Robotics and transanal total mesorectal excision
Background
The management of rectal cancer has evolved over the last three decades, with a multidisciplinary strategy being the cornerstone of care (1,2). Magnetic resonance imaging (MRI), selective use of preoperative chemoradiation and detailed operative planning have facilitated appropriate radical resections (3,4).
Surgery is still the most critical component of curative-intent treatment in rectal cancer (5). With the evolution of technology, minimally invasive options are readily available currently, making laparoscopic, robotic and transanal approaches valid alternatives to open total mesorectal excision (TME) (6).
Even under experienced hands, performing a TME is a difficult task. Poor maneuverability and visibility in the confined space of the pelvis potentially lead to suboptimal dissection with margin involvement or disruption of the mesorectal envelope, thus compromising oncologic outcomes (7,8). This is worsened in situations such as the male or narrow pelvis, obese patients, fibrosis due to radiation and low or bulky tumors.
Although recently, the ALaCaRT and ACOSOG Z6051 randomized controlled trials failed to demonstrate non-inferiority of a laparoscopic approach over an open approach for radical rectal cancer resection based on surrogate oncologic markers such as completeness of mesorectal resection, circumferential margin status and distal resection margin status (9,10), follow-up data from both, the ALaCaRT and ACOSOG Z6051 trial has demonstrated equivalent results in terms of 2-year disease-free-survival (DFS) and overall-survival (OS) between laparoscopic and open surgery for rectal cancer (11,12). Furthermore, the CLASSIC II and COREAN trials also support the equivalency of oncologic outcomes between the laparoscopic and open approach (13,14).
This reflects the fact that even though there has been undeniable, steep progress and diffusion of minimally invasive surgery worldwide, conventional laparoscopic surgery alone may be insufficient to overcome the difficulties and technical pitfalls of a challenging pathology such as rectal cancer. Moreover, this explains why robotics and transanal minimally invasive surgery (TAMIS) as minimally invasive options for rectal cancer resection have become tremendously attractive alternatives amongst colorectal surgeons during the past decade.
Robotics provides benefits such as camera stability, 3D vision, improved access and visibility in narrow spaces, precise dissection, enhanced dexterity, tremor reduction and enhanced ergonomics, amongst others. To date, there is not enough data to support an oncologic superiority of robotic surgery over other approaches for rectal cancer resection. A recently published systematic review and metanalysis of controlled trials, including the ROLARR trial (15), suggest benefits of robotics over laparoscopic surgery in terms of conversion rates (16), thus supporting ease of dissection and navigation in the pelvis over conventional laparoscopy.
On the other hand, transanal TME (taTME) has developed from a fusion of concepts and technological developments; Heald’s TME (17), Marks’ transanal-transabdominal (TATA) dissection and ‘bottom-to-up’ approach (18), the development of minimally invasive surgery including laparoscopy and transanal access platforms such as Transanal Endoscopic Microsurgery (‘TEM’—Richard Wolf, Knittlingen, Germany) (19) and more recently, TAMIS (20).
Benefits from a perineal approach for sphincter-preserving resections have been demonstrated by Denost et al. in a randomized controlled trial comparing perineal transanal approach in addition to laparoscopy versus a purely laparoscopic approach in 100 patients with low rectal cancer (21). The authors showed that the rate of positive circumferential resection margin (CRM) decreased significantly when a perineal approach was performed (4% versus 18%, P=0.025). In this scenario, a minimally invasive transanal approach would further improve visibility, maneuverability and dissection in the lower pelvis, thus, providing better quality specimens than a pure transabdominal approach with or without an open perineal approach. Recent systematic reviews and meta-analyses mainly based on retrospective comparative data support this idea, with results showing better TME quality and fewer positive CRMs in taTME specimens when compared to purely laparoscopic TME specimens (22,23).
In the setting of a rapidly evolving field, the current debate is centered in which would be the best approach to treat patients that require resection for rectal cancer. Due to their potential benefits, the natural evolution of robotics and TAMIS has been their combination in the context of technological development and decreasing costs.
To date, there is scarce data on combined robotics and taTME: hybrid transabdominal robotic and taTME (R+taTME), and robotic-assisted transanal surgery-TME (RATS-TME).
The objective of this article is to analyze the available literature, define the potential role for combined robotic and taTME approaches, and delineate future perspectives on this topic.
Hybrid transabdominal robotic and taTME (R+taTME)
Robotic surgery helps overcome difficulties encountered in the middle and lower pelvis when dissecting along the extra-fascial TME plane. Despite enhanced maneuverability and visibility obtained with robotic technology, in the context of high-risk patients (obese patient, narrow pelvis and low or bulky tumors), it may still be insufficient to provide a perfect quality specimen.
TaTME improves control of the distal margin and dissection in the lower third of the pelvis (24). Better specimen quality due to less manipulation and traction injuries or mesorectal disruptions when dissecting in the lower pelvis could eventually translate into better oncologic outcomes in high-risk patients.
Data on R+taTME is scarce. The first report of such an approach was published by Mendes et al. (25) in 2015. The authors performed a combined procedure in a 55-year-old female with a cT3N+ rectal adenocarcinoma, 5 cm from the anal verge (FAV), previously treated with chemoradiation. Their setup involved an abdominal robotic component utilizing the da Vinci Si System (Intuitive Surgery, Sunnyvale, CA, USA) followed by taTME using the TEO platform (‘Transanal Endoscopic Operations’—Karl Storz, Tuttlingen, Germany). The specimen was extracted transanally and a mechanical end-to-end anastomosis was fashioned with a circular stapler. A defunctioning colostomy was performed and no postoperative complications were reported with a postoperative stay of 2 days. Operative time and blood loss were not reported. Even though a detailed pathological report is not provided, it is stated that the mesorectal resection was complete. The authors concluded that the combined approach is safe and may be useful in technically demanding situations such as the narrow pelvis, obesity and ultra-low rectal tumors.
During the same year, Gómez Ruiz et al. (26) published the results of 5 patients (4 men) where, both transabdominal and transanal dissections, were robotically-assisted and performed in a sequential fashion using the da Vinci Si System and PAT (‘Puerto Acceso Transanal’—Developia-HUMV, Santander, Spain) combined with a GelPoint Path (Applied Medical, Rancho Santa Margarita, CA, USA). The mean age of patients was 57 [38–67] years and BMI was 25.8 [22–31] kg/m2. Tumors were located at an average of 5 [4–6] cm FAV and were preoperatively staged as T2N0M0 (one patient) and T2N1M0 (four patients). The mean operative time was 398 [270–450] minutes with no intraoperative complications or conversion, and the mean length of hospital stay was 6 [5–7] days. There was one anastomotic leakage in the series. All specimens were complete mesorectal excisions with negative margins. The authors concluded that this approach is feasible and safe for early rectal cancer and may help overcome the limitations of traditional laparoscopic resection.
Another case was reported by Bravo et al. (27) in 2017 performing a simultaneous two-team approach (‘Cecil’ approach) with the da Vinci Xi System (Intuitive Surgical, Sunnyvale, CA, USA) and the GelPoint Path in a 50-year-old man with rectal cancer, 5 cm FAV. Preoperative clinical staging was T2N0M0. Total operative time was 160 min and the estimated blood loss less than 50 mL. The patient had no postoperative complications and was discharged on postoperative day 3. Pathology revealed an intact mesorectum and a pT2N0 tumor with negative margins. They concluded that hybrid surgery with pelvic robotic dissection and transanal TME was feasible, quick and safe.
To our knowledge, the largest series was recently published by our group (28). Eight patients (7 males) underwent R+taTME via a modified Cecil approach using the da Vinci Xi System and the GelPoint Path. The median age was 60 years [47–73] with a BMI of 29.5 [20–39.1] kg/m2. Tumour height was at a median of 7.5 [4–13] cm FAV and six patients were clinical stage III cancers which received neoadjuvant chemoradiotherapy. The median length of stay was 9 [4–33] days. There were no intra-operative complications and no patients required conversion to an open approach. Complications included one anastomotic leak and one presacral collection. All patients had a complete mesorectum with negative margins.
Details of these publications are summarized in Table 1.
Table 1
| Variables | Mendes et al. | Gómez Ruiz et al. | Bravo et al. | Nikolic et al. |
|---|---|---|---|---|
| Study | ||||
| Year published | 2015 | 2015 | 2017 | 2019 |
| Kind of publication | Case report | Case series | Case report | Case series |
| Number of cases | 1 | 5 | 1 | 8 |
| Patient characteristics | ||||
| Male/female | 0/1 | 4/1 | 1/0 | 7/1 |
| Age (years) | 55 | 57 [38–67]b | 50 | 60 [47–73]a |
| BMI (kg/m2) | n/a | 25.8 [22–31]b | 28 | 29.5 [20–39.1]b |
| Tumour DAV (cm) | 5 | 5 [4–6]b | 5 | 7.5 [4–13]b |
| Clinical stage (I/II/III/IV) | 0/0/1/0 | 1/0/4/0 | 1/0/0/0 | 1/1/6/0 |
| Neoadjuvant treatment | Yes | Yes (4/5) | No | Yes (6/8) |
| Procedure details | ||||
| Cecil approach | No | No | Yes | Yes |
| Robotic systemc | da Vinci Si | da Vinci Si | da Vinci Xi | da Vinci Xi |
| Transanal platform | TEOd | PATe + GelPoint Pathf | GelPoint Path | GelPoint Path |
| Operative time (min) | n/a | 398 [270–450]b | 160 | 240 [220–500]b |
| Blood loss (mL) | n/a | 90 [25–120]b | 50 | n/a |
| Splenic flexure mobilisation | Yes | Yes | Yes | Yes |
| Defunctioning stoma | Yes | Yes | Yes | Yes |
| Anastomosis method (S/H/N)g | Stapled | 3/2/0 | Stapled | 6/2/0 |
| Conversion | No | No | No | No |
| Morbidity | No | 1 anastomotic leak | No | 1 anastomotic leak, 1 presacral collection |
| Length of stay (days) | 2 | 6 [5–7]b | 3 | 9 [4–33]a |
| Pathology | ||||
| Quality of TME | Complete | Complete | Complete | Complete |
| CRM involvement | n/a | No | No | No |
| Distal margin involvement | n/a | No | No | No |
a, median value [range]; b, mean value [range]; c, da Vinci Surgical System (Intuitive Surgery, Sunnyvale, CA); d, TEO, Transanal Endoscopic Operation (Karl Storz, Tuttlingen, Germany); e, PAT, Puerto Acceso Transanal (Developia-HUMV, Santander, Spain); f, GelPoint Path (Applied Medical, Rancho Santa Margarita, CA); g, S/H/N, stapled/handsewn/no anastomosis. n/a, not available; BMI, body mass index; DAV, distance from anal verge; TME, total mesorectal excision; CRM, circumferential resection margin.
Abdominal setup
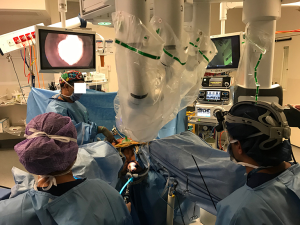
Before surgery, the patient receives mechanical bowel preparation and the operating team is organized for a two-team simultaneous procedure (Figure 1). The patient is anesthetized and prophylactic antibiotics administered at induction. An indwelling urinary catheter is inserted and the patient placed in the Lloyd-Davies position.
The transabdominal approach starts with an OptiPort entry through the right flank using a 0° 10 mm camera to establish pneumoperitoneum. Four 8-mm robotic ports are inserted under vision and robotic arms docked for a routine low anterior resection with the da Vinci Xi Surgical System, with the patient in a 15° head down and 12° left tilt position.
The inferior mesenteric vein is divided at the duodenojejunal flexure in between Hem-o-loks (Telefex Headquarters International, Ireland). The splenic flexure is then fully mobilized in a medial to lateral fashion while the patient is in a head-down position to enable synchronous operating with the perineal surgeon. The inferior mesenteric artery is then divided in between Hem-o-loks, and the descending and sigmoid colon are fully separated from the retroperitoneum, identifying and preserving the left ureter and gonadal vessels. The abdominal dissection must be efficient to be able to coordinate with the perineal surgeon. The transabdominal TME is then commenced in a standard robotic fashion. Dissection is carried out outside of the mesorectal envelope, in the extra-fascial TME plane. The abdominal dissection then meets the perineal surgeon’s dissection and a Pfannenstiel incision is made to remove the rectum trans-abdominally given that a transanal extraction can be associated with traction damage to the marginal artery.
Perineal setup
The taTME approach is set up synchronously. The anus is everted with four 0 silk sutures placed in four quadrants to retract the anus. A Lonestar Retractor (Lonestar, Cooper Surgical, Trumbull, CT, USA) is used to retract the anoderm and the anal canal washed with cetrimide.
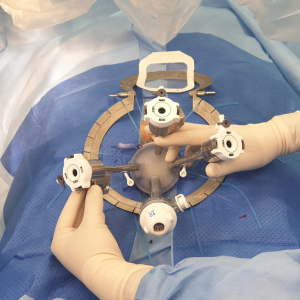
The GelPoint Path is then inserted using sponge forceps and position confirmed using the dilator (Figure 2). A 1-0 prolene suture with a 26-mm rounded needle is used to create a purse-string to close the rectum distal to the lesion, ensuring that equal proportions of tissue are taken at equidistance from the port lip in a clockwise fashion.
Three ports are then inserted via the platform with the utilization of a 0° camera. A pneumorectum is established with an AirSeal® System (Conmed, Connecticut, USA) at a flow rate of five liters per minute and a pressure of 5 mmHg. A 5 mm SILS™ Hook diathermy (Covidien, Dublin, Ireland) with articulation ability is used.
A planned circumferential mucosal mark was created with hook diathermy at the edge of the radial mucosal folds followed by a full-thickness circumferential rectotomy. The GelPoint Path is then removed and the purstring reinforced with 1-0 prolene, ideally inverting the mucosa. A cetrimide wash is again performed, the GelPoint Path repositioned, the camera is switched to a 30° laparoscope and pressure from the AirSeal is increased to 12 mmHg. ‘Down-to-up’ dissection is carried out through the TME plane circumferentially, avoiding damage to the mesorectal envelope and the nerves laterally, until dissection is completed in a combined transabdominal-transanal effort from both teams.
Anastomosis
A 33-mm haemorrhoidal stapler anvil (DST Series™ Technology—Covidien, Dublin, Ireland) is inserted into the distal end of the colon and secured with a purse-string suture. Transanally, a full-thickness purse-string suture is placed around the distal resection edge with a 0 prolene suture on a 26-mm round needle. Once the anvil has been passed through the anus, the purse-string is tied and the stapler is introduced transanally to create the anastomosis. This may be a colonic-pouch-anal, side-to-end, or end-to-end anastomosis depending on the colonic length and patient anatomy. The anastomosis is then reviewed visually and tactically. A reverse leak test is performed. A 19-Fr Blake’s drain tube is placed in the pelvis and a loop ileostomy is formed on the right side of the abdomen in a previously marked site.
Postoperatively, the ERAS (Enhanced Recovery After Surgery) protocol is followed.
Robotic-assisted taTME (RATS-TME)
An exciting new trend in colorectal surgery is the fusion of robotic technology and TAMIS. This has been coined as RATS by Atallah et al. and has been utilized for local excision of rectal neoplasms and TME with good initial results (29).
Theoretical advantages of RATS-TME over conventional taTME are related to improved visibility with high-definition 3D vision, camera stability, the use of wristed instruments that enhance dexterity and improve dissection, and ergonomics, amongst others. Also, the steep learning curve of a technically demanding approach, such as taTME, would be favored with the aid of robotic technology (30).
The first RATS-TME case was reported by Verheijen et al. (31) in 2014. A 48-year-old female with a cT3N+ rectal cancer, 8 cm FAV, underwent neoadjuvant chemoradiation and subsequently had a sequential laparoscopic and RATS-TME using the da Vinci Si System and the GelPoint Path. The specimen was extracted transanally and an end-to-end stapled anastomosis was performed using a circular stapler. A defunctioning loop ileostomy was fashioned. The total operative time was 250 min, with an estimated blood loss of 50 mL. The postoperative stay was 3 days and the patient had no complications. The authors concluded that transanal TME using the robot is feasible and may help overcome technical difficulties associated with the use of conventional instruments in the setting of a single incision port.
Since then, six case series have been published and their results are summarized in Table 2 (26,29,32-35).
Table 2
| Variables | Atallah et al. | Gómez Ruiz et al. | Huscher et al. | Kuo et al. | Monsellato et al. | Hu et al. |
|---|---|---|---|---|---|---|
| Study | ||||||
| Year published | 2015 | 2015 | 2015 | 2016 | 2019 | 2020 |
| Number of cases | 4 | 5 | 7 | 15 | 3 | 20 |
| Patient characteristics | ||||||
| Male/female | 3/1 | 4/1 | 3/4 | 8/7 | 2/1 | 13/7 |
| Age (years) | 45 [26–59] | 57 [38–67] | 63.2 [48–74]a | 60.3 [44–75]a | 61 [55–68] | 56.3 [31–79]a |
| BMI (kg/m2) | 31 [21–38] | 25.8 [22–31]a | 9 | 21.97 [range n/a]a | 26 [25–28] | 23.9 [18.7–30.1]a |
| Tumour DAV (cm) | 3.3 [1–5]a | 5 [4–6] | 2 [1–6.5] | 3.3 [2–5] a | 4 [3–6] | 5.8 [2–10] a |
| Clinical stage (I/II/III/IV) | 1/0/3/0 | 1/0/4/0 | 0/7/0/0 | 2/1/10/0, 2 benign polyps | 0/0/3/0 | 4/4/10/2 |
| Neoadjuvant treatment | Yes (3/4) | Yes (4/5) | No | Yes (11/13) | Yes (3/3) | Yes (12/20) |
| Procedure details | ||||||
| Cecil or two team approach | No | No | No | No | Yes (1/3) | Yes (20/20) |
| Abdominal approach | Laparoscopic | Robotic | Laparoscopic | Single port robotic + assistant port | Robotic 2, laparoscopic 1 | Laparoscopic |
| Robotic systemb | da Vinci Si | da Vinci Si | da Vinci Si | da Vinci Si | da Vinci Si | da Vinci Xi |
| Transanal platform | GelPoint Pathc | PATd + GelPoint Path | GelPoint Path | GelPoint Path | GelPoint Path | GelPoint Path |
| Operative time (min) | 376 [140–409] | 398 [270–450] | 165.7 [85–220]a | 473 [335–569] | 550 [440–600] | 172.3 [135–215]a |
| Blood loss (mL) | 200 [50–300] | 90 [25–120] | n/a | 33 [30–50]a | n/a | 82 [30–500] |
| Splenic flexure mobilisation | Yes | Yes | Yes | n/a | Yes | 5/20 |
| Defunctioning stoma | 3/4e | Yes | Yes | 5/15 | Yes | 14/18 |
| Anastomosis method (S/H/N) | 0/3/1 | 3/2/00 | 7/0/0 | 0/15/0 | 0/3/0 | 16/2/2 |
| Conversion | No | No | No | 2/15f | No | No |
| Morbidity | 1 wound haematoma, 1 PE | 1 anastomotic leak (CD II) | 1 anastomotic bleeding (CD II) | 1 mechanical bowel obstruction, 1 wound infection | 1 ARF | 7/20 (35%; including one pelvic abscess, no anastomotic leaks reported) |
| Length of stay (days) | 4.3 [4–5] | 6 [5–7] | 4.8 [4–6] | 12.2 [10–14] | 10 [7–15] | 8.8 [6–24] |
| Pathology | ||||||
| Quality of TME (complete/near complete/muscularis) | 1/3/0 | 5/0/0 | 6/1/0 | 15/0/0 | 3/0/0 | 18/2/0 |
| CRM involvement | No | No | No | No | No | 3/20 (15%) |
| Distal margin involvement | No | No | No | No | No | No |
Values expressed in median [range]. a, indicates mean value [range]; b, da Vinci Surgical System (Intuitive Surgery, Sunnyvale, CA); c, GelPoint Path (Applied Medical, Rancho Santa Margarita, CA); d, PAT, Puerto acceso transanal (Developia-HUMV, Santander, Spain); e, one patient had an end ileostomy; f, conversion to conventional laparoscopy. n/a, not available; BMI, body mass index; DAV, distance from anal verge; TME, total mesorectal excision; CRM, circumferential resection margin; S/H/N, stapled/handsewn/no anastomosis; ARF, acute renal failure; PE, pulmonary embolism; CD, Clavien-Dindo.
Atallah et al. (29) reported 4 cases (3 males) where a hybrid abdominal laparoscopic and RATS-TME was performed sequentially. The mean age was 44 [29–59] years with an average BMI of 29 kg/m2 and an average tumor height of 3.3 [1–5] cm FAV; three of them had neoadjuvant chemoradiation and subsequent resection and one had a pT1sm3 rectal cancer after local excision requiring completion TME. The mean operative time was 376 min with an estimated mean blood loss of 200 [50–300] mL. There was no intra-operative morbidity and the mean postoperative length of stay was 4.3 days. Mesorectal quality was reported as complete or near-complete in all cases. Resection margins were negative in all cases. Morbidity included one wound hematoma, one subsegmental pulmonary embolism (asymptomatic) and recurrent deep vein thrombosis, and a readmission for dehydration due to high ileostomy output. No local or distant recurrences were found in any of the patients after an average 8-month follow-up. The authors concluded that RATS-TME might facilitate dissection, especially of the distal two-thirds of the rectum.
Gómez Ruiz et al. (26) published results of 5 patients operated via a sequential robotic transabdominal and RATS-TME, as described in the previous section. Interestingly, they initially tested the procedure in pre-clinical cadaveric models using laparoscopic abdominal assistance before publishing their case series (36).
Huscher et al. (32) published an editorial communicating results of 7 patients (4 women) with rectal cancer who underwent a hybrid laparoscopic transabdominal and RATS- TME in a sequential fashion. The mean age was 63.2 [48–74] years and BMI was 29.9 [21.5–37.5] kg/m2. All patients were clinical stage I or II and none of them received neoadjuvant therapy. The mean operative time was 165.7 [85–120] minutes. One patient had lower gastrointestinal bleeding, presumably from the anastomosis, requiring transfusion. None of the patients had an anastomotic leak. The mean hospital stay was 4.8 [4–6] days. The macroscopic assessment revealed a complete or near-complete mesorectum in all cases. The mean number of lymph nodes resected was 14 [10–20] and an R0 resection was achieved in all cases. All patients had their defunctioning stoma closed during follow-up. They concluded that combining robotics and transanal access is feasible and could improve results in rectal cancer surgery.
Kuo et al. (33) reported a series of 15 patients (8 males) having a combined sequential single-port (plus an assistant port) robotic transabdominal approach followed by RATS-TME, performed by a single surgeon using the da Vinci Si System. The patients’ median age was 60.3 [44–75] years, with a BMI of 21.97 kg/m2. Indication for resection was rectal cancer in 13 patients and dysplastic polyps in the other two. Median operative time was 473 [335–569] min and the estimated blood loss was 33 [30–50] mL. Two patients required conversion to conventional laparoscopy, one due to a left ureteric transection and another due to bleeding during the transanal phase. Five patients had a diversion stoma. Reported morbidity included an intestinal obstruction requiring a return to theatre for adhesiolysis and another patient with a superficial wound infection. The mean length of hospital stay was 12.2 [10–14] days. All specimens were reported as complete mesorectum with clear circumferential and distal resection margins. The authors concluded that the application of robotic technology to a combined transanal and transabdominal approach for low rectal lesions is feasible and may offer benefits over conventional laparoscopy.
Monsellato et al. (35) published in 2019 a series of 3 patients (2 males) having a combined procedure. In two cases, the authors performed a sequential approach with a RATS-TME component first and a subsequent robotic transabdominal approach, and another where they performed simultaneous laparoscopic transabdominal and RATS-TME. The mean age was 61 [55–68] years and BMI was 26 [25–28] kg/m2. Tumors were located at a mean distance of 4 [3–6] cm FAV, all clinical-stage III cancers treated with upfront chemoradiation. Mean operative time was 550 [440–600] minutes and length of stay was 10 [7–15] days. There were no surgical complications reported and all specimens were complete TMEs with negative margins. The conclusion was that RATS-TME is feasible and safe, with good initial postoperative results.
The largest series to date has been recently published by Hu et al. (34). Twenty patients (12 males) underwent simultaneous laparoscopic transabdominal and RATS-TME in a two-team approach. The abdominal component was performed via a single port (through the ileostomy site), whilst the perineal approach combined a da Vinci Xi System with a GelPoint Path. The mean patient age was 56.7 [31–79], and the mean distance from tumor to anal verge was 6.0 [2–10]. Fifty percent of patients were stage III cancers and 60% had neoadjuvant treatment. Ninety percent of patients had a restorative resection. The mean intraoperative blood loss was 88 [30–500] mL and a circular stapling was utilized to restore bowel continuity in 80% of patients. The overall morbidity rate was 35%, including one pelvic abscess. One patient was reoperated due to perineal wound bleeding. They reported that all patients had complete or near-complete mesorectal resections and three patients had a positive CRM (15%). They conclude that RATS-TME assisted by laparoscopy is safe and feasible.
Setup
We have performed this approach in three low-risk cases in a totally-robotic sequential fashion, using the da Vinci Xi System and the GelPoint Path. In these cases, the operating theatre was setup for an initial transanal phase. The anus was everted, and a Lonestar retractor, GelPoint Path and purse-string suture are placed as described in the previous section.
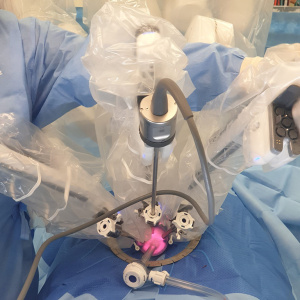
Three robotic ports are then inserted via the platform; one of them an 8-mm bariatric port for the camera superiorly, two 8-mm normal length ports laterally, and an assistant port is placed inferiorly and is used for the AirSeal® (Conmed, Connecticut, USA) (Figure 3).
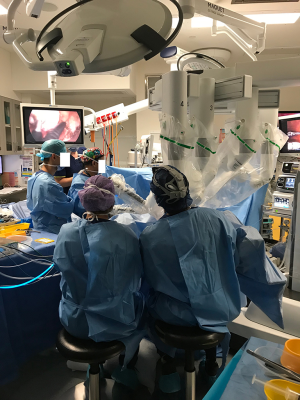
The da Vinci Xi System cart is placed laterally and the robot docked (Figure 4). We used three robotic arms with the camera in the anterior aspect, a left-handed fenestrated bipolar and a right-handed monopolar spatula. Marking of the mucosa is made circumferentially and then a full-thickness rectotomy is performed. Further dissection is carried out through the TME plane; initially in the posterior aspect, then anteriorly in order to identify the prostate and avoid urethral injuries and then circumferentially until most of the pelvic dissection is performed transanally and the peritoneal reflection is breached anteriorly. Then the abdominal component was performed as described in the previous section. A Pfannenstiel incision in made to remove the specimen transabdominally and reconstruction is performed as previously described, ideally with a colonic-pouch-anal anastomosis.
Discussion
In the setting of a rapidly evolving field such as rectal cancer management, minimally invasive options are readily available to perform radical resections. Currently and mainly based on retrospective data, laparoscopic, robotic and transanal approaches seem to be valid alternatives to open TME (6).
Approaches to rectal cancer resection vary internationally. A recently published article by the ESCP collaborating group on a prospective audit of elective rectal cancer surgery showed that amongst 2,579 patients, 6.5% had a robotic approach and 19.9% had a minimally invasive approach (laparoscopic or robotic) with a taTME component, with results suggesting equivalent anastomotic leak rates and positive resection margin in open, laparoscopic, robotic and transanal resections (37).
Robotic surgery provides several advantages over conventional laparoscopy, such as a high-definition image and 3D vision, camera stability, precise dissection, tremor reduction and enhanced ergonomics, amongst others. Even though robotics uptake for colorectal cancer surgery is still low, it is increasing worldwide, especially for rectal cancer surgery (37,38). Despite no proven oncological superiority of robotic resections over other approaches for rectal cancer surgery, randomized control data suggest benefits of robotic surgery over laparoscopic surgery in terms of conversion rates (16). Also, the learning curve of rectal resections is probably favored with robotic technology over conventional laparoscopy (39).
On the other hand, even though Buess (19) developed the TEM platform during the early 1980s and that the first taTME case published was performed using the TEM platform in 2010 (40), it was not until after the development of TAMIS in 2009 (20) that taTME was popularised worldwide.
Despite growing enthusiasm, there have been concerns around taTME and its profile of complications such as urethral and sidewall injuries and it´s oncologic safety based on recent Norwegian data (41).
The rate of urethral injuries in the first 720 cases published from the taTME international registry was 0.7% (42). Most of these complications have occurred early during the learning curve of different groups across the globe (43). Burden from urethral injuries is severe, resulting in a 22% conversion rate, an 8% rate of unplanned APR or Hartmann procedure and 26% of urethral repair complications, including a 9% need for permanent urinary diversion (43) Identifying key anatomical landmarks such as the neurovascular bundle of Walsh (44), the use of fluorescent urinary catheters (45) and identifying ‘halo signs’ or ‘O’s and ‘triangles’ during dissection (46), have all been proposed to prevent urinary tract and pelvic sidewall structure injuries to the sacral plexuses and major vessels when dissection is carried out too laterally. In order to prevent these injuries, especially during the learning curve, there has been a growing effort to develop training models and structured training programs across Europe, North America and Australasia, as highlighted by our group (47-49). Various consensus related to the current status of taTME, structured training curriculum and safe implementation has been published in the last few years (50-53).
A recent Norwegian publication has raised concerns in terms of anastomotic leak rates and recurrence rates and patterns amongst taTME patients (41). Even though randomized controlled data is awaited to solve this discrepancy, it is worth noting the low uptake of preoperative radiation in the taTME group compared with the national cohort (21% vs. 39%; P=0,001). It is also of interest, the high rate of pathologically positive or sub millimetric margin rate at 12.7% in the taTME group, which is not in keeping with most specialist centers and previously published data. As opposed to Norwegian data, recent systematic reviews and meta-analyses mainly based on comparative retrospective data have shown better TME quality and fewer rates of positive CRM in the taTME groups than the laparoscopic groups (22,23). It has also been shown in a publication based on follow-up data from 157 patients undergoing taTME for rectal cancer in two high volume centers in the Netherlands, that taTME is associated with favorable oncologic outcomes when performed in referral centers under experienced hands (54).
Also, others have shown that after adjusting for confounders, high anastomotic leak rates in taTME are mostly a result of an anastomosis in the lowest part of the rectum or the anus. Anastomotic leak rates in low rectal anastomoses remain high, regardless of the operative approach (37).
Despite these reasonable concerns, most of the literature would support taTME uptake and its utilization when performed under trained specialist surgeons in high volume centers.
In such an exciting and rapidly evolving field, still, most of the publications aim to compare outcomes of robotics and taTME and put them as competing approaches (55). The literature on combined robotics and taTME is in its infancy.
Colombo et al. evaluated the role of taTME in patients undergoing laparoscopic or robotic TME. In the context of similar conversion rates (4.8% vs. 3.2%; P=0.661), they found that the taTME use was significantly higher in the laparoscopic group than in the robotic group (16.7% vs. 1.7%; P=0.004). They conclude that the added benefit of taTME when robotic TME is performed is less significant than for laparoscopic TME, which was attributed to the enhanced ability of robotics to navigate through the pelvis during dissection (56).
Even though robotics help overcome some difficulties of conventional laparoscopy, in the context of high-risk patients, the role of taTME as a tool to control the distal resection margin, perform rectal transection and distal mesorectal dissection, justifies its utilization (57). Moreover, a single stapled anastomosis would avoid multiple staple firing and crossed staple lines that could potentially have an impact on anastomotic leak rates (58). These reasons may explain why in a recent publication by the ESCP collaborating group on a multicentric, prospective audit on implementation of taTME internationally, a taTME component was used in 25% (31/126) of the cases where a robotic restorative resection for rectal cancer was performed. Besides, with this approach, there was a significantly lower conversion rate when compared to combined conventional laparoscopic and taTME (0% versus 16.2%) (37).
Published results on R+taTME (Table 1) show that this has mainly been implemented in male patients with a BMI over 25 kg/m2 and in the context of low or locally advanced rectal cancers. Only our group presented a series of patients where a simultaneous two-team approach was systematically used. Interestingly, there are no conversions reported with this approach and publications communicate good results in terms of oncologic surrogate markers with complete TME and negative margins in all cases.
Our experience is that this approach allows improved visualization, articulation, and dissection abdominally via the application of robotic technology, facilitating the ability to perform meticulous rectal dissection along the TME plane. Distally, taTME has resulted in greater confidence to control the distal margin and to achieve an intact TME specimen, especially in high-risk patients. As a further matter, a transanal component yields control over choosing to take wider margins, such as the endopelvic fascia, when there have been concerns about CRM involvement in distal rectal cancers. We advocate on performing this kind of procedure in a two-team approach, with adequately trained surgeons, where continuous communication between teams enhances dissection, specimen quality and operative times.
More has been published on the combination of robotics and TAMIS for RATS-TME (Table 2). This approach has been mainly implemented in male patients with low, locally advanced rectal cancers, requiring neoadjuvant chemoradiation. Most of the series report a sequential procedure rather than a simultaneous two-team approach, and interestingly conversion rates are low. Only Kuo et al. (33), using a single-port robotic abdominal component and RATS-TME, reported two conversions in their series (13.3%) as previously described, where both cases were converted to conventional laparoscopy. Even though the robotic-assisted transanal component is a common factor in all reports, it is worth noting the heterogeneity of the transabdominal approach (conventional laparoscopy, robotic or single-port robotic) and the fact that this has been performed in a sequential or two-team fashion.
Based on these initial reports, RATS-TME appears to be safe and feasible when performed by experienced minimally invasive surgeons. The use of robotic technology in combination with transanal access platforms helps improve visualization with a stable high-definition 3D camera and dissection with the use of wristed instruments, potentially shortening learning curves, enhancing dissection and improving ergonomics. Despite its potential benefits, RATS-TME is still in its infancy and its disadvantages are mainly related to a complex theatre setup, docking, and probably longer operative time and costs.
In our experience, RATS-TME enhances dissection in the lower pelvis due to the added benefits of robotics, but its setup is more complex. In the scenario of being able to use a single robotic system, we would prefer the transabdominal use over the transanal, because of the ease of setup in the operating theatre and ability to perform a Cecil approach in a less complicated manner. We envisage that the increased utilization of a single-port robotic system (such as the da Vinci SP robot from below) and the possibility of dual robotics may alter this paradigm.
While technology is evolving, single-port robot prototypes are currently being tested in cadaveric models and initial human experiences (59-62) for natural orifice transluminal endoscopic surgery (NOTES). Navigation tools have also been developed and tested, eventually making it possible to apply augmented reality to pelvic dissection soon to patients with adverse pelvic anatomy or bad tumor features (63,64).
It is likely that the ongoing development of technology and technical aspects of rectal cancer surgery will make a two-field robot approach (65) or pure-robotic NOTES a reality in the near future.
Conclusions
A combination of robotic technology and taTME to perform hybrid procedures appears to be safe and feasible on the basis of initial published data.
Benefits from these approaches could be translated into a better resection quality, especially in high-risk patients, with potential improvement in oncologic and functional outcomes. These approaches need to be performed by teams with sound concepts of oncological anatomy, appropriate surgical expertise and training, familiarity with minimally invasive techniques including robotic technology and taTME, and should be undertaken ideally in high volume referral centers.
Robotics and taTME should not be considered competing techniques. Due to their potential, the natural evolution of robotics and TAMIS has been their combination in a scenario of ongoing technological and technical development.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office for the series “Transanal Total Mesorectal Excision (taTME)” published in Annals of Laparoscopic and Endoscopic Surgery. This article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2020.03.03). Mr. SKW is proctor for Intuitive Surgical Inc. and teaches and proctors taTME in Australia. The series “Transanal Total Mesorectal Excision (taTME)” was commissioned by the editorial office without any sponsorship or funding. SKW served as the unpaid Guest Editor for the series and serves as an unpaid editorial board member of Annals of Laparoscopic and Endoscopic Surgery from Jul 2019 to Aug 2021. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patients for publication of images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Berho M, Narang R, Van Koughnett JAM, et al. Modern multidisciplinary perioperative management of rectal cancer. JAMA Surg 2015;150:260-6. [Crossref] [PubMed]
- Palmer G, Martling A, Cedermark B, et al. Preoperative tumour staging with multidisciplinary team assessment improves the outcome in locally advanced primary rectal cancer. Colorectal Dis 2011;13:1361-9. [Crossref] [PubMed]
- Burton S, Brown G, Daniels IR, et al. MRI directed multidisciplinary team preoperative treatment strategy: The way to eliminate positive circumferential margins? Br J Cancer 2006;94:351-7. [Crossref] [PubMed]
- Taylor FGM, Quirke P, Heald RJ, et al. Preoperative Magnetic Resonance Imaging Assessment of Circumferential Resection Margin Predicts Disease-Free Survival and Local Recurrence: 5-Year Follow-Up Results of the MERCURY Study. J Clin Oncol 2014;32:34-43. [Crossref] [PubMed]
- Heald RJ, Husband EM, Ryall RDH. The mesorectum in rectal cancer surgery—the clue to pelvic recurrence? Br J Surg 1982;69:613-6. [Crossref] [PubMed]
- Simillis C, Lal N, Thoukididou SN, et al. Open Versus Laparoscopic Versus Robotic Versus Transanal Mesorectal Excision for Rectal Cancer: A Systematic Review and Network Meta-analysis. Ann Surg 2019;270:59-68. [Crossref] [PubMed]
- Quirke P, Steele R, Monson J, et al. Effect of the plane of surgery achieved on local recurrence in patients with operable rectal cancer: a prospective study using data from the MRC CR07 and NCIC-CTG CO16 randomised clinical trial. Lancet 2009;373:821-8. [Crossref] [PubMed]
- Tilly C, Lefèvre JH, Svrcek M, et al. R1 rectal resection: Look up and don’t look down. Ann Surg 2014;260:794-9; discussion 799-800. [Crossref] [PubMed]
- Stevenson ARL, Solomon MJ, Lumley JW, et al. Effect of laparoscopic-assisted resection vs open resection on pathological outcomes in rectal cancer: The ALaCaRT randomized clinical trial. JAMA 2015;314:1356-63. [Crossref] [PubMed]
- Fleshman J, Branda M, Sargent DJ, et al. Effect of laparoscopic-assisted resection vs open resection of stage II or III rectal cancer on pathologic outcomes the ACOSOG Z6051 randomized clinical trial. JAMA 2015;314:1346-55. [Crossref] [PubMed]
- Fleshman J, Branda ME, Sargent DJ, et al. Disease-free Survival and Local Recurrence for Laparoscopic Resection Compared With Open Resection of Stage II to III Rectal Cancer: Follow-up Results of the ACOSOG Z6051 Randomized Controlled Trial. Ann Surg 2019;269:589-95. [Crossref] [PubMed]
- Stevenson ARL, Solomon MJ, Brown CSB, et al. Disease-free Survival and Local Recurrence After Laparoscopic-assisted Resection or Open Resection for Rectal Cancer: The Australasian Laparoscopic Cancer of the Rectum Randomized Clinical Trial. Ann Surg 2019;269:596-602. [Crossref] [PubMed]
- Bonjer HJ, Deijen CL, Abis GA, et al. A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med 2015;372:1324-32. [Crossref] [PubMed]
- Jeong SY, Park JW, Nam BH, et al. Open versus laparoscopic surgery for mid-rectal or low-rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): Survival outcomes of an open-label, non-inferiority, randomised controlled trial. Lancet Oncol 2014;15:767-74. [Crossref] [PubMed]
- Jayne D, Pigazzi A, Marshall H, et al. Effect of robotic-assisted vs conventional laparoscopic surgery on risk of conversion to open laparotomy among patients undergoing resection for rectal cancer the rolarr randomized clinical trial. JAMA 2017;318:1569-80. [Crossref] [PubMed]
- Prete FP, Pezzolla A, Prete F, et al. Robotic Versus Laparoscopic Minimally Invasive Surgery for Rectal Cancer: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Ann Surg 2018;267:1034-46. [Crossref] [PubMed]
- Heald RJ. A new approach to rectal cancer. Br J Hosp Med 1979;22:277-81. [PubMed]
- Marks G, Mohiuddin M, Rakinic J. New hope and promise for sphincter preservation in the management of cancer of the rectum. Semin Oncol 1991;18:388-98. [PubMed]
- Buess G, Hutterer F, Theiss J, et al. A system for a transanal endoscopic rectum operation. Chirurg 1984;55:677-80. [PubMed]
- Atallah S, Albert M, Larach S. Transanal minimally invasive surgery: A giant leap forward. Surg Endosc 2010;24:2200-5. [Crossref] [PubMed]
- Denost Q, Adam JP, Rullier A, et al. Perineal transanal approach: A new standard for laparoscopic sphincter-saving resection in low rectal cancer, a randomized trial. Ann Surg 2014;260:993-9. [Crossref] [PubMed]
- Zhang X, Gao Y, Dai XL, et al. Short- and long-term outcomes of transanal versus laparoscopic total mesorectal excision for mid-to-low rectal cancer: a meta-analysis. Surg Endosc 2019;33:972-85. [Crossref] [PubMed]
- Wu Z, Zhou W, Chen F, et al. Short-term outcomes of transanal versus laparoscopic total mesorectal excision: A systematic review and meta-analysis of cohort studies. J Cancer 2019;10:341-54. [Crossref] [PubMed]
- Jiang HP, Li Y, Sen , Wang B, et al. Pathological outcomes of transanal versus laparoscopic total mesorectal excision for rectal cancer: a systematic review with meta-analysis. Surg Endosc 2018;32:2632-42. [Crossref] [PubMed]
- Mendes CRS, Valadão M, Araújo R, et al. Transanal minimally invasive surgery for total mesorectal excision (ETM) through transanal approach (TaETM) with robotic and Transanal Endoscopic Operations (TEO) combined access: step by step surgery. Arq Bras Cir Dig 2015;28:117-20. [Crossref] [PubMed]
- Gómez Ruiz M, Parra IM, Palazuelos CM, et al. Robotic-assisted laparoscopic transanal total mesorectal excision for rectal cancer: A prospective pilot study. Dis Colon Rectum 2015;58:145-53. [Crossref] [PubMed]
- Bravo R, Trépanier JS, Arroyave MC, et al. Combined transanal total mesorectal excision (taTME) with laparoscopic instruments and abdominal robotic surgery in rectal cancer. Tech Coloproctol 2017;21:233-5. [Crossref] [PubMed]
- Nikolic A, Waters PS, Peacock O, et al. Hybrid abdominal robotic approach with conventional transanal total mesorectal excision (TaTME) for rectal cancer: feasibility and outcomes from a single institution. J Robot Surg 2020;14:633-41. [Crossref] [PubMed]
- Atallah S, Martin-Perez B, Parra-Davila E, et al. Robotic transanal surgery for local excision of rectal neoplasia, transanal total mesorectal excision, and repair of complex fistulae: clinical experience with the first 18 cases at a single institution. Tech Coloproctol 2015;19:401-10. [Crossref] [PubMed]
- Koedam TWA, Veltcamp Helbach M, van de Ven PM, et al. Transanal total mesorectal excision for rectal cancer: evaluation of the learning curve. Tech Coloproctol 2018;22:279-87. [Crossref] [PubMed]
- Verheijen PM, Consten EC, Broeders IA. Robotic transanal total mesorectal excision for rectal cancer: Experience with a first case. Int J Med Robot 2014;10:423-6. [Crossref] [PubMed]
- Huscher CGS, Bretagnol F, Ponzano C. Robotic-assisted Transanal Total Mesorectal Excision: The Key Against the Achilles’ Heel of Rectal Cancer? Ann Surg 2015;261:e120-1. [Crossref] [PubMed]
- Kuo LJ, Ngu JCY, Tong YS, et al. Combined robotic transanal total mesorectal excision (R-taTME) and single-site plus one-port (R-SSPO) technique for ultra-low rectal surgery—initial experience with a new operation approach. Int J Colorectal Dis 2017;32:249-54. [Crossref] [PubMed]
- Hu JM, Chu CH, Jiang JK, et al. Robotic transanal total mesorectal excision assisted by laparoscopic transabdominal approach: A preliminary twenty-case series report. Asian J Surg 2020;43:330-8. [Crossref] [PubMed]
- Monsellato I, Morello A, Prati M, et al. Robotic transanal total mesorectal excision: A new perspective for low rectal cancer treatment. A case series. Int J Surg Case Rep 2019;61:86-90. [Crossref] [PubMed]
- Gomez Ruiz M, Martin Parra I, Calleja Iglesias A, et al. Preclinical cadaveric study of transanal robotic proctectomy with total mesorectal excision combined with laparoscopic assistance. Int J Med Robot 2015;11:188-93. [Crossref] [PubMed]
- 2017 European Society of Coloproctology (ESCP) collaborating group. An international multicentre prospective audit of elective rectal cancer surgery; operative approach versus outcome, including transanal total mesorectal excision (TaTME). Colorectal Dis 2018;20:33-46. [Crossref] [PubMed]
- Schootman M, Hendren S, Ratnapradipa K, et al. Adoption of robotic technology for treating colorectal cancer. Dis Colon Rectum 2016;59:1011-8. [Crossref] [PubMed]
- Jiménez-Rodríguez RM, Rubio-Dorado-Manzanares M, Díaz-Pavón JM, et al. Learning curve in robotic rectal cancer surgery: current state of affairs. Int J Colorectal Dis 2016;31:1807-15. [Crossref] [PubMed]
- Sylla P, Rattner DW, Delgado S, et al. NOTES transanal rectal cancer resection using transanal endoscopic microsurgery and laparoscopic assistance. Surg Endosc 2010;24:1205-10. [Crossref] [PubMed]
- Wasmuth HH, Færden AE, Myklebust T, et al. Transanal total mesorectal excision for rectal cancer has been suspended in Norway. Br J Surg 2020;107:121-30. [Crossref] [PubMed]
- Penna M, Hompes R, Arnold S, et al. Transanal Total Mesorectal Excision: International Registry Results of the First 720 Cases. Ann Surg 2017;266:111-7. [Crossref] [PubMed]
- Sylla P, Knol JJ, D’Andrea AP, et al. Urethral Injury and Other Urologic Injuries During Transanal Total Mesorectal Excision. Ann Surg 2019; [Epub ahead of print]. [Crossref] [PubMed]
- Atallah S, Albert M. The neurovascular bundle of Walsh and other anatomic considerations crucial in preventing urethral injury in males undergoing transanal total mesorectal excision. Tech Coloproctol 2016;20:411-2. [Crossref] [PubMed]
- Mabardy A, Lee L, Valpato AP, et al. Transanal total mesorectal excision with intersphincteric resection and use of fluorescent angiography and a lighted urethral stent for distal rectal cancer. Tech Coloproctol 2017;21:581-2. [Crossref] [PubMed]
- Bernardi MP, Bloemendaal ALA, Albert M, et al. Transanal total mesorectal excision: dissection tips using “O”s and “triangles.” Tech Coloproctol 2016;20:775-8. [Crossref] [PubMed]
- Abbott SC, Stevenson ARL, Bell SW, et al. An assessment of an Australasian pathway for the introduction of transanal total mesorectal excision (taTME). Colorectal Dis 2018;20:O1-6. [Crossref] [PubMed]
- Penna M, Whiteford M, Hompes R, et al. Developing and assessing a cadaveric training model for transanal total mesorectal excision: initial experience in the UK and USA. Colorectal Dis 2017;19:476-84. [Crossref] [PubMed]
- Atallah SB, DuBose AC, Burke JP, et al. Uptake of transanal total mesorectal excision in North America: Initial assessment of a structured training program and the experience of delegate surgeons. Dis Colon Rectum 2017;60:1023-31. [Crossref] [PubMed]
- Motson RW, Whiteford MH, Hompes R, et al. Current status of trans-anal total mesorectal excision (TaTME) following the Second International Consensus Conference. Colorectal Dis 2016;18:13-8. [Crossref] [PubMed]
- Penna M, Hompes R, Mackenzie H, et al. First international training and assessment consensus workshop on transanal total mesorectal excision (taTME). Tech Coloproctol 2016;20:343-52. [Crossref] [PubMed]
- Francis N, Penna M, Mackenzie H, et al. Consensus on structured training curriculum for transanal total mesorectal excision (TaTME). Surg Endosc 2017;31:2711-9. [Crossref] [PubMed]
- Adamina M, Buchs NC, Penna M, et al. St.Gallen consensus on safe implementation of transanal total mesorectal excision. Surg Endosc 2018;32:1091-103. [Crossref] [PubMed]
- Hol JC, van Oostendorp SE, Tuynman JB, et al. Long-term oncological results after transanal total mesorectal excision for rectal carcinoma. Tech Coloproctol 2019;23:903-11. [Crossref] [PubMed]
- Grass JK, Perez DR, Izbicki JR, et al. Systematic review analysis of robotic and transanal approaches in TME surgery- A systematic review of the current literature in regard to challenges in rectal cancer surgery. Eur J Surg Oncol 2019;45:498-509. [Crossref] [PubMed]
- Colombo PE, Bertrand MM, Alline M, et al. Robotic Versus Laparoscopic Total Mesorectal Excision (TME) for Sphincter-Saving Surgery: Is There Any Difference in the Transanal TME Rectal Approach?: A Single-Center Series of 120 Consecutive Patients. Ann Surg Oncol 2016;23:1594-600. [Crossref] [PubMed]
- Spinelli A, Carvello M, D’Hoore A, et al. Integration of transanal techniques for precise rectal transection and single-stapled anastomosis: a proof of concept study. Colorectal Dis 2019;21:841-6. [PubMed]
- Ito M, Sugito M, Kobayashi A, et al. Relationship between multiple numbers of stapler firings during rectal division and anastomotic leakage after laparoscopic rectal resection. Int J Colorectal Dis 2008;23:703-7. [Crossref] [PubMed]
- Marks J, Ng S, Mak T. Robotic transanal surgery (RTAS) with utilization of a next-generation single-port system: a cadaveric feasibility study. Tech Coloproctol 2017;21:541-5. [Crossref] [PubMed]
- Atallah S. Assessment of a flexible robotic system for endoluminal applications and transanal total mesorectal excision (taTME): Could this be the solution we have been searching for? Tech Coloproctol 2017;21:809-14. [Crossref] [PubMed]
- Samalavicius NE, Janusonis V, Smolskas E, et al. Transanal and robotic total mesorectal excision (robotic-assisted TaTME) using the Senhance® robotic system - a video vignette. Colorectal Dis 2020;22:114-5. [Crossref] [PubMed]
- Carmichael H, D’Andrea AP, Skancke M, et al. Feasibility of transanal total mesorectal excision (taTME) using the Medrobotics Flex® System. Surg Endosc 2020;34:485-91. [Crossref] [PubMed]
- Atallah S, Parra-Davila E, Melani AGF, et al. Robotic-assisted stereotactic real-time navigation: initial clinical experience and feasibility for rectal cancer surgery. Tech Coloproctol 2019;23:53-63. [Crossref] [PubMed]
- Atallah S, Zenoni S, Kelly J, et al. A blueprint for robotic navigation: pre-clinical simulation for transanal total mesorectal excision (taTME). Tech Coloproctol 2016;20:653-4. [Crossref] [PubMed]
- Atallah S, DuBose A, Larach SW. Towards the development of simultaneous two-field robotic surgery. Tech Coloproctol 2016;20:71-3. [Crossref] [PubMed]
Cite this article as: Larach JT, Warrier SK. Robotics and transanal total mesorectal excision. Ann Laparosc Endosc Surg 2020;5:41.