomosis (n=40) were included in the quantitative synthesis.
Studies quality assessment
The assessment of the included RCT (33) classified the study as having “some concerns” according to RoB 2 tool (8). Three NRCTs were assessed as having moderate risk of bias (18,26,30). Twenty-one NRCTs were classified at serious risk of bias (13-16,19-25,27-29,31,32,34-38), all due to potential risks of confounding as none of these studies matched the laparoscopic group to robotic group before inclusion of the patients. Two NRCTs were classified at a critical risk of bias (12,17); in both studies it was due to critical bias of confounding that was assessed to be problematic to provide any useful evidence on the effects of RRC and LRC. These two studies were excluded from quantitative synthesis.
Outcomes
Operative variables
All included studies reported comparison of one or more operative variables. Quantitative synthesis of data about operative time (OT) was performed from twenty-two studies. Meta-analysis revealed that the OT was significantly longer for RRC with a MD of 45.36 minutes (95% CI: 31.75, 58.97; P<0.00001). The considered studies had very high heterogeneity (I2=95%) (Figure 2A). Data about conversion rate were available from 21 study. Conversion rate was significantly lower in the RRC group with 38 events over 1519 RRC (2.5%) vs. 746 conversions over 7,805 LRC (9.56%). The estimated RR was 0.47 (95% CI: 0.27, 0.81; P=0.007, I2=33%) (Figure 2B). Twenty-one study included data on lymph node harvesting. The studies by deSouza et al. (31) and Deutsch et al. (19) were excluded from the analysis as mean and SD could not be calculated. The study by de’Angelis et al. (32) was not included because the mean values for each group were not reported. Meta-analysis from 18 studies showed that the number of harvested lymph node was significantly higher in the RRC group with a MD of 2.03 (95% CI: 0.45, 3.61; P=0.01, I2=68%) (Figure 2C).

Estimated blood loss for RRC and LRC groups was calculated from 13 studies. Quantitative synthesis showed more favorable outcomes for RRC with a MD of −8.68 (95% CI: −17.27, −0.08; P=0.05, I2=46%) (Figure 2D).
Postoperative variables
Based on 18 studies that reported overall postoperative complications, 16 were included in the meta-analysis. Two studies (28,32) that were reporting 90-day morbidity were excluded. There was no difference in the overall complication rate between RRC and LRC (RR 0.91; 95% CI: 0.80, 1.04; P=0.18, I2=0%). Similarly, comparing severe complications [i.e., grade 3 or more by Clavien-Dindo classification (39)] as reported in 7 studies, there was no significant difference between the two groups with thirteen patients over 427 in RRC group (3.04%) and 30 over 562 in LRC group (5.34%). The RR was 0.69 without reaching the significant threshold (95% CI: 0.36, 1.34; P=0.28, I2=0%) (Figure 3A).

Reoperation and readmission at 30 days after surgery were analyzed in 12 and 9 studies respectively. There was no significant difference between RRC and LRC groups with an RR for reoperation of 0.76 (95% CI: 0.41, 1.41; P=0.38, I2=0%) and an RR for readmission of 1.46 (95% CI: 0.71, 3.01; P=0.31, I2=33%).
Data about anastomotic leak (AL) were extracted and analyzed only from 16 studies in which the type of anastomosis was clearly described for each group. Rate of AL did not differ significantly between RRC and LRC with an RR of 0.86 (95% CI: 0.52, 1.41; P=0.55, I2=0%) (Figure 3B). The quantitative synthesis of surgical site infection and postoperative ileus rates was not performed as the case definition for these complications was not consisted throughout the included studies.
Sixteen studies reported 30 days mortality. With 6 events over 1,051 patients (0.57%) in RRC group and 40 events over 9,759 patients (0.04%) in LRC, no significant difference was observed in the pooled data analysis (RR 1.84; 95% CI: 0.84, 4.01; P=0.12, I2=0%) (Figure 3C).
For the time to flatus, studies describing the outcome as “first flatus” were included, studies using other descriptions (e.g., first bowel movement) were excluded. The time to first flatus after surgery was reported in 11 studies, and was comparable between RRC and LRC with a MD of −0.16 (95% CI: −0.61, 0.29; P=0.49). However, a high heterogeneity was found (I2=95%).
Data about the length of hospital stay (LOS) was available and analyzed only from 22 studies. The meta-analysis showed a significantly shorter LOS in the RRC group. The MD was −0.60 (95% CI: −1.01, −0.19; P=0.004, I2=64%) (Figure 3D).
Costs
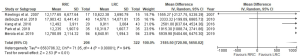
Six studies compared the total cost per patient (19,23,26,27,31,32). de’Angelis et al. (32) reported the average costs per group and thus these data were not included in meta-analysis. Merola et al. (26) reported the mean cost in euros thus it was converted in American dollars (USD) according to actual conversion rate on February 2020. The USD value from two studies published more than 10 years ago (20,31) was converted to actual USD value as in February 2020. Total costs were significantly higher in the RRC group with a MD of 3,185.50 USD (95% CI: 720.98, 5,650.02; P=0.01, I2=94%) (Figure 4).
No subgroup analysis was performed analyzing surgical and hospitalization cost separately due to the lack of consistency in the calculations and descriptions of these costs in each study.
Oncological outcomes
Three studies reported 3-year and/or 5-year OS and DFS for operated colon cancer patients (23,29,33). Meta-analyses on these outcomes were not performed due to insufficient statistical data provided in these studies. However, in all three studies oncological outcomes were comparable between the two surgical groups. Park et al. (33) reported a 91.1% (95% CI: 78.8–100%) 5-year OS for all tumor stages in the RRC group and 91.0% (95% CI: 81.3–100%) in the LRC group (P=0.678). Five years DFS for all tumor stages were 77.4% (95% CI: 60.6–92.1%) for RRC and 83.6% (95% CI: 72.1–0.97.0%) for LRC (P=0.442). In the study by Spinoglio et al. (29), the 5-year OS for RRC was 77% and for LRC 73% (P=0.64). The 5-year DFS for RRC and LRC was 85% and 83% respectively (P=0.36). No statistical difference was found in the study of Kang et al. (23) who reported a 5-year OS for RRC of 73.1% and for LRC of 79.2%, whereas the 5-year DFS was 89.5% and 84% respectively.
Discussion
Operative outcomes and learning curve
The present systemic review and meta-analysis provides the latest and the most comprehensive literature analysis on the comparison between robotic and LRC including 27 studies in the qualitative synthesis and 25 studies in the quantitative one. Overall, 13 studies (48%) (12,13,21,26-30,33,34,36-38) were published between 2018 and 2019, including the only RCT (33), highlighting the rising interest in the outcomes and potential benefits of RRC as compared to conventional laparoscopy.
In the present meta-analysis, we demonstrated significantly better operative outcomes for RRC regarding conversion rate and estimated blood loss, which confirm the safety of the robotic approach. Similar results have been reported in other meta-analyses published in last 5 years (4,5,40); however, this is the first meta-analysis to report a significantly higher number of harvested lymph node in the RRC group. The number of retrieved lymph node during an oncological colon resection has been widely discussed as one of the prognostic factors for oncological results. Recent studies (41,42) have reported that lymph node harvesting has a significant association with survival outcomes for both lymph node negative (stage I and II) and positive (stage III) colon cancers, confirming the lymph node harvesting as one of the quality criteria for oncological resection.
Operative time was significantly longer in RRC group as it has been shown in previous meta-analyses (4,5,40,43). As suggested by Solaini et al. (4) the type of anastomosis (intracorporeal or extracorporeal) could be one the factors impacting on the operative time. They performed a subgroup analysis on extracorporeal anastomosis and found no significant difference in the operative time between RRC and LRC. This finding suggests that future comparisons of RRC and LRC should be carried out only when the same type of anastomosis is performed, especially when the aim is to evaluate the impact of the surgical technique on operative time. This more consistent evaluation may potentially even demonstrate a benefit of the robotic technique when performing an intracorporeal anastomosis. Another important factor that affects operative time is surgeon’s and surgical team’s knowledge and experience with the robotic platform. The docking and targeting of the robotic system is most of the time included in the operative time count as these procedures are performed after the skin incision. Surgeon’s control and familiarity with the robotic console as well as the scrub nurse’s knowledge of the robotic instruments and their replacement also has a great impact on the overall operative time. Three studies (29,32,38) included in this systemic review took into account the possible impact of the learning curve on the operative time; in each of these studies the RRC was performed by a single surgeon. Spinoglio et al. (29) and Megevand et al. (38) reported a significant decrease in the operative time during the learning curve of the RRC with a mean operative time of 329 min in the earlier RRC procedures and 266 min in latest RRC series (P≤0.001) (29). de’Angelis et al. (32) performed subgroup analyses categorizing RRC and LRC patients in 3 groups: cases 1–10, cases 11–20, cases 21+. They showed a trend toward a significant difference in the 11–20 case series, in favor of RRC with a mean operative time of 193.8 min (SD 24.24) in RRC group and 214.5 min (12.57) in LRC group (P=0.07). It could be expected that once RRC will have become a routine procedure in many centers where surgeons and surgical teams have overpassed the learning curve, future studies will report substantially shorter operative time.
There are two studies published in 2017 that analyzed the learning curve for RRC. Parisi et al. (44) reported a series of 108 patients undergoing RRC with intracorporeal anastomosis. Based on a cumulative sum (CUSUM) analysis, 3 phases of learning curve were detected: cases 1–44: initial learning phase; cases 45–90: consolidation phase; cases 91–108: mastery phase. Mean operative time in phase 1 was 313.2 min (SD 72.8), in phase 2–285.8 min (62.1) and in phase 3–216.4 min (37.2); a significant difference was found between both phase 1 and phase 2 and between phase 2 and phase 3 (P<0.0001). Similar results but with shorter learning curve were presented by Raimondi et al. (45). In a series of 23 RRC with intracorporeal anastomosis, the CUSUM analysis detected 2 phases of learning curve: cases 1–13: acquisition phase and cases 14–23: consolidation phase.
Mean operative time differed significantly: it was 281.5 min (SD 6.59) in the acquisition phase and 244.3 (8.959) in the consolidation phase (P=0.0026).
Postoperative and oncological outcomes
In the present meta-analysis, we did not find any differences regarding 30 days morbidity (total and Grade ≥3 complications), anastomotic leak rate, mortality, reoperation and readmission rates. Only meta-analysis by Ma et al. (5) has reported a decrease in overall complications in RRC group (P=0.05). However, when the analysis was performed regarding the postoperative ileus, bleeding and anastomotic leakage rates, no differences were found between RRC and LRC. There exists an important inconsistency of definitions of postoperative complications as well as a wide range of non-surgical complications included in the total morbidity that may explain differences in study results. Although no decrease of morbidity was detected in our quantitative synthesis, we found a significantly shorter LOS in the RRC group as was previously described by Ma et al. (5). Two other meta-analyses have reported significantly shorter time to first flatus; our analysis did not show differences between the groups. This could be explained by different study selection for this outcome; we strictly included only studies where postoperative bowel activity was defined as “time to (first) flatus”, studies with other descriptions, e.g., first bowel movement or return of bowel activity, were not considered.
To our knowledge, the present systemic review is the first attempt to perform a qualitative and quantitative synthesis comparing oncological outcomes (OS and DFS) between robotically and laparoscopically treated colon cancer patients. Three studies (23,29,33) provided data on 3-year and/or 5-year OS and DFS that were comparable between RRC and LRC groups. Although it was not possible to perform meta-analysis due to a lack of provided statistical variables, to date there exist no evidence of inferiority of RRC and it can be considered as oncologically safe approach as laparoscopy. Moreover, the finding of higher number of harvested lymph node during RRC may translate into improved oncological outcomes observable in futures studies. Further well-designed studies with larger cohort of patients and sufficient follow-up are necessary to make concrete conclusions regarding this outcome.
Costs
Similarly to other meta-analyses (4,40), the present quantitative synthesis showed a significant higher costs for RRC as compared with LRC, with a mean difference of more than 3,000 USD per patient case. Increased cost is one of the most important drawbacks for implementing the robotic systems in routine practice, therefore high-quality studies comparing expenses for robotic and laparoscopic approach are crucial. Unfortunately, the existing studies that were analyzed in the present systemic review (20,23,26,31,33), showed very high heterogeneity (I2=95%) and high risk of bias. One of the most important reasons for heterogeneity is the different variables included in the cost analyses in each study (e.g., use of surgical and medical instruments, operating room time, cost of operating personnel, cost of anesthesia, length of hospital stay, costs of medicaments and/or consultation received after operation, laboratory and radiological exams etc.). It is clear that a cost of RRC can be a burden for its future implementation despite the increasing evidence of the safety and feasibility of this approach. However, upcoming years can make a dramatical change and decrease the cost as new robotic systems are appearing in the market.
Limitations
The present meta-analysis is based on 24 NRCTs, which were retrospective, and only one RCT.
NRCTs are susceptible for selection, confounding and reporting bias and in general provide low level of evidence (46). Twenty-one of NRCTs were evaluated as having serious risk of bias, mainly due to confounding bias; only two studies (26,30) performed case matching between RRC and LRC before the analysis and one study (18) used propensity score matching.
In some of the outcome analysis, we observed high heterogeneity among the included studies. It can be explained by different sample size, study populations, insufficient or uncomplete data, and interstudy and/or intergroup differences in the surgical technique. Operative, postoperative outcomes and cost can be significantly affected by anastomosis type that varied among the studies or was not specified in large retrospective cohorts where data were extracted from national databases (14,21).
Future directions
The main attention is currently focusing on the comparison of different types of anastomosis during RRC. There are four ongoing studies about RRC that are registered in Clinicaltrials.gov medical library; three of them aim to compare outcomes of intracorporeal and extracorporeal anastomosis. Intracorporeal Versus Extracorporeal Anastomosis in Robotic Right Colectomy (INEXA) (ClinicalTrials.gov ID: NCT03130166) is a bicentric Danish RCT with a primary outcome measure: postoperative recovery. Robotic-assisted and Laparoscopic Right Colectomy Study (ANCOR) (ID: NCT03312569) is a multicentric prospective comparison study in USA of both anastomosis types that has set an incisional hernia rate as their first outcome measure.
Minimally Invasive Right Colectomy Anastomosis Study (MIRCAST) (ID: NCT03650517) is a large European observational, prospective, parallel cohorts study to compare both anastomosis type for 30 days postoperative morbidity. Finally, monocentric Danish prospective single-arm study with historical controls - Prospective Study of Extended Robotic Right Hemicolectomy With Complete Mesocolic Excision for Cancer (ID: NCT04190589)—aims to compare oncological quality of robotic versus open procedure.
New robotic systems for abdominal surgery
Intuitive da Vinci ® robotic system has been dominating the market since its invention in 1994.
The latest generation—the da Vinci Xi—was introduced in 2014 and brought in several technical improvements compared to the pervious robotic platforms. The first single site RRC was described by Morelli et al. in 2013 (47). Da Vinci single site technology provides a possibility to perform single incision surgical procedures by adapted curved cannulas that triangulates instruments internally and separate arms externally, 5 lumen ports that can be introduced by 2.5 cm incision and semi rigid instrument. Authors of several case series on single site RRC (48-50) have stated that this approach is safe and feasible.
There is some evidence in the literature of surgical procedures performed by alternative robotic systems. Samalavicius et al. (51) has reported a right hemicolectomy performed with Senhance® Surgical System with Digital Laparoscopy. This is a multi-port system with independent robotic arms that can be moved and docked separately to standard laparoscopic trocars. Surgeon works at a console using 3D-HD glasses and using handles that are based on laparoscopic instrument principles. Instruments used on the patients work similarly to the fundaments of laparoscopy.
A more elaborate system names “Micro Hand S” has been developed in Central South University in collaboration with Tianjin University (China). It was produced to overcome the financial limitations that are often responsible for slow implementation of existing robotic system in developing countries. It resembles principles of da Vinci system with a surgeon’s console with 3D stereo image screen, two master manipulators, control panel and several pedals and a slave cart with three active slave manipulators (arms). Yi et al. (52) reported a detailed description on this technology and a first case series of abdominal procedures, among them—one successfully performed right colectomy. As stated by the authors, studies on larger patient series are crucial to confirm safety and feasibility of this system.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Marco Milone and Ugo Elmore) for the series “Right Colectomy 2.0” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at http://dx.doi.org/10.21037/ales-20-36
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales-20-36). The series “Right Colectomy 2.0” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Weber PA, Merola S, Wasielewski A, et al. Telerobotic-assisted laparoscopic right and sigmoid colectomies for benign disease. Dis Colon Rectum 2002;45:1689-94. [Crossref] [PubMed]
- Protyniak B, Jorden J, Farmer R. Multiquadrant robotic colorectal surgery: the da Vinci Xi vs Si comparison. J Robot Surg 2018;12:67-74. [Crossref] [PubMed]
- Jimenez-Rodriguez RM, Quezada-Diaz F, Tchack M, et al. Use of the Xi robotic platform for total abdominal colectomy: a step forward in minimally invasive colorectal surgery. Surg Endosc 2019;33:966-71. [Crossref] [PubMed]
- Solaini L, Bazzocchi F, Cavaliere D, et al. Robotic versus laparoscopic right colectomy: an updated systematic review and meta-analysis. Surg Endosc 2018;32:1104-10. [Crossref] [PubMed]
- Ma S, Chen Y, Chen Y, et al. Short-term outcomes of robotic-assisted right colectomy compared with laparoscopic surgery: A systematic review and meta-analysis. Asian J Surg 2019;42:589-98. [Crossref] [PubMed]
- Higgins JPT, Chandler J, Cumpston M, et al. Cochrane Handbook for Systematic Reviews of Interventions. 2nd edition. Chichester (UK): John Wiley & Sons, 2019.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097 [Crossref] [PubMed]
- Higgins JPT, Savović J, Page MJ, et al. Revised Cochrane risk-of-bias tool for randomized trials (RoB 2.0) 2019. Available online: https://www.unisa.edu.au/contentassets/72bf75606a2b4abcaf7f17404af374ad/rob2-0_indiv_main_guidance.pdf
- Sterne JA, Hernan MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [Crossref] [PubMed]
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:13. [Crossref] [PubMed]
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [Crossref] [PubMed]
- Khorgami Z, Li WT, Jackson TN, et al. The cost of robotics: an analysis of the added costs of robotic-assisted versus laparoscopic surgery using the National Inpatient Sample. Surg Endosc 2019;33:2217-21. [Crossref] [PubMed]
- Nolan HR, Smith BE, Honaker MD. Operative time and length of stay is similar between robotic assisted and laparoscopic colon and rectal resections. J Robot Surg 2018;12:659-64. [Crossref] [PubMed]
- Dolejs SC, Waters JA, Ceppa EP, et al. Laparoscopic versus robotic colectomy: a national surgical quality improvement project analysis. Surg Endosc 2017;31:2387-96. [Crossref] [PubMed]
- Ferrara F, Piagnerelli R, Scheiterle M, et al. Laparoscopy Versus Robotic Surgery for Colorectal Cancer: A Single-Center Initial Experience. Surg Innov 2016;23:374-80. [Crossref] [PubMed]
- Guerrieri M, Campagnacci R, Sperti P, et al. Totally robotic vs 3D laparoscopic colectomy: A single centers preliminary experience. World J Gastroenterol 2015;21:13152-9. [Crossref] [PubMed]
- Davis BR, Yoo AC, Moore M, et al. Robotic-assisted versus laparoscopic colectomy: cost and clinical outcomes. JSLS 2014;18:211-24. [Crossref] [PubMed]
- Casillas MA, Leichtle SW, Wahl WL, et al. Improved perioperative and short-term outcomes of robotic versus conventional laparoscopic colorectal operations. Am J Surg 2014;208:33-40. [Crossref] [PubMed]
- Deutsch GB, Sathyanarayana SA, Gunabushanam V, et al. Robotic vs. laparoscopic colorectal surgery: an institutional experience. Surg Endosc 2012;26:956-63. [Crossref] [PubMed]
- Rawlings AL, Woodland JH, Vegunta RK, et al. Robotic versus laparoscopic colectomy. Surg Endosc 2007;21:1701-8. [Crossref] [PubMed]
- Haskins IN, Ju T, Skancke M, et al. Right Colon Resection for Colon Cancer: Does Surgical Approach Matter? J Laparoendosc Adv Surg Tech A 2018;28:1202-6. [Crossref] [PubMed]
- Widmar M, Keskin M, Strombom P, et al. Lymph node yield in right colectomy for cancer: a comparison of open, laparoscopic and robotic approaches. Colorectal Dis 2017;19:888-94. [Crossref] [PubMed]
- Kang J, Park YA, Baik SH, et al. A Comparison of Open, Laparoscopic, and Robotic Surgery in the Treatment of Right-sided Colon Cancer. Surg Laparosc Endosc Percutan Tech 2016;26:497-502. [Crossref] [PubMed]
- Trastulli S, Coratti A, Guarino S, et al. Robotic right colectomy with intracorporeal anastomosis compared with laparoscopic right colectomy with extracorporeal and intracorporeal anastomosis: a retrospective multicentre study. Surg Endosc 2015;29:1512-21. [Crossref] [PubMed]
- Widmar M, Keskin M, Beltran P, et al. Incisional hernias after laparoscopic and robotic right colectomy. Hernia 2016;20:723-8. [Crossref] [PubMed]
- Merola G, Sciuto A, Pirozzi F, et al. Is robotic right colectomy economically sustainable? a multicentre retrospective comparative study and cost analysis. Surg Endosc 2020;34:4041-7. [Crossref] [PubMed]
- Blumberg D. Robotic colectomy with intracorporeal anastomosis is feasible with no operative conversions during the learning curve for an experienced laparoscopic surgeon developing a robotics program. J Robot Surg 2019;13:545-55. [Crossref] [PubMed]
- Solaini L, Cavaliere D, Pecchini F, et al. Robotic versus laparoscopic right colectomy with intracorporeal anastomosis: a multicenter comparative analysis on short-term outcomes. Surg Endosc 2019;33:1898-902. [Crossref] [PubMed]
- Spinoglio G, Bianchi PP, Marano A, et al. Robotic Versus Laparoscopic Right Colectomy with Complete Mesocolic Excision for the Treatment of Colon Cancer: Perioperative Outcomes and 5-Year Survival in a Consecutive Series of 202 Patients. Ann Surg Oncol 2018;25:3580-6. [Crossref] [PubMed]
- Ngu JC, Ng YY. Robotics confers an advantage in right hemicolectomy with intracorporeal anastomosis when matched against conventional laparoscopy. J Robot Surg 2018;12:647-53. [Crossref] [PubMed]
- deSouza AL, Prasad LM, Park JJ, et al. Robotic assistance in right hemicolectomy: is there a role? Dis Colon Rectum 2010;53:1000-6. [Crossref] [PubMed]
- de'Angelis N, Lizzi V, Azoulay D, et al. Robotic Versus Laparoscopic Right Colectomy for Colon Cancer: Analysis of the Initial Simultaneous Learning Curve of a Surgical Fellow. J Laparoendosc Adv Surg Tech A 2016;26:882-92. [Crossref] [PubMed]
- Park JS, Kang H, Park SY, et al. Long-term oncologic after robotic versus laparoscopic right colectomy: a prospective randomized study. Surg Endosc 2019;33:2975-81. [Crossref] [PubMed]
- Lujan HJ, Plasencia G, Rivera BX, et al. Advantages of Robotic Right Colectomy With Intracorporeal Anastomosis. Surg Laparosc Endosc Percutan Tech 2018;28:36-41. [Crossref] [PubMed]
- Lujan HJ, Maciel VH, Romero R, et al. Laparoscopic versus robotic right colectomy: a single surgeon's experience. J Robot Surg 2013;7:95-102. [Crossref] [PubMed]
- Gerbaud F, Valverde A, Danoussou D, et al. Experience With Transitioning From Laparoscopic to Robotic Right Colectomy. JSLS 2019;23:e2019.00044.
- Yozgatli TK, Aytac E, Ozben V, et al. Robotic Complete Mesocolic Excision Versus Conventional Laparoscopic Hemicolectomy for Right-Sided Colon Cancer. J Laparoendosc Adv Surg Tech A 2019;29:671-6. [Crossref] [PubMed]
- Mégevand JL, Amboldi M, Lillo E, et al. Right colectomy: consecutive 100 patients treated with laparoscopic and robotic technique for malignancy. Cumulative experience in a single centre. Updates Surg 2019;71:151-6. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Rondelli F, Balzarotti R, Villa F, et al. Is robot-assisted laparoscopic right colectomy more effective than the conventional laparoscopic procedure? A meta-analysis of short-term outcomes. Int J Surg 2015;18:75-82. [Crossref] [PubMed]
- Foo CC, Ku C, Wei R, et al. How does lymph node yield affect survival outcomes of stage I and II colon cancer? World J Surg Oncol 2020;18:22. [Crossref] [PubMed]
- Lykke J, Rosenberg J, Jess P, et al. Lymph node yield and tumour subsite are associated with survival in stage I–III colon cancer: results from a national cohort study. World J Surg Oncol 2019;17:62. [Crossref] [PubMed]
- Petrucciani N, Sirimarco D, Nigri GR, et al. Robotic right colectomy: A worthwhile procedure? Results of a meta-analysis of trials comparing robotic versus laparoscopic right colectomy. J Minim Access Surg 2015;11:22-8. [Crossref] [PubMed]
- Parisi A, Scrucca L, Desiderio J, et al. Robotic right hemicolectomy: Analysis of 108 consecutive procedures and multidimensional assessment of the learning curve. Surg Oncol 2017;26:28-36. [Crossref] [PubMed]
- Raimondi P, Marchegiani F, Cieri M, et al. Is right colectomy a complete learning procedure for a robotic surgical program? J Robot Surg 2018;12:147-55. [Crossref] [PubMed]
- Song JW, Chung KC. Observational studies: cohort and case-control studies. PLast Reconstr Surg 2010;126:2234-42. [Crossref] [PubMed]
- Morelli L, Guadagni S, Caprili G, et al. Robotic right colectomy using the Da Vinci Single-Site(R) platform: case report. Int J Med Robot 2013;9:258-61. [Crossref] [PubMed]
- Chang TC, Chen YT, Yen MH, et al. Single-Incision Robotic Colectomy: Comparison of Short-Term Outcomes with Multiport Robotic Colectomy. J Laparoendosc Adv Surg Tech A 2020;30:183-7. [Crossref] [PubMed]
- Spinoglio G, Lenti LM, Ravazzoni F, et al. Evaluation of technical feasibility and safety of Single-Site robotic right colectomy: three case reports. Int J Med Robot 2015;11:135-40. [Crossref] [PubMed]
- Juo YY, Agarwal S, Luka S, et al. Single-Incision Robotic Colectomy (SIRC) case series: initial experience at a single center. Surg Endosc 2015;29:1976-81. [Crossref] [PubMed]
- Samalavicius NE, Smolskas E, Deduchovas O, et al. Robotic right colectomy using the new Senhance(R) robotic platform: a three-trocar technique - a video vignette. Colorectal Dis 2019;21:1092-3. [Crossref] [PubMed]
- Yi B, Wang G, Li J, et al. Domestically produced Chinese minimally invasive surgical robot system “Micro Hand S” is applied to clinical surgery preliminarily in China. Surg Endosc 2017;31:487-93. [Crossref] [PubMed]
Cite this article as: Lauka L, Brunetti F, Beghdadi N, Notarnicola M, Sommacale D, de’Angelis N. Advantages of robotic right colectomy over laparoscopic right colectomy beyond the learning curve: a systematic review and meta-analysis. Ann Laparosc Endosc Surg 2020;5:33.


