
Training and accreditation in transanal total mesorectal excision in the United States
Introduction
The United States (US) has been at the forefront of transanal total mesorectal excision (taTME) since the initial description of the first NOTES (natural orifice transluminal endoscopic surgery) transanal sigmoid resection in a cadaver model by Whiteford et al. in 2007 (1). In 2008, Sylla et al. performed the first pilot study of transanal endoscopic rectosigmoid resection in a swine model using the transanal endoscopic microsurgery (TEM) platform in Boston, Massachusetts (2). Soon after, Trunzo and Delaney replicated the porcine study—with the addition of flexible endoscopic assistance (3). Following a number of feasibility studies in human cadavers (1,4-6), the first clinical case of taTME was performed in 2009 as a collaboration between surgical teams from the US and Spain (7). The US continued to lead the advancement of taTME and in 2013, the results of an IRB-approved pilot study of five patients with rectal cancer demonstrated the feasibility and safety of taTME (8). Additional experiences from the US, including a case series of 50 patients in Orlando confirmed that taTME resulted in acceptable pathological outcomes and perioperative morbidity (9).
Experts in taTME have been eager to disseminate this innovative approach to TME, but questions remain regarding how to best train and certify those new to the procedure and ensuring its safe introduction into clinical practice. In the US, a taTME training pathway was first described by McLemore et al. in 2013 which included establishing a transanal endoscopic surgery practice, training in taTME using male cadavers, and performing initial taTME cases for benign disease before initiating a Phase I, IRB-approved clinical trial of taTME for rectal cancer. The authors emphasized the importance of rehearsing the procedure in a cadaveric model with the entire OR team the day prior to the first live clinical case. This additional training session would help review the steps of the procedure and equipment needed, with debriefing at the end to discuss concerns and questions. The results of this experience were published in 2016 as the first taTME training pathway, where the authors summarized the prerequisites for safe adoption of taTME including expertise in (I) minimally invasive TME (laparoscopic or robotic), (II) transanal endoscopic surgery, and (III) intersphincteric resection for ultra-low rectal cancer. Additionally, to maximize safety, it was recommended that participants train in cadavers and perform their first cases under IRB protocol, and/or participate in IRB-approved data collection or a clinical registry (10).
The importance of human cadaveric training in taTME is crucial to provide hands-on experience and understanding of the pelvic anatomy from a “bottom-up” perspective. In 2017, the results of four human cadaveric workshops for taTME training in the United Kingdom (UK) and US were described, which together trained 52 surgeons. The workshops consisted of didactic lectures and videos, rectal purse string closure practice on a simulator, and one or two hands-on cadaveric sessions. The 20 surgeons who attended the US workshop completed post-course surveys and 18 surgeons reported performing taTME with a total of 85 cases over two years (11).
Though the United States spends nearly twice as much as Europe on cancer care-related costs, colorectal cancer outcomes in America vary widely compared to many European countries (12), many of which have launched nationwide initiatives to standardize training and practices. To date, there is no standardized protocol for taTME training and accreditation in the United States. Studies advise that between 40–50 cases are required to overcome the initial learning curve for taTME, but this has not been incorporated into formal credentialing standards by units that have adopted taTME (13,14). Simultaneous close mentorship by taTME experts throughout the initial learning period is another pillar of quality training, as is participation in national quality assurance databases. However, like during the advent of laparoscopic or robotic surgery in the US, protocols for training and accreditation for taTME are still in the early stages of development. An understanding of how well-established US models of accreditation and formal training were conceived will reveal the difficulties faced for taTME accreditation and may illuminate future directions.
History of surgical accreditation in the US: bariatrics and robotic surgery
Historically, accreditation procedures and measures of competency for specific surgical skills in the US has ranged from government-driven to industry-driven programs. The introduction of laparoscopy in the 1990s highlights the challenges of adopting new surgical techniques with new technologies. When laparoscopy was first introduced for cholecystectomy, bile duct injuries increased nearly fivefold (15). As the technique became widely adopted, studies demonstrated a volume-outcome relationship both for individual surgeons as well as for groups of surgeons (15,16). Similar trends were demonstrated when bariatric surgery and robotic surgery were popularized in the US. When 277,760 laparoscopic stapling bariatric procedures from the Nationwide Inpatient Sample Database were reviewed, low-volume centers (<50 cases per year) had higher rates of mortality (OR 2.5; 95% CI, 1.3–4.8; P<0.01) and serious morbidity (OR 1.2; 95% CI, 1.1–1.4; P<0.01) than high volume centers (≥50 cases per year) (17). Similarly, at the individual level, one study demonstrated a 10% reduction in risk ratio for an adverse event (defined as venous thromboembolism, pulmonary embolism, reoperation, non-discharge at 30 days or rehospitalization within 30 days) for every 10 cases per year increase in surgeon volume (18). Early studies in robotic surgery exhibited similar relationship between case volume and outcomes. A retrospective review of 957 colon and rectal procedures performed between 2013–2017 at 32 centers found that at high volume centers (>30 resections per year), operative times, length of stay, rates of conversion and total direct costs all significantly decreased when compared to low volume centers (19).
While the volume-outcome relationship appears to be consistent amongst all procedures, methods of training and accreditation in the US vastly differ. The creation of formal bariatric surgery and robotic accreditation pathways exemplify two different models for certification. Bariatric surgery accreditation policies have evolved drastically over the past 15 years. Initially, individual third-party payers endorsed their own bariatric centers of excellence. In 2004, prompted by concerns for safety at low-volume centers, the American Society for Metabolic and Bariatric Surgery (ASMBS) and the American College of Surgeons (ACS) established national facility accreditation standards (20). The ACS-sponsored Bariatric Surgery Center Network, born out of the ACS-ASMBS partnership, stipulated that in order to become a center of excellence, the hospital must meet the following criteria: (I) perform a minimum case volume of 125 surgeries per year, (II) have a multidisciplinary, experienced team of staff and at least two surgeons, and (III) participate in a prospective database for data collection (21). A year later, the Centers for Medicare and Medicaid Services (CMS) assumed control of accreditation and mandated that Medicare patients undergo surgery at an accredited center. This decision was overturned in 2013 because it was thought to impede access to care.
Now, a single organization, the Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program (MBSAQIP) sets the standards for participation, which includes 100% case reporting to the National Surgical Quality Improvement Program and local institutional committees (20). The MBSAQIP’s aim is to expand access to bariatric surgery, and they have done so by creating two levels of accreditation. Level one centers are higher volume with larger teams and thus deemed capable of managing complex patients; level two centers have a lower volume of annual cases and thus are limited to lower-risk patients and cannot perform elective revisional cases. Currently, there are more than 800 accredited centers in the US and Canada combined (22).
Multiple studies using large datasets demonstrate a swift improvement in complication rates, mortality rates, length of stay and cost when bariatric surgery is performed at an accredited center (16,23-25). As expected, the majority of such centers in the US are high volume centers (HVCs). However, a study that examined all cases in the 2010 Nationwide Inpatient Sample found a higher likelihood of mortality (OR 2.26; 95% CI, 1.24–4.10; P<0.007) at unaccredited centers, independent of whether the institution was high volume or a teaching hospital (16). While it is well understood that surgery at HVCs reduces the odds of morbidity and mortality, the value of accreditation may be of equal, if not greater, importance in some cases. The credentialing of bariatric surgery centers has proven to be essential for the safety of this surgical procedure. The evolution of the governing body for bariatric surgery, though, has been in flux, driven by a combination of data, financial, and governmental incentives.
Training and certification in robotic surgery have followed a different model propelled almost exclusively by industry with little oversight by surgical societies. In contrast to training for laparoscopic surgery, which mandates the completion of the Fundamentals of Laparoscopic Surgery modules for laparoscopic credentialing (26), training and accreditation in robotic surgery are institution-specific and overseen by the robot manufacturer (27).
For the past nine years, the Association of Program Directors for Colon and Rectal Surgery (APDCRS) has offered cadaver courses for training in colorectal robotics, funded by Intuitive Surgical (Sunnyvale, CA). To qualify for the course, the trainee must have completed the “da Vinci Technology Online Modules,” the “da Vinci Technology Overview In-Service and Skills Simulation,” have entered all robotic cases into the case log system provided by the APDCRS, and have participated in five da Vinci cases as console surgeon. There is also the option for a “Training Certificate” issued by Intuitive Surgical (in lieu of the advanced course) for participants who have completed 20 console cases and 10 bedside cases. To promote the maintenance of robotic skills, the company sponsors a “SurgeOn Premier” program, which includes refresher training courses during a fellow’s first year of practice (28).
In summary, credentialing in colorectal robotics is sponsored and regulated by Intuitive Surgical, with oversight by the APDCRS. Despite the availability of training programs, however, a 2018 study that surveyed the practice patterns of the American Society of Colon and Rectal Surgeons (ASCRS) Young Surgeons Committee found that while robotic experience was limited during training (84% of respondents performed fewer than five cases during residency, with only 12% performing more than 25 cases), 92% of respondents reported performing robotic assisted colorectal surgery in independent practice (29).
Laparoscopic TME training in the US
Laparoscopic TME was popularized in the 1990s and quickly showed improved short-term postoperative outcomes. Several randomized control trials compared laparoscopic TME to open TME and demonstrated that a laparoscopic approach was associated with reduced postoperative pain, wound infection rates, and decreased length of hospital stay (30). However, high conversion rates were reported with laparoscopic TME in the COLOR II and ACOSOG Z6051 trials (17% and 11.3%, respectively) (31,32). The most common factors associated with conversion to open surgery included a narrow pelvis, obesity, and tumor fixation. Additional patient and tumor-related factors predictive of intraoperative difficulty and positive circumferential resection margin (CRM) include male sex, obesity, narrow pelvis, bulky tumors, and advanced T-stage (33-35). These factors can complicate laparoscopic TME by limiting visualization, and access to the mesorectal plane and dissection within the confined space of the narrow pelvis. These challenges can lead to dissection along the wrong tissue planes, incomplete TME and worse oncologic outcomes.
Laparoscopic TME for rectal cancer can be a technically difficult procedure and failure to obtain a complete TME has been associated with worse oncologic outcomes. Yet there is currently no formal accreditation process for surgeons performing laparoscopic TME. While some studies have suggested that at least 50 cases are required to achieve proficiency in laparoscopic colectomy, several clinical trials have mandated a minimum of 20 laparoscopic colectomies be performed to meet the inclusion requirements (36-38). In addition to lack of formal accreditation in laparoscopic TME, there currently is no requirement for self-reporting of operative results, such as TME specimen grade, rate of positive CRM or DRM, number of lymph nodes harvested, or rate of R0 resection.
The National Accreditation Program for Rectal Cancer (NAPRC), which awards accreditation to rectal cancer programs, has attempted to address the lack of performance metrics. The NAPRC was born out of a collaboration between the OSTRiCH Consortium (Optimizing the Surgical Treatment of Rectal Cancer) and the American College of Surgeons Commission on Cancer. A rectal cancer program is accredited as a center of excellence if it demonstrates compliance with the standards of the NAPRC, including establishing a multidisciplinary team approach to each case, adhering to evidence-based practices, and data collection for performance improvement. In the near future, it is likely that centers will be required to comply with submission of all rectal cancer cases for performance measures through the Rapid Quality Report System (RQRS). Currently, no studies have examined the utility of NAPRC accreditation with respect to improved operative or oncologic outcomes. Nonetheless, a 2014 study examining rectal cancer cases from the National Cancer Database showed there was a stricter adherence to evidence-based guidelines in high volume centers (>30 cases per year) (39).
taTME training in the US
An understanding of the United States’ models for accreditation in well-established procedures, like bariatric and robotic surgery, and lack of accreditation for laparoscopic TME provides a framework for developing protocols for taTME training and accreditation. Presently, the international taTME community collaborates to host courses that pairs experts with qualified trainees to provide essential guidance to surgeons with a high likelihood of implementing taTME. These courses typically include classroom-based didactic lessons, live cases, and hand-on cadaver training.
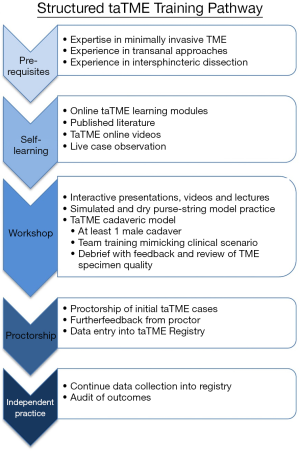
As described earlier, early human cadaveric courses were hosted by the UK and the US starting in 2013. A formal taTME training pathway was proposed with an emphasis on expertise in laparoscopic TME, transanal surgery and intersphincteric resection, live case observation, simulation and hands-on cadaveric experience, proctorship and data collection/audit of outcomes [Figure 1 (11)]. Despite the proposal, no formalized training program has been established in the US to date. However, other countries have implemented their own national training programs. In 2014, the Netherlands established a structured program for colorectal surgeons wishing to pursue taTME. Minimal pre-requisites included (I) at least 50 laparoscopic TME cases, (II) prior TAMIS experience, (III) at least 20 taTME cases per year, and (IV) appropriate surgical equipment and instruments with the ability to perform a two-team approach (40). The current training course consists of a two-day program with didactic lessons, hands-on training with box simulator models and human cadavers, and the observation of a live taTME surgery. Additionally, a structured proctoring program is implemented at the trainees’ hospital until proficiency to proceed alone is demonstrated. In 2018, results from 120 patients, representing the first 10 cases from 12 hospitals following completion of the Dutch training program, were published. One hundred percent of TME specimens were graded as complete/near complete, 100% had a negative DRM, 5% had a positive CRM, and intraoperative complications occurred in 4.9% of cases. The overall postoperative morbidity rate was 45% and major complications occurred in 19.2%. Furthermore, the anastomotic leak rate among these first 120 cases was 17.3%, higher than the early anastomotic leak rate of 7.8% reported by the international taTME registry. These results highlighted that the Dutch model of taTME training results in safe pathological and operative outcomes, although postoperative complications were high among the surgeons in the early stages of their taTME learning curve.
Although the US does not have a standardized training pathway, a number of taTME courses for taTME have been conducted annually. Small industry-sponsored workshops are held four to eight times per year at various sites in the US and typically can accommodate between 10–20 colorectal surgeons at a time. However, strict prerequisites in advanced laparoscopic TME and transanal surgery and post-workshop follow-up are lacking when compared to the training programs in the Netherlands. Additionally, sponsors for these courses vary. The American Society of Colon and Rectal Surgeons (ASCRS) has held formal taTME training workshops during their last four annual meetings, while most other courses have been sponsored by industry sponsors.
In 2017, Atallah et al. reported the Orlando experience in training 81 surgeons in taTME over a 12-month period (41). The 2-day industry-sponsored courses consisted of didactic sessions, a hands-on laboratory with male cadaveric training, live taTME surgery observation, and surgeons were encouraged to enter taTME cases in the OSTRiCH taTME registry. Among the surgeons who participated in the survey, only 52.6% reported performing taTME at least once since course completion, with 45% reporting they were not comfortable performing live taTME surgery. Among those who had performed taTME, 25% reported a urethral complication. In response to these findings, the Orlando training program was modified to include more detailed didactics and hands-on training to improve surgeons’ understanding of key landmarks in avoiding urethral injury. They reported that the rate of inadvertent prostate mobilization during cadaver dissection decreased from 20% to 3.3%, highlighting the need for training programs to be amenable to restructuring to improve surgeon learning and avoid potential complications (41).
While the Dutch training program and several others have mandated proctoring during the first live cases, none of the taTME training programs in the US have made proctoring an absolute requirement for taTME implantation, or a prerequisite for attending a course (40,42). While proctoring is recommended, it has not been widely implemented across the country, in part due to the lack of formal institutional credentialing requirements to perform taTME. The lack of mentorship opportunities may explain why nearly half of the surveyed surgeons who attended the Orlando training course remained uncomfortable performing taTME at their institution. Moreover, in 2018, a consensus statement was released by the St. Gallen Colorectal Consensus Expert Group that endorsed proctoring as an essential requirement for safe taTME implementation. Among this group of international experts in rectal cancer and taTME, 97% agreed that proctoring with an expert surgeon is strongly advised and 95% agreed that the first one to five taTME cases should be proctored before proceeding independently (43). While some US surgeons have independently arranged for proctoring of their initial taTME case(s), the lack of a standardized protocol deprives the trainee of the expertise and oversight that a mentor can provide during the early learning period (10,41).
A major obstacle that impedes routine taTME mentorship in the US is the cost of proctoring. There is no formal funding mechanism to cover the costs of proctoring, which thereby fall on institutions and/or individual units or surgeons who wish to be proctored. One possible way to offset the cost of proctoring includes collaborations between surgical societies and industry, as seen with Intuitive Surgical in the adoption of robotic surgery. While this may be the most cost-effective solution, it has the potential to lead to an unintended bias in a surgeon’s instrument choice for taTME. Nevertheless, industry sponsorship has been crucial for many of the early training programs, including the joint UK/US cadaver course and the current training program in Orlando, Florida (11,41).
Barriers to formal taTME training
While most will agree that formal training and credentialing for taTME in the US is necessary, a number of further challenges exist in the establishment of an accreditation program. In addition to the cost of proctoring, another barrier is the steep learning curve for taTME and the need for ample case volume to master the technical complexity of taTME. According to a study by Koedam et al., when the learning curve is defined as a decrease in major postoperative complications and anastomotic leaks, proficiency does not occur until after 40 cases (13). Another study reported a minimum of 45–51 taTME cases to reach proficiency in producing high-quality TME specimens (14).
Many taTME structured training programs have a prerequisite minimum number of rectal cases per year for surgeons to be considered eligible for taTME training, and several consensus statements have recommended an annual volume of 10–20 cases [Table 1 (43-45)]. In the United States, it is estimated that over 400 surgeons have received taTME training, yet only 25 would be classified as high volume rectal cancer surgeons (41). Previous studies have shown that outcomes are improved when rectal cancer surgery is performed by high volume surgeons at high volume centers (46-48). Despite participating in training, many surgeons outside of tertiary referral centers do not experience enough clinical volume to develop the technical competency required to overcome the significant learning curve for taTME.
Table 1
| Prerequisite training | Recommended taTME case volume | Recommended taTME training | Recommended post taTME training | |
|---|---|---|---|---|
| St. Gallen Colorectal Consensus Expert Group (43) 2018 | Experience in oncological rectal surgery with annual center volume of ≥10 cases | 20 cases within 2 years | Dedicated courses with hands-on/cadaveric courses | Initial 1–5 cases proctored |
| Prospective monitoring and participation in clinical studies | ||||
| International taTME Educational Collaborative Group (44) 2017 | Centers with ≥0 rectal cancers per year and ≥2 surgeons trained in taTME | ≥20 cases per year | Self-learning modules, interactive didactic sessions, live case observation, dry lab workshop and cadaveric training | Initial 5–10 cases proctored |
| Training and accreditation in laparoscopic colorectal surgery | Data collection into registry with audit of outcomes | |||
| ≥30 independent laparoscopic rectal cases | ||||
| ≥5 TEMS or TAMIS cases | ||||
| Second International taTME Conference Expert Group (45) 2016 | Experience in laparoscopic colon and rectal surgery, particularly laparoscopic anterior resection for cancer | N/A | Didactic training, live case observation, pursestring suture training, cadaveric training | Period of proctoring advisable |
| Experience in transanal surgery | Data contribution into a registry | |||
| Experience with handsewn coloanal anastomosis |
TEMS, transanal endoscopic microsurgery; TAMIS, transanal minimally invasive surgery; N/A, not available.
It is also critical that surgeons track their cases to better improve taTME outcomes. Data collection and monitoring of treatment, patient outcomes, and pathologic specimens in a taTME registry has been a foundation of taTME training since the early cadaveric courses held in the US and the UK (11). Unfortunately, reporting of taTME outcomes into the registry is not mandated and is self-reported, with the potential for underreporting of adverse outcomes. In the case of urethral injuries during taTME, only 46% of injuries that were known to have occurred around the world were reported to either the LOREC (Low Rectal Cancer Development Program) or OSTRiCH registries (49). Without mandatory reporting of taTME data and internal auditing of results, data collected from these registries may be incomplete or biased, with significant repercussions on the validity of reported outcomes. Ideally part of the accreditation criteria for the NAPRC will be participation in the Rapid Quality Reporting System, as well as complying with the Estimated Performance Rate to ensure the highest quality care.
A recent example of discordance between registry data and nationally audited rectal cancer data with respect to taTME outcomes is that of Norway. An audit of taTME outcomes over a four-year period led to the suspension of taTME in Norway. Early data demonstrated a 7.6% rate of local recurrence following taTME, with an estimated local recurrence rate of 11.6% at 2.4 years relative to 2.4% local recurrent rate observed in the national Norwegian Colorectal Cancer Registry (50). Previous single center series had reported local recurrence rates ranging from 2.3–5.7% and, in 2019, a long-term study from the Netherlands reported a three-year and five-year local recurrence rate of 2% and 4%, respectively (9,51-56). Multifocal recurrence was seen in 50% of those with local recurrence and all recurrences occurred within two years of surgery, at a median of 9.5 months. Two centers in Norway were reportedly entering cases into to the international taTME registry, yet the occurrence of multifocal recurrence had never been reported in the LOREC registry until the Norwegian moratorium was published. The majority (152/157) of patients undergoing taTME, including those who developed local recurrence, had surgery performed at one of four hospitals by surgeons who had trained in international workshops and completed structured training programs, with one of the sites receiving proctoring of their initial cases. The four hospitals performed between 32–57 taTME cases over the four-year period, however, the actual annual case volume of these centers is unknown and may have been below the annual volume of HVCs. Case volume is an important component of safety and efficacy: a number of studies have shown a relationship between volume and clinical outcomes in taTME. A 2016 study comparing low volume (≤30 total cases) and high volume taTME centers (>30 total cases) demonstrated higher conversion rates (4.3% vs. 2.7%), lower rates of complete TME (80.5% vs. 89.7%), and higher rates of local recurrence (8.9% vs. 2.8%) at low volume centers (48).
The results from Norway highlight the importance of appropriate case volume, patient selection, surgical technique and postoperative surveillance. The suspension of taTME in Norway is reminiscent of the early 1990s, when a moratorium was issued following a report of a 21% incidence of port site metastases after laparoscopic colectomy (57). Further research found no significant difference in the risk of port site metastases between laparoscopic and open surgery, and the incidence of port site metastases was attributed to suboptimal surgical techniques among surgeons early in their training for the procedure (58). In response to incidence of port site metastases, surgical societies, including the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) and the ASCRS, created guidelines for the safe training and implementation of laparoscopic colorectal surgery (32).
While none of the American surgical societies have yet issued a formal response to the Norwegian suspension on taTME, it is likely that they will recommend that taTME be performed by surgeons at high volume rectal cancer centers who have extensive laparoscopic TME and transanal endoscopic surgery experience. These surgeons will be expected to have to have completed hands-on human cadaveric training as part of a structured training curriculum, followed by proctoring until the surgeon is deemed technically competent and safe to proceed independently.
An ongoing North American multicenter prospective phase II clinical trial aims to validate the peri-procedural and oncologic safety of taTME by including strict site eligibility requirements with respect to taTME case volume, as well as strict inclusion criteria for trial enrollment (NCT03144765). This prospective trial, which emphasizes surgical expertise at high volume centers as well as stringent patient selection, along with other ongoing phase II and III multicenter trials—will clarify local recurrence rates following taTME and elucidate the true incidence of multifocal local recurrence experienced in Norway (48,53).
Conclusion
Transanal TME is a promising yet technically challenging operation for the treatment of distal rectal cancer. The international taTME community has established well-defined training pathways with emphasis on hands-on cadaveric models and short-term proctoring of a surgeon’s first live taTME cases. Currently, United States surgeons who wish to incorporate taTME into practice can attend workshops sponsored by industry or various surgical societies. However, no standardization exists among these courses and proctoring tends to be cost-prohibitive, which often becomes a major barrier for surgeons. Ongoing prospective phase II and III multicenter clinical trials aim to validate taTME as an oncologically safe treatment for distal rectal cancer when performed by experienced surgeons at high volume centers that adhere to strict outcomes reporting.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Satish Kumar Warrier and Alexander Heriot) for the series “taTME” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2020.01.03). The series “taTME” was commissioned by the editorial office without any funding or sponsorship. PS reports that she has received consulting fees from the following commercial entities: Karl Storz, Ethicon, Medtronic, Olympus (as a consultant). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Whiteford MH, Denk PM, Swanstrom LL. Feasibility of radical sigmoid colectomy performed as natural orifice translumenal endoscopic surgery (NOTES) using transanal endoscopic microsurgery. Surg Endosc 2007;21:1870-4. [Crossref] [PubMed]
- Sylla P, Willingham FF, Sohn DK, et al. NOTES rectosigmoid resection using transanal endoscopic microsurgery (TEM) with transgastric endoscopic assistance: a pilot study in swine. J Gastrointest Surg 2008;12:1717-23. [Crossref] [PubMed]
- Trunzo JA, Delaney CP. Natural orifice proctectomy using a transanal endoscopic microsurgical technique in a porcine model. Surg Innov 2010;17:48-52. [Crossref] [PubMed]
- Rieder E, Spaun GO, Khajanchee YS, et al. A natural orifice transrectal approach for oncologic resection of the rectosigmoid: an experimental study and comparison with conventional laparoscopy. Surg Endosc 2011;25:3357-63. [Crossref] [PubMed]
- Telem DA, Han KS, Kim MC, et al. Transanal rectosigmoid resection via natural orifice translumenal endoscopic surgery (NOTES) with total mesorectal excision in a large human cadaver series. Surg Endosc 2013;27:74-80. [Crossref] [PubMed]
- McLemore EC, Coker AM, Devaraj B, et al. TAMIS-assisted laparoscopic low anterior resection with total mesorectal excision in a cadaveric series. Surg Endosc 2013;27:3478-84. [Crossref] [PubMed]
- Sylla P, Rattner DW, Delgado S, et al. NOTES transanal rectal cancer resection using transanal endoscopic microsurgery and laparoscopic assistance. Surg Endosc 2010;24:1205-10. [Crossref] [PubMed]
- Sylla P, Bordeianou LG, Berger D, et al. A pilot study of natural orifice transanal endoscopic total mesorectal excision with laparoscopic assistance for rectal cancer. Surg Endosc 2013;27:3396-405. [Crossref] [PubMed]
- Burke JP, Martin-Perez B, Khan A, et al. Transanal total mesorectal excision for rectal cancer: early outcomes in 50 consecutive patients. Colorectal Dis 2016;18:570-7. [Crossref] [PubMed]
- McLemore EC, Harnsberger CR, Broderick RC, et al. Transanal total mesorectal excision (taTME) for rectal cancer: a training pathway. Surg Endosc 2016;30:4130-5. [Crossref] [PubMed]
- Penna M, Whiteford M, Hompes R, et al. Developing and assessing a cadaveric training model for transanal total mesorectal excision: initial experience in the UK and USA. Colorectal Dis 2017;19:476-84. [Crossref] [PubMed]
- Philipson T, Eber M, Lakdawalla DN, et al. An analysis of whether higher health care spending in the United States versus Europe is 'worth it' in the case of cancer. Health Aff (Millwood) 2012;31:667-75. [Crossref] [PubMed]
- Koedam TWA, Veltcamp Helbach M, van de Ven PM, et al. Transanal total mesorectal excision for rectal cancer: evaluation of the learning curve. Tech Coloproctol 2018;22:279-87. [Crossref] [PubMed]
- Lee L, Kelly J, Nassif GJ, et al. Defining the learning curve for transanal total mesorectal excision for rectal adenocarcinoma. Surg Endosc 2020;34:1534-42. [PubMed]
- Moore MJ, Bennett CL. The learning curve for laparoscopic cholecystectomy. The Southern Surgeons Club. Am J Surg 1995;170:55-9. [Crossref] [PubMed]
- Morton JM, Garg T, Nguyen N. Does hospital accreditation impact bariatric surgery safety? Ann Surg 2014;260:504-8; discussion 508-9. [Crossref] [PubMed]
- Jafari MD, Jafari F, Young MT, et al. Volume and outcome relationship in bariatric surgery in the laparoscopic era. Surg Endosc 2013;27:4539-46. [Crossref] [PubMed]
- Smith MD, Patterson E, Wahed AS, et al. Relationship between surgeon volume and adverse outcomes after RYGB in Longitudinal Assessment of Bariatric Surgery (LABS) study. Surg Obes Relat Dis 2010;6:118-25. [Crossref] [PubMed]
- Bastawrous A, Baer C, Rashidi L, et al. Higher robotic colorectal surgery volume improves outcomes. Am J Surg 2018;215:874-8. [Crossref] [PubMed]
- Blondet JJ, Morton JM, Nguyen NT. Hospital Accreditation and Bariatric Surgery: Is It Important? Adv Surg 2015;49:123-9. [Crossref] [PubMed]
- Champion JK, Pories WJ. Centers of Excellence for Bariatric Surgery. Surg Obes Relat Dis 2005;1:148-51. [Crossref] [PubMed]
- Surgeons ACo. Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program. 2019. Available online: https://www.facs.org/search/bariatric-surgery-centers?allresults=. Accessed September 25, 2019.
- Kohn GP, Galanko JA, Overby DW, et al. High case volumes and surgical fellowships are associated with improved outcomes for bariatric surgery patients: a justification of current credentialing initiatives for practice and training. J Am Coll Surg 2010;210:909-18. [Crossref] [PubMed]
- Nguyen NT, Nguyen B, Nguyen VQ, et al. Outcomes of bariatric surgery performed at accredited vs nonaccredited centers. J Am Coll Surg 2012;215:467-74. [Crossref] [PubMed]
- Kwon S, Wang B, Wong E, et al. The impact of accreditation on safety and cost of bariatric surgery. Surg Obes Relat Dis 2013;9:617-22. [Crossref] [PubMed]
- Hafford ML, Van Sickle KR, Willis RE, et al. Ensuring competency: are fundamentals of laparoscopic surgery training and certification necessary for practicing surgeons and operating room personnel? Surg Endosc 2013;27:118-26. [Crossref] [PubMed]
- Sood A, Jeong W, Ahlawat R, et al. Robotic surgical skill acquisition: What one needs to know? J Minim Access Surg 2015;11:10-5. [Crossref] [PubMed]
- Surgery AoPDfCaR. Robotic Colorectal Surgery Training Program. 2019. Available online: http://www.apdcrs.org/wp/member-resources/robotic-colorectal-surgery-training-program/. Accessed September 19, 2019.
- Keller DS, Zaghiyan K, Mizell JS. Use of robotic technology: a survey of practice patterns of the ASCRS Young Surgeons Committee. Tech Coloproctol 2018;22:715-7. [Crossref] [PubMed]
- Breukink S, Pierie J, Wiggers T. Laparoscopic versus open total mesorectal excision for rectal cancer. Cochrane Database Syst Rev 2006;CD005200 [PubMed]
- van der Pas MH, Haglind E, Cuesta MA, et al. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol 2013;14:210-8. [Crossref] [PubMed]
- Fleshman J, Marcello P, Stamos MJ, et al. Focus Group on Laparoscopic Colectomy Education as endorsed by The American Society of Colon and Rectal Surgeons (ASCRS) and The Society of American Gastrointestinal and Endoscopic Surgeons (SAGES). Dis Colon Rectum 2006;49:945-9. [Crossref] [PubMed]
- Targarona EM, Balague C, Pernas JC, et al. Can we predict immediate outcome after laparoscopic rectal surgery? Multivariate analysis of clinical, anatomic, and pathologic features after 3-dimensional reconstruction of the pelvic anatomy. Ann Surg 2008;247:642-9. [Crossref] [PubMed]
- Sohn DK, Park SC, Kim MJ, et al. Feasibility of transanal total mesorectal excision in cases with challenging patient and tumor characteristics. Ann Surg Treat Res 2019;96:123-30. [Crossref] [PubMed]
- Oh SJ, Shin JY. Risk factors of circumferential resection margin involvement in the patients with extraperitoneal rectal cancer. J Korean Surg Soc 2012;82:165-71. [Crossref] [PubMed]
- Li JC, Hon SS, Ng SS, et al. The learning curve for laparoscopic colectomy: experience of a surgical fellow in an university colorectal unit. Surg Endosc 2009;23:1603-8. [Crossref] [PubMed]
- Nelson H, Petrelli N, Carlin A, et al. Guidelines 2000 for colon and rectal cancer surgery. J Natl Cancer Inst 2001;93:583-96. [Crossref] [PubMed]
- Clinical Outcomes of Surgical Therapy Study G. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med 2004;350:2050-9. [Crossref] [PubMed]
- Monson JR, Probst CP, Wexner SD, et al. Failure of evidence-based cancer care in the United States: the association between rectal cancer treatment, cancer center volume, and geography. Ann Surg 2014;260:625-31; discussion 631-2. [Crossref] [PubMed]
- Veltcamp Helbach M, van Oostendorp SE, Koedam TWA, et al. Structured training pathway and proctoring; multicenter results of the implementation of transanal total mesorectal excision (TaTME) in the Netherlands. Surg Endosc 2020;34:192-201. [Crossref] [PubMed]
- Atallah SB, DuBose AC, Burke JP, et al. Uptake of Transanal Total Mesorectal Excision in North America: Initial Assessment of a Structured Training Program and the Experience of Delegate Surgeons. Dis Colon Rectum 2017;60:1023-31. [Crossref] [PubMed]
- Abbott SC, Stevenson ARL, Bell SW, et al. An assessment of an Australasian pathway for the introduction of transanal total mesorectal excision (taTME). Colorectal Dis 2018;20:O1-O6. [Crossref] [PubMed]
- Adamina M, Buchs NC, Penna M, et al. St.Gallen consensus on safe implementation of transanal total mesorectal excision. Surg Endosc 2018;32:1091-103. [Crossref] [PubMed]
- Francis N, Penna M, Mackenzie H, et al. Consensus on structured training curriculum for transanal total mesorectal excision (TaTME). Surg Endosc 2017;31:2711-9. [Crossref] [PubMed]
- Motson RW, Whiteford MH, Hompes R, et al. Current status of trans-anal total mesorectal excision (TaTME) following the Second International Consensus Conference. Colorectal Dis 2016;18:13-8. [Crossref] [PubMed]
- Aquina CT, Probst CP, Becerra AZ, et al. High volume improves outcomes: The argument for centralization of rectal cancer surgery. Surgery 2016;159:736-48. [Crossref] [PubMed]
- Xu Z, Becerra AZ, Justiniano CF, et al. Is the Distance Worth It? Patients With Rectal Cancer Traveling to High-Volume Centers Experience Improved Outcomes. Dis Colon Rectum 2017;60:1250-9. [Crossref] [PubMed]
- Deijen CL, Tsai A, Koedam TW, et al. Clinical outcomes and case volume effect of transanal total mesorectal excision for rectal cancer: a systematic review. Tech Coloproctol 2016;20:811-24. [Crossref] [PubMed]
- Sylla P, Knol JJ, D'Andrea AP, et al. Urethral Injury and Other Urologic Injuries During Transanal Total Mesorectal Excision: An International Collaborative Study. Ann Surg 2019; [Epub ahead of print]. [Crossref] [PubMed]
- Wasmuth HH, Faerden AE, Myklebust TA, et al. Transanal total mesorectal excision for rectal cancer has been suspended in Norway. Br J Surg 2020;107:121-30. [Crossref] [PubMed]
- de’Angelis N, Portigliotti L, Azoulay D, et al. Transanal total mesorectal excision for rectal cancer: a single center experience and systematic review of the literature. Langenbecks Arch Surg 2015;400:945-59. [Crossref] [PubMed]
- Lacy AM, Tasende MM, Delgado S, et al. Transanal Total Mesorectal Excision for Rectal Cancer: Outcomes after 140 Patients. J Am Coll Surg 2015;221:415-23. [Crossref] [PubMed]
- Lelong B, de Chaisemartin C, Meillat H, et al. A multicentre randomised controlled trial to evaluate the efficacy, morbidity and functional outcome of endoscopic transanal proctectomy versus laparoscopic proctectomy for low-lying rectal cancer (ETAP-GRECCAR 11 TRIAL): rationale and design. BMC Cancer 2017;17:253. [Crossref] [PubMed]
- Lelong B, Meillat H, Zemmour C, et al. Short- and Mid-Term Outcomes after Endoscopic Transanal or Laparoscopic Transabdominal Total Mesorectal Excision for Low Rectal Cancer: A Single Institutional Case-Control Study. J Am Coll Surg 2017;224:917-25. [Crossref] [PubMed]
- Veltcamp Helbach M, Deijen CL, Velthuis S, et al. Transanal total mesorectal excision for rectal carcinoma: short-term outcomes and experience after 80 cases. Surg Endosc 2016;30:464-70. [Crossref] [PubMed]
- Hol JC, van Oostendorp SE, Tuynman JB, et al. Long-term oncological results after transanal total mesorectal excision for rectal carcinoma. Tech Coloproctol 2019;23:903-11. [Crossref] [PubMed]
- Berends FJ, Kazemier G, Bonjer HJ, et al. Subcutaneous metastases after laparoscopic colectomy. Lancet 1994;344:58. [Crossref] [PubMed]
- Zanghì A, Cavallaro A, Piccolo G, et al. Dissemination metastasis after laparoscopic colorectal surgery versus conventional open surgery for colorectal cancer: a metanalysis. Eur Rev Med Pharmacol Sci 2013;17:1174-84. [PubMed]
Cite this article as: Peyser D, Hersh EH, Sylla P. Training and accreditation in transanal total mesorectal excision in the United States. Ann Laparosc Endosc Surg 2020;5:26.


