Robotic spleen-preserving distal pancreatectomy
Introduction
Nearly 25 years after its first introduction in clinical practice, minimally invasive surgery (MIS) of the pancreas is gaining momentum, also as a result of increased experience in this field and the availability of innovative technologies (1-3). With distal pancreatectomy (DP) in particular, the application of MIS (both laparoscopic and robotic surgery has shown a number of clinical advantages over conventional surgery, especially with reference to enhanced postoperative recovery (4-6).
At the same time, although more technically demanding and more time-consuming as compared to conventional DP with splenectomy, spleen-preserving procedures are gaining wide diffusion in selected patients (7-10). In particular, minimally invasive spleen-preserving DP is regarded as a demanding intervention, for which there is still no consensus on the optimal surgical procedure. Herein we report our technique of robotic spleen-preserving DP, as performed to treat a patient with a low-grade neuroendocrine tumor of the pancreas.
Case presentation and surgical technique
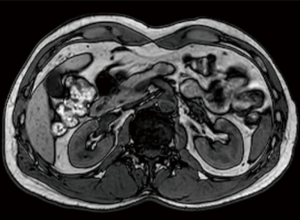
A 44-year old, otherwise healthy male was referred to the surgical department for further investigation of an incidentally discovered pancreatic nodule at abdominal ultrasonography. Physical examination was unremarkable. Routine blood investigations were substantially normal. Computed tomography and magnetic resonance imaging confirmed the presence of a solid nodule measuring 20 millimeters in diameter within the body of the pancreas (Figure 1). Endoscopic ultrasound-guided fine-needle aspiration biopsy was suggestive of pancreatic neuroendocrine tumor. The expression of Ki67 was <2%. The patient was scheduled to receive robotic DP with preservation of the spleen and splenic vessels.
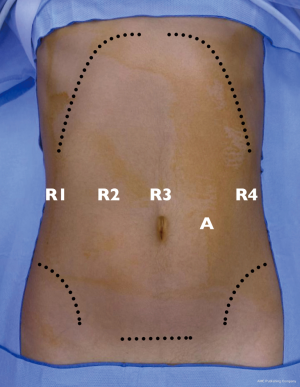
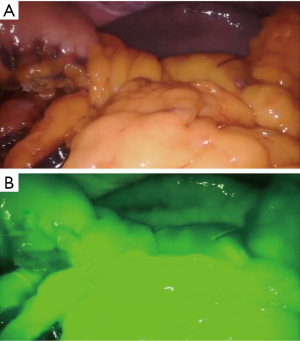
A fourth generation, four-arm Da Vinci Surgical Robot (Da Vinci Xi, Intuitive Surgical, Sunnyvale, CA, USA) is employed. The patient is placed in the supine position, with the legs apart. An anti-Trendelenburg, left-side up position is given. Pneumoperitoneum is obtained using the Veress needle in the left hypochondrium. One laparoscopic assistant port and four standard robotic ports are thus placed. Ports placement strategy is depicted in Figure 2. The procedure commences with conventional laparoscopic exploration of the abdominal cavity. The peritoneum, omentum, mesentery and viscera are inspected for signs of peritoneal disease. The stomach is retracted upward and to the right by the third arm. The gastrocolic ligament is thus divided below the gastroepiploic arcade and the lesser omentum is accessed. The dissection is carried to the origin of the right gastroepiploic artery. When present, adhesions between the posterior wall of the stomach and the anterior layer of the pancreatic capsule (supramesocolic fascia) are divided. A robot-integrated ultrasound inspection follows (11), to confirm the exact location of the tumor and evaluate its relationship with the main pancreatic duct and splenic vessels. The splenic artery is thus identified and dissected at the upper edge of the pancreas. Once the first tract of splenic artery is dissected circumferentially, it is encircled with a silicon loop, to allow rapid control in the case of vascular injury and bleeding. The peritoneum overlying the inferior border of the pancreas is incised transversely to reach the avascular plane on the pre-adrenal fascia. From the inferior border of the pancreas, the pancreatic neck is dissected off the superior mesenteric vein (SMV) and the portal vein (PV). The pancreatic parenchyma is now dissected from the splenic vein and divided using the Harmonic® scalpel and monopolar scissors (Video 1). During pancreatic division, the main pancreatic duct of Wirsung is identified and suture-ligated with monofilament suture. Once the pancreas is completely divided at its neck, it is gently rotated laterally, to facilitate the meticulous dissection required to separate the parenchyma from the splenic artery and vein. The dissection proceeds from medial to lateral, in the direction of the splenic hilum. All communicating branches between the pancreas and the splenic vessels are divided between ties or carefully cauterized until the pancreas is completely freed from the remaining attachments of the lienorenal ligament. The specimen is thus retrieved through a protected Pfannenstiel incision. The viability of the spleen is now appraised with extemporaneous fluorescence-angiography (FireflyTM) by injecting intravenously a small bolus of indocyanine green (Figure 3). A closed-suction drain is left adjacent to the pancreatic bed. The patient had an uneventful postoperative course and was discharged home on the 7th postoperative day. Final pathology confirmed the diagnosis of a neuroendocrine tumor 19 millimeters in diameter with 24 harvested lymph nodes.
Discussion
In the case of benign or premalignant conditions, DP can be performed with conservation of the spleen to avoid the well-known postsplenectomy sequelae on immunologic and hematologic function (3,4,12,13). Traditionally, spleen-preserving DP is regarded as a more demanding procedure compared with the conventional DP with splenectomy (4). Actually, meticulous and time-consuming dissection is usually required due to a higher risk of vascular injury and bleeding (4). When the spleen is to be conserved, DP can be performed either with preservation (SVP, also known as the Kimura technique) either with sacrifice of the splenic vessels [also known as the Warshaw technique (WT)]. Currently, both SVP and the WT are considered safe and effective techniques to perform DP. Nevertheless, there are several reports suggesting that SVP should be preferred to reduce postoperative morbidity (10,12). Song et al. recently aggregated the available data from the literature comparing the WT vs. SVP technique of spleen-preserving DP. The authors meta-analyzed the outcomes of more than 1,000 patients from 18 studies, mostly reporting on MIS (>90%) (12). Despite a significantly higher amount of estimated blood loss, and a tendency toward increased operation time, the SVP technique was superior to the WT with regard to clinically relevant postoperative pancreatic fistula, splenic infarct, intra/postoperative splenectomy and gastric varices.
As for the method of surgical approach, the application of MIS to DP, has shown some advantages over open surgery in terms of postoperative recovery, length of hospital stay, and quality of life (4,6,13). In order to evaluate possible differences on the rate of spleen preservation, we recently analyzed the data provided in an elegant meta-analysis by Mehrabi et al. comparing laparoscopic versus open DP for the treatment of benign and malignant pancreatic lesions (3,13). Were considered only those studies in which both laparoscopic and conventional DPs were well-matched for benign and malignant disease, including the data of 950 patients derived from 14 studies. Overall, the rate of spleen preservation was 23%, with significant difference between laparoscopic and conventional surgery (34% and 12%, respectively, P<0.01). Interestingly, in the specific subgroup of patients receiving surgery for benign or premalignant disease, the rate of spleen preservation was 43% and 14%, respectively. This data is consistent with that observed by Xie et al. in a recent, well-conducted meta-analysis comparing the relative results of open vs. laparoscopic DP on more than 1,300 patients from 9 studies (14). The open and laparoscopic groups were homogeneously distributed for the relative proportions of benign conditions and malignancies Overall, there was a significant difference (risk ratio, 2.38) in the rate of spleen preservation favoring MIS over conventional surgery (13% vs. 30%, respectively).
Currently, minimally invasive spleen-preserving DP is performed at specialized centers both by conventional laparoscopy and robotic surgery. However, a number of reports, including meta-analyses have shown possible advantages associated with the application of robotic platform on the rate of spleen preservation and conversion to open surgery as compared to conventional laparoscopy (15-20). Hong et al. recently evaluated a consecutive series of minimally invasive DP performed by a single, experienced surgeon (4). The difference in the percentage of spleen-conserving procedures between robotic and laparoscopic PD was analyzed on an intent-to-treat basis for splenic preservation. Overall, there was a significantly higher rate of splenic preservation favoring the robot (96.8%) over conventional laparoscopy (82.5%). This finding was already observed by two recent reviews by Guerrini et al. (20), and Røsok et al. (2) comparing robotic and laparoscopic PD. Overall, the meta-analysis of data showed a significantly higher proportion of spleen-preservation and a lower rate of conversion to laparotomy for the robotic group.
Actually, minimally invasive spleen-preserving DP, and especially the SVP technique is regarded as a technically highly-demanding surgery (3,4,13,16). Several crucial maneuvers, such as pancreatic dissection, and especially splenic vessels dissection are likely facilitated by the use of the robotic system (4,16,17). The magnified 3D-view and the augmented dexterity enable not only easier dissection and suture, but also simplify the management of intraoperative complications such as vascular injuries, which are main factors increasing the rate of conversion to open surgery (2,17,20).
Conclusions
The relative lack of high-level studies still preclude definitive conclusions on the argument. However, increasing evidence is supporting the role of the robot in performing minimally invasive DP, especially when surgery is performed with the intent to preserve the spleen. As shown in the present report, the technology of the robotic system is a valid option to perform minimally invasive DP with preservation of the spleen and splenic vessels competently.
Supplementary: Discussion
1. Dr. Giovanni Battista Levi Sandri: Did authors had conversion during robotic pancreatectomy? If yes, it was an elective conversion for an unfavorable intraoperative finding or an emergency conversion secondary to an unfavorable intraoperative event?
Over the last years our group has reached a significant experience on robotic pancreatectomy made up of about 150 cases including pancreaticoduodenectomies, distal pancreatectomies (with or without splenectomy) and enucleations. In the case of pancreaticoduodenectomy, 12% of conversion to an open procedure were recently reported by our group concerning the recent experience with the technique. These cases were converted to laparotomy mainly in relation with severe peritoneal adhesions or the presence of advanced disease. There was one procedure which was converted due to an uncontrolled bleeding from the splenic vein. With regard to distal pancreatectomy and pancreatic enucleation, all surgeries were completed with the robot and no conversion to open surgery occurred.
2. Dr. Giovanni Battista Levi Sandri: It is necessary a learning curve of previous distal pancreatectomy with splenectomy?
It is quite difficult to assess the actual learning curve of surgical procedures which are relatively uncommon such as robotic distal pancreatectomy with preservation of the spleen. Conventionally, certain endpoints of pancreatic surgery such as intraoperative blood loss, operating time, length of postoperative hospital stay, the rate of postoperative morbidity and particularly POPF are considered as primary measures of interest. Nevertheless, to define a reliable learning process for distal pancreatectomy with preservation of the spleen, other crucial factors are to be taken into account beside the specific experience of the operating surgeon. Among these, patients clinical characteristics, the rate of splenic vessels preservation, and, importantly, the level of training of the entire surgical team are to be considered. With specific reference to the surgical procedure, in the early period non-experienced surgeons must consider cases requiring splenectomy or, theoretically, the Warshaw technique in those where the spleen should be preserved.
Supplementary: Discussion
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Giovanni Battista Levi Sandri and Massimo Carlini) for the series “Minimally Invasive Spleen Surgery” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales-19-267). The series “Minimally Invasive Spleen Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Edwin B, Sahakyan MA, Abu Hilal M, et al. Laparoscopic surgery for pancreatic neoplasms: the European association for endoscopic surgery clinical consensus conference. Surg Endosc 2017;31:2023-41. [Crossref] [PubMed]
- Røsok BI, de Rooij T, van Hilst J, et al. Minimally invasive distal pancreatectomy. HPB (Oxford) 2017;19:205-14. [Crossref] [PubMed]
- Guerra F, Pesi B, Fatucchi LM, et al. Splenic preservation during open and minimally-invasive distal pancreatectomy. Surgery 2015;158:1743-4. [Crossref] [PubMed]
- Hong S, Song KB, Madkhali AA, et al. Robotic versus laparoscopic distal pancreatectomy for left-sided pancreatic tumors: a single surgeon's experience of 228 consecutive cases. Surg Endosc 2020;34:2465-73. [PubMed]
- Klompmaker S, de Rooij T, Koerkamp BG, et al. International validation of reduced major morbidity after minimally invasive distal pancreatectomy compared with open pancreatectomy. Ann Surg 2019; [Epub ahead of print]. [Crossref] [PubMed]
- de Rooij T, van Hilst J, van Santvoort H, et al. Minimally invasive versus open distal pancreatectomy (LEOPARD): a multicenter patient-blinded randomized controlled trial. Ann Surg 2019;269:2-9. [Crossref] [PubMed]
- Dokmak S, Aussilhou B, BenSafta Y, et al. Double Gastric Hanging for Gastric Exposure in Laparoscopic Distal Pancreatectomy. Dig Surg 2019;36:449-54. [Crossref] [PubMed]
- Butturini G, Inama M, Malleo G, et al. Perioperative and long-term results of laparoscopic spleen-preserving distal pancreatectomy with or without splenic vessels conservation: a retrospective analysis. J Surg Oncol 2012;105:387-92. [Crossref] [PubMed]
- Justin V, Wisiak S, Waha JE, et al. Laparoscopic distal pancreatectomy with preservation of the splenic vessels. Ann Laparosc Endosc Surg 2019;4:98. [Crossref]
- Yongfei H, Javed AA, Burkhart R, et al. Geographical variation and trends in outcomes of laparoscopic spleen-preserving distal pancreatectomy with or without splenic vessel preservation: A meta-analysis. Int J Surg 2017;45:47-55. [Crossref] [PubMed]
- Guerra F, Amore Bonapasta S, Annecchiarico M, et al. Robot-integrated intraoperative ultrasound: Initial experience with hepatic malignancies. Minim Invasive Ther Allied Technol 2015;24:345-9. [Crossref] [PubMed]
- Song J, He Z, Ma S, et al. Clinical comparison of spleen-preserving distal pancreatectomy with or without splenic vessel preservation: a systematic review and meta-analysis. J Laparoendosc Adv Surg Tech A 2019;29:323-32. [Crossref] [PubMed]
- Mehrabi A, Hafezi M, Arvin J, et al. A systematic review and meta-analysis of laparoscopic versus open distal pancreatectomy for benign and malignant lesions of the pancreas: it's time to randomize. Surgery 2015;157:45-55. [Crossref] [PubMed]
- Xie K, Zhu YP, Xu XW, et al. Laparoscopic distal pancreatectomy is as safe and feasible as open procedure: a meta-analysis. World J Gastroenterol 2012;18:1959-67. [Crossref] [PubMed]
- Kang CM, Kim DH, Lee WJ, et al. Conventional laparoscopic and robot-assisted spleen-preserving pancreatectomy: does da Vinci have clinical advantages? Surg Endosc 2011;25:2004-9. [Crossref] [PubMed]
- Daouadi M, Zureikat AH, Zenati MS, et al. Robot-assisted minimally invasive distal pancreatectomy is superior to the laparoscopic technique. Ann Surg 2013;257:128-32. [Crossref] [PubMed]
- Guerra F, Pesi B, Amore Bonapasta S, et al. Challenges in robotic distal pancreatectomy: systematic review of current practice. Minerva Chir 2015;70:241-7. [PubMed]
- Alfieri S, Butturini G, Boggi U, et al. Short-term and long-term outcomes after robot-assisted versus laparoscopic distal pancreatectomy for pancreatic neuroendocrine tumors (pNETs): a multicenter comparative study. Langenbecks Arch Surg 2019;404:459-68. [Crossref] [PubMed]
- Lyman WB, Passeri M, Sastry A, et al. Robotic-assisted versus laparoscopic left pancreatectomy at a high-volume, minimally invasive center. Surg Endosc 2019;33:2991-3000. [Crossref] [PubMed]
- Guerrini GP, Lauretta A, Belluco C, et al. Robotic versus laparoscopic distal pancreatectomy: an up-to-date meta-analysis. BMC Surg 2017;17:105. [Crossref] [PubMed]
Cite this article as: Guerra F, Paolini C, Coratti A. Robotic spleen-preserving distal pancreatectomy. Ann Laparosc Endosc Surg 2020;5:24.