Optimal workup for a hiatal hernia
A hiatal hernia (HH) is a partial or total migration of the stomach across the diaphragmatic hiatus up to the mediastinum, alone or together with other abdominal organs.
The estimated prevalence in the United States is between 10% and 80% (1). It’s usually related to high body mass index (BMI) and older age. However, the real prevalence of hiatal hernias is not easy to establish because many patients are asymptomatic and, hence, are not diagnosed.
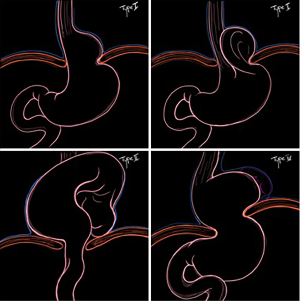
Hiatal hernias are classified into four types (I to IV) (Figure 1):
- Type I, or "sliding hernia" is one where the gastroesophageal junction migrates proximal to the esophageal hiatus. This type occurs in the presence of the enlargement of the esophageal hiatus and the relaxation of the phrenoesophageal ligament; it represents about 95% of hiatal hernias (2).
- Type II hernia is paraesophageal (PEH), due to the enlargement of the esophageal hiatus in the anterior and lateral part of the phrenoesophageal membrane. The gastric fundus or body herniates through this defect whereas the gastroesophageal junction remains in the abdomen (3). This type of hernia is rare, since it accounts for less than 1% of all hiatal hernias.
- Type III is the most common paraesophageal hiatal hernia. It combines the characteristics of both type I and type II hernias. The phrenoesophageal membrane is loose and elongated, the esophagogastric junction is displaced in the thorax and a defect in the antero-lateral portion of the membrane allows the stomach to migrate into the mediastinum (2). It represents about 5% of all hiatal hernia (4).
- Type IV hiatal hernias are characterized by a large diaphragmatic hiatal defect. The stomach and other intra-abdominal organs can herniate in the mediastinum (4). The most common herniated organs are small and large intestine with or without associated omentum. However, spleen, pancreas and liver can also migrate in the mediastinum. This type is the least common and accounts for about 0.1% of hiatal hernias (4).
- Finally a Type V hernia has been described by some authors and is a herniated fundoplication or wrap migration.
In type III and IV the hernia is a risk for gastric volvulus. An organoaxial rotation occurs in about 60% of cases and consists in the rotation around the axis that connects the esophago-gastric junction (EGJ) and the pylorus. Instead in the case of a mesenteroaxial rotation, the motion is around the short axis of the stomach: the antrum rotates anteriorly and superiorly so that the posterior surface of the stomach flips anteriorly. This is sometimes called the "upside down" stomach (5).
Hiatal hernias are common and again, are often asymptomatic. There is no indication to pursue a diagnosis of hiatal hernia in asymptomatic patients, but symptomatic ones need evaluation and should be considered for elective surgical repair, especially if large and associated with obstructive symptoms or volvulus. Obstructed or gangrenous PEH require emergent repair and can have significant morbidity and mortality.
The optimal work up changes depending on patient’s history and clinical presentation.
First diagnosis
Elective patients suspected for Hiatal Hernia are primarily examined for history of previous surgery, especially upper GI surgery, and comorbidities. Then a thorough analysis of signs and symptoms is mandatory. Although small hernias may be associated with typical gastro-esophageal reflux disease (GERD) symptoms such as heartburn or regurgitations, patients presenting with a large HH more often complain of obstructive symptoms such as chest or epigastric pain, dysphagia, postprandial pain or vomiting, due to compression of adjacent mediastinal and thoracic structures and organs. Extra digestive symptoms are also frequently reported including respiratory symptoms such as recurrent aspiration, pneumonia, cough, shortness of breath and dyspnea on exertion. Furthermore, fatigue and chronic iron-deficiency anemia can be an indirect sign of hiatal hernia as large hernias are sometimes associated with mechanical (Cameron's) ulcers where the stomach drapes over the hiatus (6).
Radiologic studies
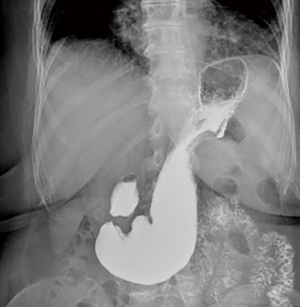
The first part of the work-up is common for all patients. Chest X-ray and upper gastrointestinal series are the initial tests (Figure 2). Chest X-ray may identify some opacity of the soft tissue with or without air-fluid levels. Also, a retrocardiac air-fluid level is characteristic for a paraesophageal hiatal hernia, and endoluminal gas may be seen in cases of intestinal herniation. Intestinal loops may be seen in an unusual vertical pattern toward the chest with a typical displacement or ascending deformity of the transverse colon in cases of colon herniation (7). Contrast-swallow studies help to identify the size and the position of the EGJ in relation to the hiatus and the stomach (8). Also, they display the axis of a volvulus, if present. In addition, contrast studies provide information about gastric outlet or esophageal obstruction and may suggest abnormal esophageal motility and associated alterations such as esophageal lesions, strictures or diverticula.
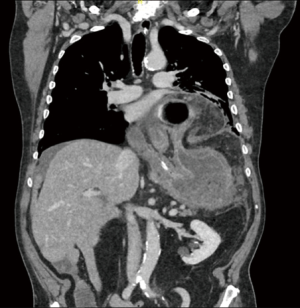
Although not part of a standard work-up, a large number of patients are referred to the surgeon with a CT scan performed for dysphagia and dyspnea. CT images allow to assess the dimensions of the hernia, the width of the hiatus, the migration of other abdominal organs in the mediastinum and to point out complications such as gastric volvulus (9). However, a CT scan does not replace the radiological examinations necessary for a correct preoperative workup, because the incidental diagnosis of hiatal hernia, especially if small, must always be supported by further investigations (2). In fact, some studies have reported an augmented incidence of hiatal hernia during the execution of colon CT, not confirmed with abdominal CT without colonic distention (10,11) (Figure 3).
Endoscopy
All patients with suspected or confirmed symptomatic hiatal hernia should undergo an esophagogastroduodenoscopy (EGD); however, given the diffusion of endoscopy, hiatal hernias are frequently diagnosed when endoscopy has been already performed for other symptoms and/or reasons. Endoscopy helps defining the anatomy, the size and type of the hernia, any associated esophageal and gastric mucosal disease such as esophagitis, Barrett’s esophagus and cancer. It can also suggest a delayed gastric emptying when retained food is found in the stomach.
A hiatal hernia is diagnosed by EGD evaluating the distance between the EGJ and the diaphragmatic incisura, which is the impression of the diaphragmatic hiatus on the gastric wall. The endoscopic hiatal hernia diagnosis is defined as a distance greater than 2 cm (3). However, some pathological conditions can complicate the endoscopic diagnosis: in patients with Barrett’s esophagus the identification of the EGJ can be difficult (6) while in presence of a wide separation of the crura, the diaphragmatic impression can be hard to recognize (3).
The EGJ position can be also assessed in retroflexion using the Hill classification (12). This classification evaluates the EGJ and hiatal integrity considering a “flap-valve” mechanism and could be used also to predict reflux.
According to this classification, grade I flap-valve is the normal configuration and it is defined by the presence of a prominent fold of tissue closely approximated to the shaft of the endoscope and extending 3–4 cm along the lesser curve at the entrance of the esophagus into the stomach; there is no hiatal hernia.
In grade II, the fold of tissue is flattened and there are occasional periods of opening and rapid closing around the endoscope with respiration.
In Hill grade III flap-valve there is no fold at the entrance of the esophagus into the stomach and the endoscope is not tightly gripped by the tissues. This condition is frequently associated with sliding hiatal hernias.
Lastly, Hill grade IV valve is defined by the diaphragmatic hiatus making an extrinsic compression on the gastric mucosa; essentially no fold where the lumen of the esophagus is gaping open, allowing the squamous epithelium to be viewed from below. This grade is always associated with a hiatal hernia.
Functional and motility studies
In our institution whenever possible we study all the patients scheduled for a hiatal hernia repair with a high resolution manometry (HRM); this exam provides important details about the motility of the esophagus and the EGJ.
HMR can also identify and calculate the size of the sliding part of the hernia by assessing the spatial dissociation between the lower esophageal sphincter (LES) and the diaphragmatic sphincter, visualized as a double peak pressure profile at the EGJ (13). The Chicago Classification of hiatal hernia by HRM is based on this spatial separation of the two “high pressure zones” (14,15). However, the accurate position of the probe for HRM can be challenging, especially in patients with large hernia (16,17).
LES relaxation in patients with paraesophageal HH can be impaired, resulting in an increased intrabolus pressure and ultimately, according to the Chicago classification, EGJ outflow obstruction.
HRM can help to tune the operative strategy since findings of severe dysmotility or pseudoachalasia may indicate a simple hiatal repair without fundoplication.
In case of sliding hiatal hernias, a pH test is useful to identify the presence of reflux and so the patients that might benefit from antireflux surgery in addition to hernia repair. In symptomatic patients presenting with large hiatal hernia, the benefit of performing a pH study is controversial because a negative result would not change the need for operative repair (18).
In patients complaining of respiratory symptoms, particularly shortness of breath and dyspnea on exertion, pulmonary function testing (PFT) may offer important information and also provide risk assessment. PFTs may be useful for assessing the degree of pulmonary impairment and to rule out underlying pulmonary disease; however, in case of coexisting pulmonary disease, it may be difficult to determine whether the hernia or the lung disease is responsible for the patients’ symptoms.
An echocardiogram could also be useful to rule out cardiac dysfunction as the culprit of symptoms (19).
In patients complaining of chest pain, a cardiac stress test could be performed to exclude any myocardial ischemia.
After assessing the anatomy of the hernia and the function of the esophagus, patients are evaluated by the anesthesiologists for a general risk assessment especially since this condition affects primarily the elderly.
Recurrence
Recurrence can occur from 5% to 42% (20,21). The mechanism of recurrence is still not well understood, but technical aspects of the primary repair, age, perhaps elevated body mass index (BMI), and pulmonary disease has been considered as possible risk factors for recurrence (22).
Recurrences could present with intact hiatus, lateral defect, anterior defect, posterior defect or anteroposterior defect, listed in increasing order of frequency (20).
In addition, apparently well-done fundoplications and cruroplasties in symptomatic patients on occasion require revision because of over-tightness.
In patients with a suspected hiatal hernia recurrence, a careful workup is mandatory.
Despite recurrence being frequent, radiological recurrence alone is not an indication for a redo-surgery, since the quality of life is impacted by symptoms and not by the radiological recurrence (23). Only symptomatic patients are surgical candidates. Obstructive symptoms are the most commonly reported in case of recurrence and include dysphagia, early satiety, anorexia, regurgitation, vomiting, weight loss and postprandial bloating.
In the elderly population, alterations in eating habits, postprandial dyspnea and early satiety may be related to aging, in presence of a negative workup (24).
If redo-surgery is necessary, a thorough reading of the previous operatory report is mandatory to understand the mechanism of failure and help plan the surgical strategy in the clinical context of the patient. The operative strategy could change depending on the size of the hernia, the presence of residual sac, the length of the intra-abdominal esophagus, the presence and the type of fundoplication, the presence of a mesh.
The work-up is similar to the non-recurrent patients.
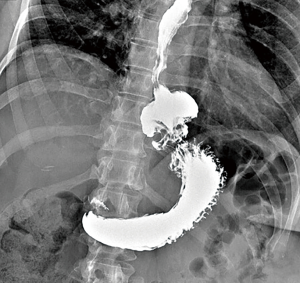
Contrast swallow and EGD are useful to assess the size of the recurrent hernia, the position of the EGJ, detect mechanical and functional obstruction related to the hiatal repair and fundoplication if present and esophageal and gastric mucosal disease (Figure 4).
A CT scan with sagittal, coronal, and 3D reformatted images is very useful in patients with altered anatomy because of previous upper-GI or thoracic surgery (9).
Emergency
Hiatal hernia may present as a rare acute complication requiring urgent surgical management mainly to correct acute gastric volvulus or ischemia with perforation.
Early recognition and intervention are the key. In the case of emergency presentation, an excessive investigation may lead to delay in treatment and suboptimal outcomes (25).
Patients with emergency presentations of hiatal hernia may present the Borchardt’s triad of symptoms: severe epigastric pain, retching with inability to vomit and impossibility to position a nasogastric tube into the stomach.
A CT scan may be useful for patients with suspected complications from a gastric volvulus; it allows to visualize the herniated organs within the chest cavity. Furthermore, in the case of intestinal obstruction and strangulation, dilated intestinal segments with air-fluid levels can be visualized within the chest cavity and abdomen. The CT scan can also point out the presence of gastric necrosis through some suggestive findings such as pneumatosis of the gastric wall, free gas and fluid outside the gastric wall within the hernia sac, and lack of contrast enhancement of the gastric wall (26).
An EGD can also have a therapeutic role in the case of gastric volvulus, helping to decompress the stomach and position of a nasogastric tube. Insufflation of the stomach can sometimes unfold the volvulus, changing the operative strategy on a semi-urgent operation in daylight time. In the meantime, a nasogastric tube is placed to maintain the decompression (26).
Conclusions
Patients’ symptoms, clinical presentations and hiatal hernia type drive the selection of the most appropriate workup for hiatal hernia. For elective HH repair we advocate the use of UGI series, EGD and HRM as first line pre-operative tests. More specific functional and morphological studies such as pH testing, PTF, and CT scan should be used case by case depending on the hernia size, patients’ symptoms and setting.
Acknowledgments
We would like to thank Catherine Cers for the graphic support.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2020.03.02). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wu AH, Tseng CC, Bernstein L. Hiatal hernia, reflux symptoms, body size, and risk of esophageal and gastric adenocarcinoma. Cancer 2003;98:940-8. [Crossref] [PubMed]
- Abbara S, Kalan MMH, Lewicki AM. Intrathoracic stomach revisited. AJR Am J Roentgenol 2003;181:403-14. [Crossref] [PubMed]
- Kahrilas PJ, Kim HC, Pandolfino JE. Approaches to the diagnosis and grading of hiatal hernia. Best Pract Res Clin Gastroenterol 2008;22:601-16. [Crossref] [PubMed]
- Krause W, Roberts J, Garcia-Montilla RJ. Bowel in Chest: Type IV Hiatal Hernia. Clin Med Res 2016;14:93-6. [Crossref] [PubMed]
- Rashid F, Thangarajah T, Mulvey D, et al. A review article on gastric volvulus: A challenge to diagnosis and management. Int J Surg 2010;8:18-24. [Crossref] [PubMed]
- Wallner B, Sylvan A, Janunger KG. Endoscopic assessment of the “Z-line” (squamocolumnar junction) appearance: reproducibility of the ZAP classification among endoscopists. Gastrointest Endosc 2002;55:65-9. [Crossref] [PubMed]
- Eren S, Gümüş H, Okur A. A rare cause of intestinal obstruction in the adult: Morgagni’s hernia. Hernia 2003;7:97-9. [Crossref] [PubMed]
- Kohn GP, Price RR, DeMeester SR, et al. Guidelines for the management of hiatal hernia. Surg Endosc 2013;27:4409-28. [Crossref] [PubMed]
- Eren S, Ciriş F. Diaphragmatic hernia: diagnostic approaches with review of the literature. Eur J Radiol 2005;54:448-59. [Crossref] [PubMed]
- Pickhardt PJ, Boyce CJ, Kim DH, et al. Should small sliding hiatal hernias be reported at CT colonography? AJR Am J Roentgenol 2011;196:W400-4 [Crossref] [PubMed]
- Revelli M, Furnari M, Bacigalupo L, et al. Incidental physiological sliding hiatal hernia: a single center comparison study between CT with water enema and CT colonography. Radiol Med 2015;120:683-9. [Crossref] [PubMed]
- Hill LD, Kozarek RA, Kraemer SJ, et al. The gastroesophageal flap valve: in vitro and in vivo observations. Gastrointest Endosc 1996;44:541-7. [Crossref] [PubMed]
- Bredenoord AJ, Weusten BLAM, Carmagnola S, et al. Double-peaked high-pressure zone at the esophagogastric junction in controls and in patients with a hiatal hernia: a study using high-resolution manometry. Dig Dis Sci 2004;49:1128-35. [Crossref] [PubMed]
- Weijenborg PW, van Hoeij FB, Smout AJ, et al. Accuracy of hiatal hernia detection with esophageal high-resolution manometry. Neurogastroenterol Motil 2015;27:293-9. [Crossref] [PubMed]
- Kahrilas PJ, Bredenoord AJ, Fox M, et al. The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil 2015;27:160-74. [Crossref] [PubMed]
- Swanstrom LL, Jobe BA, Kinzie LR, et al. Esophageal motility and outcomes following laparoscopic paraesophageal hernia repair and fundoplication. Am J Surg 1999;177:359-63. [Crossref] [PubMed]
- Boushey RP, Moloo H, Burpee S, et al. Laparoscopic repair of paraesophageal hernias: a Canadian experience. Can J Surg 2008;51:355-60. [PubMed]
- Broeders JA, Draaisma WA, Bredenoord AJ, et al. Oesophageal acid hypersensitivity is not a contraindication to Nissen fundoplication. Br J Surg 2009;96:1023-30. [Crossref] [PubMed]
- Naoum C, Falk GL, Ng ACC, et al. Left atrial compression and the mechanism of exercise impairment in patients with a large hiatal hernia. J Am Coll Cardiol 2011;58:1624-34. [Crossref] [PubMed]
- Suppiah A, Sirimanna P, Vivian SJ, et al. Temporal patterns of hiatus hernia recurrence and hiatal failure: quality of life and recurrence after revision surgery. Dis Esophagus 2017;30:1-8. [Crossref] [PubMed]
- Rathore MA, Andrabi SIH, Bhatti MI, et al. Metaanalysis of recurrence after laparoscopic repair of paraesophageal hernia. JSLS 2007;11:456-60. [PubMed]
- Nason KS, Luketich JD, Qureshi I, et al. Laparoscopic Repair of Giant Paraesophageal Hernia Results in Long-Term Patient Satisfaction and a Durable Repair. J Gastrointest Surg 2008;12:2066-75; discussion 2075-7. [Crossref] [PubMed]
- Dallemagne B, Kohnen L, Perretta S, et al. Laparoscopic repair of paraesophageal hernia. Long-term follow-up reveals good clinical outcome despite high radiological recurrence rate. Ann Surg 2011;253:291-6. [Crossref] [PubMed]
- Carrott PW, Hong J, Kuppusamy M, et al. Clinical ramifications of giant paraesophageal hernias are underappreciated: making the case for routine surgical repair. Ann Thorac Surg 2012;94:421-6; discussion 426-8. [Crossref] [PubMed]
- Shafii AE, Agle SC, Zervos EE. Perforated gastric corpus in a strangulated paraesophageal hernia: a case report. J Med Case Rep 2009;3:6507. [Crossref] [PubMed]
- Lidor AO, Kawaji Q, Stem M, et al. Defining recurrence after paraesophageal hernia repair: correlating symptoms and radiographic findings. Surgery 2013;154:171-8. [Crossref] [PubMed]
Cite this article as: Laracca GG, Spota A, Perretta S. Optimal workup for a hiatal hernia. Ann Laparosc Endosc Surg 2021;6:20.