Sleeve gastrectomy and its modifications
Introduction
Sleeve gastrectomy (SG), also called longitudinal gastrectomy, is performed with increasing frequency in the treatment of morbid obesity in recent years. The method is generally considered to be a totally restrictive technique because it reduces gastric capacity, but it also provides a decrease in post-prandial ghrelin levels. Ghrelin is secreted from the fundus. Gastric fundus contains more ghrelin per gram of tissue than the duodenum (1,2). In addition, Laparoscopic SG (LSG) does not cause malabsorption but increased intestinal motility, and the rate at which food reaches the small intestine, and thus affect circulating levels of other intestinal hormones (3). This consequently decreases the sensation of hunger. LSG has been the most common bariatric surgical procedure in many countries. But it is difficult to compare studies on preoperative preparation and outcomes because of the variations of technique (4).
Patient selection
LSG is very effective and useful for morbidly obese patients and patients with BMI greater than 50. It can be planned as the first stage procedure. It can also be safely performed in patients with comorbidities and awaiting for transplantation (5,6). SG has a shorter operative time than other gastric bypass procedures. It is really difficult to reach the biliary tract with gastric bypass methods, but there is no technical difficulty with SG. It may also be preferred in patients with diseases like Crohn and ulcerative colitis, or who need regular upper gastrointestinal system examination. It may be useful in patients who have undergone lower gastrointestinal system surgery and have small bowel adhesions. It is also recommended for the patients as gastric bypass can cause changes in serum levels of some kind of drugs. Many bariatric surgeons accept gastroesophageal reflux disease (GERD), Barrett’s esophagus as contraindications (7). None of the studies have shown the risk of Barrett’s conversion to high-grade dysplasia or adenocarcinoma after SG. SG can be performed in adolescents with severe comorbidities whose body mass index higher than 35. This is effective when compared to diet and exercise. It allows the healing or ameliorate of both somatic and physiological issues related with obesity (8-10). LSG has theoretical advantages, especially for this patient group. The incidence of surgical or non-surgical complications is lower in LSG than in gastric bypass. The need for mineral and vitamin supplements may be shorter in LSG groups than bypass groups. The main advantage of SG in adolescent obese patients is that it can be easily converted to other alternative methods in later years. We believe bariatric surgery for adolescent should be considered by a special adolescent bariatric. All patients must be informed about the technique of SG and accept the risks of operation. Patients should know that they will be on a long-term diet program and they should change their lifestyle.
Technique
Patient position
The patient was placed supine with the French position. Lower extremity compression is very important to avoid deep vein thrombosis. We use compression socks. Also compression devices can be used for prophylaxis. We use belts from the soles of the feet to fix the patient to the operation table.
Pneumoperitoneum
In our practice, we prefer the closed method using Veress needle. It is in most cases placed from the supraumbilical region. We maintain intraabdominal pressure between 13 and 14 mmHg. For patients who cannot be placed safely, we use a 0 degree telescope and optic trocar. If necessary open (Hasson) technique can be used it is the safest method.
Insertion of trocars
In our practice, we usually use two 12 mm and one 10 mm trocar. However, extra trocars may be required in difficult situations. The first trocar is placed in the superior-lateral aspect of the umbilicus. We prefer 30° camera because it provides perfect visualization of the angle of His. We use two 12 mm ports placed in the right and left midclavicular line.
Liver retraction
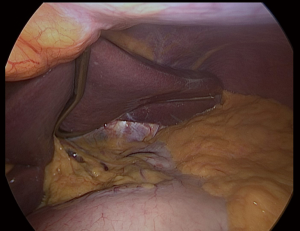
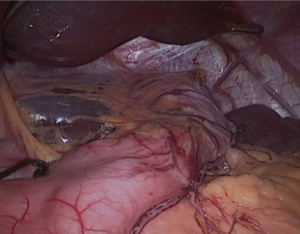
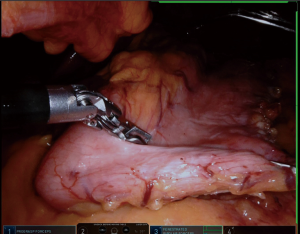
We placed in Nathanson®’s retractor through a 5 mm incision. Then we retract the left lobe of the liver that also allows the visualization of the hiatus and vagus nerves (Figure 1). Then it is evaluated for any looseness or hiatus hernia (HH). If there is a HH, we usually perform crus repair after completion of SG.
Dissection and mobilization of greater curvature
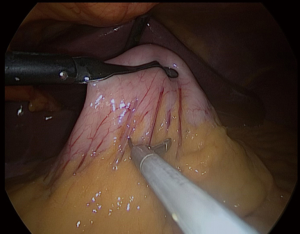
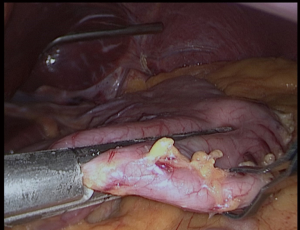
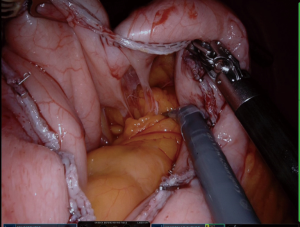
We always put a nasogastric (NG) tube to decompression the stomach. The NG tube should not be forgotten in the stomach after decompressing the stomach. It is essential to verify that the vessels located around the greater curvature are properly sealed (Figure 2). The greater curvature is freed from 4–6 cm proximal to the pylorus. Entering the lesser sac is easier here. Any 5 mm energy-based device of can be used. Sealing should be done close to the stomach (11,12).
Dissection near angle of His
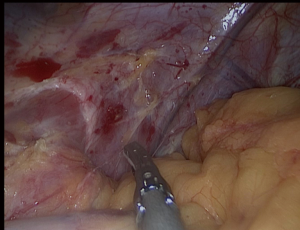
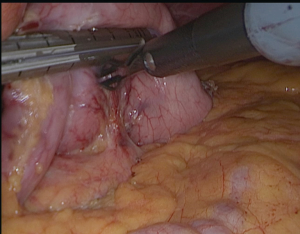
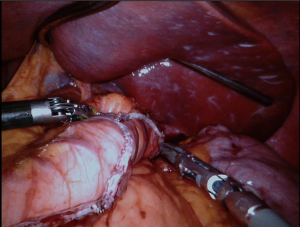
All attachments between the fundus and the diaphragm should be divided (Figure 3); the fat pad should be dissected but should not resected. We should be avoided from the dissection of the GE junction. After totally mobilizing the greater curvature in the presence of HH, both crus dissected for repair after completion SG.
Creation of gastric tube
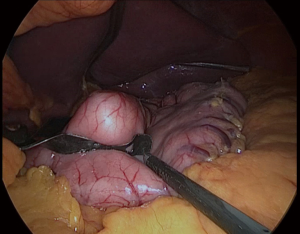
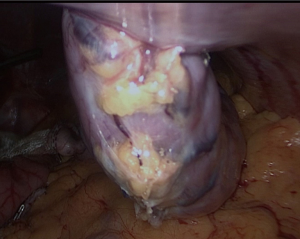
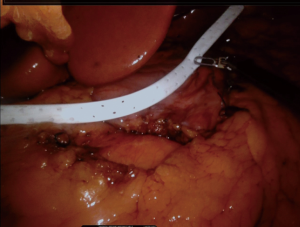
First, the placement of all tubes except the bougie into the stomach should be performed by the anesthesiologist. The bougie is then advanced to the first part of the duodenum and its alignment should be straight (Figure 4). We usually place it after the first stapler (Figures 5,6). There should be communication between the anesthesiologist and the surgeon, as forced placement may cause injury. Instead, an endoscope may be used to calibrate the sleeve formation. There are many different types of bougie ranging from 32–60 F to form the tube. We use 36 F bougie in our current practice. Technically, weight loss is not related with the bougie size (13,14). Using a thinner size bougie can provide more effective weight loss, but may result in higher stricture and leakage rates. The transection of the stomach starts 3–5 cm proximal to the pylorus. We usually use a 60 mm stapler extra thick cartridge (black) for first firing. The first stapler should be placed in from the right trocar tangential to the antrum and we should take care of closure the jaws. After the first firing, left trocar can used for the ongoing stapling. Usually 4–8 staplers are enough to complete the sleeve formation. Antrum is the thicker part of the stomach. The first two cartridges should be green or preferably black, the rest can be blue, purple or green. We recommend using black or green cartridges for the first two fires and purple cartridges for the other four fires. In our practice we usually prefer purple color cartridges (for the first two firings. Lateral traction of the stomach is essential to create a straight staple line. One of the most important point during transection is to avoid incisura angularis and remove the all fundus at a distance of 1 cm from the esophagus.
Hemostasis and reinforcement
Staple line reinforcement can be used to reduce bleeding and leaks and there are some techniques to support this line like over sewing, using buttressing material, fibrin glue along the staple line. Buttressing materials include glycol trimethyl carbonate copolymer, bovine pericardium or porcine small intestine submucosa can be used to decrease the bleeding rates from the staple line. But use of buttressing material is still controversial. As a result of our study, 145 patients underwent LSG; we used Tisseel® for 57 patients and Seamguard® for 73 patients. These reinforcement materials were effective methods to decrease staple line bleeding. Hospitalization, complications and drainage volumes were similar in both groups. Seamguard® was reduced operating time. Use of Tisseel® was decreased the hospital costs. And we can say both reinforcement techniques can be safely use during LSG (15). In our center, Seamguard® is used in patients with high BMI and using anticoagulants. It should be noted that the staples are not hemostatic and the staple line needs to be checked. Hemorrhage should be stopped with clips and sutures. Surgeon should be avoided from the thermal damage.
Leak test and extraction of specimen
In our practice the leak test is a routine test for all patients. We use methylene blue through the nasogastric tube (Figure 7). Also we use endoscopy to check the sleeve tube for leaks, bleeding and patency. The specimen can be extracted from 15 mm trocar (Figure 8). A suction drain (Jackson Pratt 14 F) is placed under the liver. We always close the fascia of the 15 mm trocars to avoid hernia occurrence and for skin closure we use subcutaneous Monocryl sutures.
Concomitant HH and reflux
HH and GERD treatment which can be emerged after SG operations and it is still very controversial. Nearly half of morbidly obese patients have GERD. Symptomatic GERD after SG is associated with increased relaxation of the lower esophageal sphincter and high intra-abdominal pressure. Prevalence of esophagitis and low incidence of esophageal and junctional adenocarcinomas are increased in morbidly obese patients compared to the general population (16). In a consensus meeting 83% of specialist surgeons decided that HH should be identified intraoperatively and, if present, repaired; 69% of surgeons participating in the 4th International LSG Consensus found that they were actively looking for and the 89% of surgeons said they would repair it if identified intraoperatively (4,5). In our current practice, we recommend that intraoperative HH repair when we detect HH preoperatively or perioperatively. After dissection of the large curvature is completed, the left crus should be exposed and the left and right crus should be repaired using non-absorbable sutures in the presence of HH. Meanwhile, a bougie or a preferred endoscope can be inserted. After SG decreased intra-abdominal pressure with weight loss helps to reduce the incidence rate of GERD. Symptomatic GERD and/or a large HH diagnosed preoperatively, care should be taken against LSG. Technically, if possible, gastric bypass methods may be recommended. In cases of reflux after LSG, the wrong surgical technique should be considered and most patients can be initially treated with proton pump inhibitors. If GERD symptoms persist, LSG can be converted to gastric bypass methods.
Banding the sleeve
This approach has been recommended to decrease dilatation of sleeve tube. However, many authors believe that routine use after LSG cannot be recommended. Adding a silastic ring or band around the sleeve tube has no proven benefit and may increase short and long-term morbidity. Banding the sleeve can only be evaluate with larger randomized controlled trials with the patient›s approval process (17,18).
Robotic SG (RSG)
RSG is being a popular surgical approach with many advantages such as high image quality and sensitivity. The learning curve and the high cost are the main drawbacks. For robotic surgery we use da Vinci Xi. We placed the patients in supine position for RSG. We placed endoscope port close to the umbilicus (2 cm laterally and 2 cm above). After pneumoperitoneum, the patients were placed in the reverse Trendelenburg position. We use a Nathanson retractor from the epigastrium in all cases. We place two 8 mm ports on both sides of the hypochondrium and place a 12 mm assistant port between the previous ports and the left side of the lateral abdominal region. EndoWrist instruments (Fenestrated Bipolar and Vessel Sealer) and robotic arms by connecting the ports, we begin the operation. The next steps are almost identical to the laparoscopic technique (Figures 9-14). In our study we compared the first 30 RSG cases with the last 30 laparoscopic cases and study revealed no significant difference between the groups in terms of complications, bleeding, leakage, stenosis, length of hospital stay, and weight loss (19). However, the duration of the operation and the amount of drainage were different. Robotic surgery has facilitated many interventions because of the better visibility of EndoWrist devices and higher maneuverability. Robotic surgical techniques have also been included in obesity surgery and have been shown to be more effective and provide excellent anastomosis and access to difficult areas in some complex bariatric procedures.
Single port SG (SPSG)
SPSG procedure could performed with a single incision with limited range of movements. But extraction of specimen can be a problem without enlargement of incision. Last 10 years several series of SPSG have been published (20-22). The cosmetic benefit, less pain after surgery, hospital stay and fast return to work can be expected advantages of procedure (23,24). There are some difficulties and disadvantages like triangulation, dissection and exposure, and the need for liver retraction. The present studies show SPSG can be performed safely with similar intraoperative and postoperative morbidity compared to the LSG. Weight loss and comorbidities resolution at 1 year are was equivalent. Operative duration was longer in the SPSG group. In conclusion evaluation of the potential benefits of SPSG requires further evaluation by large, prospective, randomized studies.
N-sleeve
SG decrease volume of stomach and that increase intragastric pressure. This is the reason of some patients have reflux after SG. N-sleeve starts with dissection and reduction of HH. Esophagus should be mobilized. Para esophageal space should be dissected. The greater curvature is freed from 4–6 cm proximal to the pylorus. Two non-absorbable sutures are used to close the HH and a bougie is inserted routinely. 360° valve of 3 cm is created by using silk and the wrap valve is fixed to the anterior part of the esophagus. Then SG should be performed as usual. There are some important technical points contrary to standard SG, we should be careful about ischemia of the gastric wall and not to stapling double the part of fundus. In a recent study, 3 months after N-sleeve, 76% of the patients were asymptomatic and there was no need to use proton pump inhibitor; 12% of the patients were still have complaining with reflux symptoms (25,26).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Mehmet Mahir Ozmen) for the series “Bariatric and Metabolic Surgery” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales-20-30). The series “Bariatric and Metabolic Surgery” was commissioned by the editorial office without any funding or sponsorship. MMÖ served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Annals of Laparoscopic and Endoscopic Surgery from Mar 2019 to Feb 2021. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ozmen F, Şahin TT, Dolgun A, et al. Changes in Ghrelin and Resistin Levels Following Bariatric Surgery: One Anastomosis Gastric Bypass Vs Sleeve Gastrectomy. Surg Obes Relat Dis 2016;12:S198-S199. [Crossref]
- Ozmen F, Ozmen MM. Obesity, immunology and obesity surgery. Adv Obes Weight Manag Control 2016;4:80.
- Baumann T, Kuesters S, Grueneberger J, et al. Time-resolved MRI after ingestion of liquids reveals motility changes after laparoscopic sleeve gastrectomy—preliminary results. Obes Surg 2011;21:95-101. [Crossref] [PubMed]
- Gagner M, Deitel M, Erickson AL, et al. Survey of laparoscopic sleeve gastrectomy (LSG) at the Fourth International Consensus Summit on sleeve gastrectomy. Obes Surg 2013;23:2013-7. [Crossref] [PubMed]
- Rosenthal RJ. International sleeve gastrectomy expert panel consensus statement best practice guidelines based on experience of >12,000 cases. Surg Obes Relat Dis 2012;8:8-19. [Crossref] [PubMed]
- Schaeffer DF, Yoshida EM, Buczkowski AK, et al. Surgical morbidity in severely obese liver transplant recipients—a single Canadian Centre Experience. Ann Hepatol 2009;8:38-40. [Crossref] [PubMed]
- DuPree CE, Blair K, Steele SR, Martin MJ. Laparoscopic sleeve gastrectomy in patients with preexisting gastroesophageal reflux disease a national analysis. JAMA Surg 2014;149:328-34. [Crossref] [PubMed]
- Gündoğdu E, Bilgiç Cİ, Moran M, et al. Evaluation of the effects of laparoscopic adjustable gastric banding versus laparoscopic sleeve gastrectomy on weight loss. EuRJ 2019;6:36-42.
- Inge TH, Miyano G, Bean J, et al. Reversal of type 2 diabetes mellitus and improvements in cardiovascular risk factors after surgical weight loss in adolescents. Pediatrics 2009;123:214-22. [Crossref] [PubMed]
- Olbers T, Gronowitz E, Werling M, et al. Two-year outcome of laparoscopic Rouxen- Y gastric bypass in adolescents with severe obesity: results from a Swedish Nationwide Study (AMOS). Int J Obes (Lond) 2012;36:1388-95. [Crossref] [PubMed]
- Özmen MM, Gagner M. Laparoscopic sleeve gastrectomy: pitfalls and techniques to prevent complications. Eur J Endosc Laparosc Surg 2014;1:55-8.
- Aggarwal S, Priyadarshini P, Gagner M. LSG: The Technique. In: Agrawal S. editor. Obesity, Bariatric and Metabolic Surgery: A Practical Guide, First Edition. Publisher: Springer, 2015:247-57.
- Parikh M, Gagner M, Heacock L, et al. Laparoscopic sleeve gastrectomy: does bougie size affect mean %EWL? Short-term outcomes. Surg Obes Relat Dis 2008;4:528-33. [Crossref] [PubMed]
- Gagner M. Leaks after sleeve gastrectomy are associated with smaller bougies: prevention and treatment strategies. Surg Laparosc Endosc Percutan Tech 2010;20:166-9. [Crossref] [PubMed]
- Geyik SG, Ozmen M, Bilgic I, et al. Comparison of TISSEEL and Gore-Seamguard Usage in Laparoscopic Sleeve Gastrectomy. In: Obesity surgery. New York, USA: Springer, 2013:1206.
- Ramar S, Ramamoorthy R, Agrawal S. LSG: Outcomes. In: Agrawal S. editor. Obesity, Bariatric and Metabolic Surgery: A Practical Guide, First Edition. Publisher: Springer, 2015:277.
- Agrawal S, Van Dessel E, Akin F, et al. Laparoscopic adjustable banded sleeve gastrectomy as a primary procedure for the super-super obese (body mass index > 60 kg/m2). Obes Surg 2010;20:1161-3. [Crossref] [PubMed]
- Alexander JW, Martin Hawver LR, Goodman HR. Banded sleeve gastrectomy—initial experience. Obes Surg 2009;19:1591-6. [Crossref] [PubMed]
- Ozmen MM, Gundogdu E, Guldogan CE. First 30 Robotic Versus Last 30 Laparoscopic Sleeve Gastrectomy. Bariatr Surg Pract P 2019;14:102-6.
- Tranchart H, Rebibo L, Gaillard M, et al. Short-term outcomes of single-port versus conventional laparoscopic sleeve gastrectomy: a propensity score matched analysis. Surg Endosc 2019; [Crossref] [PubMed]
- Maluenda F, Leon J, Csendes A, et al. Single-incision laparoscopic sleeve gastrectomy: initial experience in 20 patients and 2-year follow-up. Eur Surg 2014;46:32-7. [Crossref] [PubMed]
- Porta A, Aiolfi A, Musolino C, et al. Prospective comparison and quality of life for single-incision and conventional laparoscopic sleeve gastrectomy in a series of morbidly obese patients. Obes Surg 2017;27:681-7. [Crossref] [PubMed]
- De Santé, Haute Autorité. Obésité: prise en charge chirurgicale chez l'adulte. Recommandations de bonne pratique. Saint-Denis La Plaine: HAS;2009. Ref ID, 4679.
- Pourcher G, Di Giuro G, Lafosse T, et al. Routine single-port sleeve gastrectomy: a study of 60 consecutive patients. Surg Obes Relat Dis 2013;9:385-9. [Crossref] [PubMed]
- Nocca D, Skalli EM, Boulay E, et al. Nissen Sleeve (N-Sleeve) operation: preliminary results of a pilot study. Surg Obes Relat Dis 2016;12:1832-7. [Crossref] [PubMed]
- Palermo M, Serra E, Duza G. N-sleeve gastrectomy: an option for obesity and GERD. ABCD Arq Bras Cir Dig 2019;32:e1482 [Crossref] [PubMed]
Cite this article as: Guldogan CE, Ozozan OV, Ozmen MM. Sleeve gastrectomy and its modifications. Ann Laparosc Endosc Surg 2020;5:27.