
Important outcomes for transanal total mesorectal excision in a Canadian population after using transanal minimally invasive surgery (flexible) or transanal endoscopic microsurgery (rigid) platforms
Introduction
Access to the pelvis for radical excision of the rectum continues to pose a technical challenge for surgeons. The description of the mesorectal plane by Professor Heald represents the most important advancement impacting technical standards and oncologic outcomes for patients undergoing surgery for rectal cancer (1). Transanal total mesorectal excision (TaTME) was first described by Sylla et al. using the transanal endoscopic microsurgery (TEMS) rigid platform designed by professor Buess et al.; this new technique may facilitate a minimally invasive approach in patients where laparoscopy would not otherwise be possible (2,3). A contemporary alternative to the TEMS platform is the flexible transanal minimally invasive surgery (TAMIS) port developed and introduced by Atallah et al. (4). Both devices have been extensively used for local excision of early neoplastic lesions in the rectum (5,6).
Nearly 10 years after its initial description, registry and case series data suggest TaTME has similar short term oncologic outcomes and potential to overcome some of the technical challenges of conventional approaches (7-11). Regardless of the platform, TaTME is technically demanding with a prolonged learning curve (9,12-14). In contrast to its perceived benefits, it has become apparent that TaTME carries a significant potential for devastating injuries that were not previously common in rectal cancer surgery (12-14).
While both TEMS and TAMIS devices are used to perform TaTME surgery, there are no comparative studies to elucidate whether one platform leads to better results over the other. The objective of the current study was to compare the perioperative outcomes of patients undergoing TaTME surgery when using the flexible (TAMIS) versus the rigid (TEMS) platforms at two high volume specialized rectal cancer surgery centers in Canada.
Methods
Patients
Demographic, operative, pathologic and follow up data for all patients treated by TaTME technique at St. Paul’s Hospital and Health Sciences North were independently and prospectively collected and maintained in separate databases at each institution since the time of inception (April 2014 and Jun 2015 respectively). After a data alignment strategy was developed, both databases were merged and queried for this study. Both hospitals had accumulated significant experience on transanal endoscopic surgery (TES) with their respective platforms, prior to introducing the TaTME procedure (15,16). Approval of the study protocol from the local ethics review board (ERB) was obtained at both institutions.
For inclusion criteria, only patients older than 18 years of age with a diagnosis of neoplasia of the rectum confirmed by histopathology were considered. Patients included required radical resection of the rectum and were offered TaTME at one of the two participating centers during the study period (2014 to 2018). Excluded from this study were patients treated by TaTME for diagnoses other than rectal neoplasia (e.g., ulcerative colitis, Crohn’s disease) or patients with incomplete data regarding the surgical intervention, the primary outcome or relevant covariate data despite secondary chart review (Figure 1).
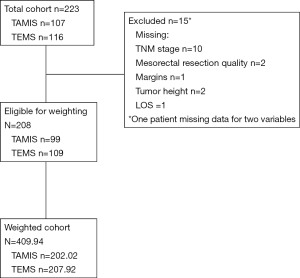
Preoperative evaluation included complete colonoscopy with focused endoscopic assessment and biopsy of the lesion, CT scan of the chest, abdomen and pelvis, regional pelvic MRI, and carcinoembryonic antigen (CEA) level. All cases were presented at multidisciplinary cancer conference (MCC) and those requiring neoadjuvant therapy were referred to the local cancer center. Restaging with repeat MRI was performed at the discretion of the managing team. Timing of surgery was determined according to accepted standards (17,18), and the multidisciplinary conference (MCC) recommendation. Those who did not require pre-operative chemo-radiation went straight for surgery. All operations were performed by subspecialty trained colorectal surgeons using a single-team (sequential) approach (19). Patients underwent full bowel preparation and were enrolled in multimodality perioperative ERAS interventions according to institutional protocols.
Outcomes measures
The primary outcome measure of this cohort study was the presence of good quality mesorectal specimens (complete/near complete). Completeness of the mesorectum was scored by specialized pathologists as per the standardized mesorectal grading system (20). In the recent ACOSOG Z6051 trial acceptable rates for good quality mesorectal specimens were determined to be 81.7% and 86.9% for open and laparoscopic surgery, respectively (21). Secondary outcomes of interest were rates of uninvolved circumferential radial margin (CRM), defined by a minimum of 1 mm clearance from any identified tumour from the mesorectal margin, and distal resection margin (DRM), defined by the absence of cancer cells at the distal margin of the specimen.
Other outcomes evaluated include perioperative morbidity (within 30 days of surgery), conversion rate, operative time, length of stay (LOS) and hospital readmission. Anastomotic leak was defined as clinical evidence of dehiscence, given by pelvic pain and/or signs of sepsis, with radiologic or endoscopic confirmation of anastomotic disruption required.
Statistical analysis
Propensity scores (i.e., the probability of TAMIS compared to TEMS given a set of baseline patient characteristics) were calculated using logistic regression analysis with age, tumor height, body mass index (BMI), neoadjuvant chemotherapy, neoadjuvant radiotherapy, and TNM stage as covariates, and treatment group (TAMIS or TEMS) as the outcome. Scores were then transformed to inverse probability of treatment weights (IPTW) (22) and subsequent IPTW were truncated based on values of their 1% and 99% quantiles. This allows estimation of the average treatment effect (ATE) after eliminating potential confounding of the included covariates. However, this method does not account for unmeasured confounders.
Most baseline characteristics were unbalanced (d ≥10%) between TAMIS and TEMS groups in the original study cohort. Covariate balance between treatment groups was assessed before and after weighting using standardized differences (d); because hypothesis testing is dependent on sample size, standardized differences are preferred for assessing covariate balance (23) where d ≥10% indicates a clinically relevant difference that requires further balancing (23,24). Adequate balance (d <10%) was achieved for all covariates after weighting using the IPTW approach. Patient and tumor characteristics are shown on Table 1. Analysis of the association between surgical procedures (TAMIS or TEMS) with binary outcome variables was conducted using weighted chi-square tests. For continuous outcomes, we used weighted quantile regression to accommodate weighted median testing. Results were considered statistically significant where P<0.05. For binary outcomes, we also calculated relative risks (RR) and their 95% confidence intervals (CI). All analyses were performed using SAS v9.4 software (SAS Institute, Carry NC).
Table 1
| Characteristic | Pre-weight | Post-weight | |||||
|---|---|---|---|---|---|---|---|
| TAMIS (n=99) | TEMS (n=109) | d, % | TAMIS (n=202.02) | TEMS (n=207.92) | d, % | ||
| Age (years), mean ± SD | 64.31±10.12 | 61.26±12.07 | 27.38 | 62.93±15.10 | 62.66±15.93 | 1.74 | |
| Tumor height (cm), mean ± SD | 6.18±2.45 | 6.13±2.62 | 1.97 | 6.16±3.49 | 6.17±3.62 | 0.28 | |
| BMI, mean ± SD | 27.68±6.93 | 27.58±5.61 | 1.59 | 27.32±9.89 | 27.58±7.28 | 2.99 | |
| Male, n (%) | 72 (72.73) | 71 (65.14) | 16.46 | 132.93 (65.80) | 140.88 (67.76) | 4.16 | |
| TNM stage I, n (%) | 15 (15.15) | 35 (32.11) | 40.74 | 51.54 (25.51) | 50.12 (24.11) | 3.24 | |
| TNM stage II, n (%) | 27 (27.27) | 25 (22.94) | 10.0 | 51.15 (25.32) | 52.10 (25.06) | 0.60 | |
| TNM stage III, n (%) | 49 (49.49) | 46 (42.20) | 14.67 | 88.37 (43.74) | 95.04 (45.71) | 3.96 | |
| TNM stage IV, n (%) | 8 (8.08) | 3 (2.75) | 23.72 | 10.97 (5.43) | 10.66 (5.13) | 1.39 | |
| Neoadjuvant chemotherapy, n (%) | 66 (66.67) | 50 (45.87) | 42.88 | 114.59 (56.72) | 116.22 (55.90) | 1.67 | |
| Neoadjuvant radiotherapy, n (%) | 70 (70.71) | 67 (61.47) | 19.61 | 126.14 (62.44) | 129.66 (62.36) | 0.17 | |
d, standardized difference (in percent %). SD, standard deviation; BMI, body mass index; TAMIS, transanal minimally invasive surgery; TEMS, transanal endoscopic microsurgery.
A sensitivity analysis on the outcomes for the operators (two) with the largest number of cases on each platform was conducted on data beyond the learning curve (40 cases) as previously calculated by Koedam et al. (25) Available data resulted on an experience of n=61 vs. n=27 cases respectively per TAMIS vs. TEMS surgeon. For this analysis we used a weighted chi-square test where samples were big enough and Fisher’s exact test for small samples. For the sensitivity analysis unweighted sample (no adjustment for cohort differences) were used, due to the size of the samples.
Results
Between March 2014 and October 2018, 223 patients treated by TaTME were identified at the two participating centers (Figure 1). After exclusion criteria were applied, a total study cohort of 208 patients were included for analysis and two cohorts established: TAMIS (n=99) and TEMS (n=109).
After weighting the sample using IPTW, estimation of effects for surgical procedure on outcomes indicated a significant difference (P=0.019) for intraoperative complications where the TAMIS group was at lower risk of experiencing complications during the procedure compared to those undergoing TEMS (RR 0.353, 95% CI: 0.142–0.880). In the TAMIS group, 82.4% had a complete specimen vs. 87.1% in the TEMS, the near complete specimens were 16.5% and 6.3% respectively. When measured together the rate of complete/near complete was 98.9% for TAMIS and 91.5% for TEMS. No significant effects were observed for positive margins, postoperative complications, anastomotic leak, or readmission. There were no patients in the TAMIS group who experienced conversion, so hypothesis testing could not be accommodated in the weighted sample. Despite this caveat, the risk of conversion was lower in the TAMIS group compared to the TEMS group with regard to the overall proportions (Table 2). A higher proportion of patients in the TAMIS group underwent primary abdominoperineal resection (APR) 17% vs. 6% for the TEMS. For those that underwent restoration of bowel continuity, a stapled anastomosis was slightly more prevalent in the TAMIS group and transabdominal extraction more frequent in the TEMS group (Table 3).
Table 2
| Outcome | TAMIS, % | TEMS, % | P | RR (95% CI) | β (95% CI) |
|---|---|---|---|---|---|
| Mesorectal resection | 0.001 | ||||
| Incomplete | 1.12 | 8.54 | 0.131 (0.033–0.516) | – | |
| Complete/near complete | 98.88 | 91.46 | |||
| Margins | 0.656 | ||||
| CRM positive | 2.34 | 3.05 | 0.766 (0.237–2.480) | – | |
| CRM negative | 97.66 | 96.95 | |||
| Intraoperative complications | 0.019 | ||||
| Yes | 2.94 | 8.31 | 0.353 (0.142–0.880) | – | |
| No | 97.06 | 91.69 | |||
| Postoperative complications | 0.296 | ||||
| Yes | 37.76 | 42.82 | 0.882 (0.696–1.117) | – | |
| No | 62.24 | 57.18 | |||
| Anastomotic leak | 0.051 | ||||
| Yes | 7.90 | 13.91 | 0.568 (0.318–1.014) | – | |
| No | 92.10 | 86.09 | |||
| Readmission | 0.201 | ||||
| Yes | 15.72 | 20.60 | 0.763 (0.503–1.157) | – | |
| No | 84.28 | 79.40 | |||
| Conversion* | – | ||||
| Yes | 0.0 | 11.80 | – | – | |
| No | 100.0 | 88.20 | |||
| OR time (minutes), median [IQR] | 312 [92] | 269 [94] | 0.003 | – | 43.0 (14.498 to 71.502) |
| Length of stay (days), median [IQR] | 3 [2] | 6 [6] | <0.001 | −3.0 (−4.134 to −1.866) |
*, conversion outcomes were all negative for TAMIS group; weighted hypothesis testing could not be accommodated. OR, operating room; IQR, interquartile range; P, probability; RR, relative risk; CI, confidence interval; β, estimate (median difference); TAMIS, transanal minimally invasive surgery; TEMS, transanal endoscopic microsurgery.
Table 3
| Surgical characteristics | Pre-weight | Post-weight | |||
|---|---|---|---|---|---|
| TAMIS | TEMS | TAMIS | TEMS | ||
| Anastomosis, n (%) | |||||
| Abdominoperineal reconstruction | 17 (17.17) | 6 (5.50) | 29.83 (14.77) | 11.0 (5.29) | |
| Stapled | 71 (71.72) | 69 (63.30) | 146.32 (72.43) | 131.42 (63.21) | |
| Hand sewn | 11 (11.11) | 34 (31.19) | 25.86 (12.80) | 65.49 (31.50) | |
| Extraction site, n (%) | |||||
| Transanal | 51 (51.52) | 17 (15.60) | 114.02 (56.44) | 30.56 (14.70) | |
| Transabdominal | 47 (47.47) | 85 (77.98) | 86.83 (42.98) | 165.02 (79.37) | |
| Other | 1 (1.01) | 1 (0.92) | 1.17 (0.58) | 1.49 (0.71) | |
| Not documented | 0 (0.00) | 6 (5.50) | 0 (0.00) | 10.86 (5.22) | |
TAMIS, transanal minimally invasive surgery; TEMS, transanal endoscopic microsurgery.
When analyzing continuous outcomes for median differences, the TAMIS group showed longer OR time (median difference 43.0, 95% CI: 14.498–71.502). The incidence of intraoperative complications was lower for TAMIS, the difference was not significant between groups. Most complications were related to bleeding from the presacral space or the side walls. One case of hypercarbia was identified in the TEMS group requiring a break to reduce the levels of CO2.
Postoperative morbidity was similar in both groups 37.5% vs. 42.6% (P=0.286) and no significant difference was identified (RR 0.808, 95% CI: 0.547–1.195). Other than leaks, urinary retention, ileus and high output from the ileostomy were prevalent. Anastomotic leak was identified on 7.90% vs. 13.91% (P=0.051) for TAMIS vs. TEMS (RR 0.568, 95% CI: 0.318–1.014). Patients in the TAMIS group experienced a shorter LOS (median difference −3.0, 95% CI: −4.134 to −1.866). There was no significant difference in hospital readmission between groups 15.72% vs. 20.60% (P=0.201) (Table 2).
Subgroup sensitivity analysis
Data from the two surgeons beyond 40 cases was used to perform a subgroup analysis of the results. This demonstrated no significant difference for most outcomes, including intraoperative complications [n=0 for TAMIS vs. n=2 (7.4%) for TEMS, P=0.092] Incomplete specimens [n=1 for TAMIS (1.6%) vs. n=1 for TEMS (3.7%), P=0.522] and positive CRM [n=2 TAMIS (3.3%) vs. n=2 TEMS (7.4%), P=0.583]. The significant differences corresponded to anastomotic leaks [n=1 TAMIS (1.6%) vs. n=4 TEMS (14.8%), P=0.029] and conversions TAMIS [n=0 conversion (0%) and TEMS n=2 (7.4%), P=0.092].
Postoperative complications were weighted (IPTW) TAMIS 32.14% and TEMS had 38.66%, weighted chi-square test P=0.370. Readmission was weighted (IPTW) TAMIS 11.82% and TEMS 31.49%, weighted chi-square test P=0.002.
Discussion
Minimally invasive rectal cancer surgery is a technical challenge for surgeons, mainly due to the difficult access to the pelvis and the number of close critical structures necessary to preserve in this narrow space (26). The introduction of TEMS in the early 80’s provided a great alternative for local excision of early lesions (2). The development of the flexible TAMIS platform has made transanal surgery more accessible to surgeons and has likely facilitated the rapid development of the TaTME technique (4). TaTME has been adopted by many surgeons worldwide using both flexible and rigid platforms (7,11,27-30).
There are clinical studies comparing the use of TAMIS vs. TEMS on patients undergoing local excision, results showed no difference in performance (5,31). TaTME is a more technically challenging procedure than local excision. To our knowledge, there are no comparative studies of TAMIS vs. TEMS ports for this procedure. In 2015, Kim et al. compared the performance of TaTME with flexible vs. rigid platforms using cadaveric models (32). The authors concluded that both devices are feasible for the procedure. According to their publication, some of the attributes of the flexible platform include a short set-up time, relatively atraumatic insertion and easy application. However, a narrow operative field is considered the main limiting factor. Conversely, the rigid platform offers a larger and more stable operative field, the rigidity of the channel, long time set-up and the narrow view were listed as limiting factors. In this study the authors concluded that both pieces of equipment are equivalent
Our study focused on the quality of surgical specimens and margin positivity (Table 2). These outcomes are well established surrogates for local recurrence and survival in rectal cancer (20,33-35). We identified a higher likelihood of achieving a complete specimen when using the TEMS platform. However, complete and near complete pathologic TME specimens share a similar low risk of local recurrence (34-36). When considered together, our TAMIS cohort demonstrated a statistically significant advantage when used for TaTME.
While we have used propensity scores to account for the numerous clinically measurable differences between the groups, we are cautious in overinterpreting these results. The unmeasured differences between the centers [e.g., referral pattern, patient selection, surgeon(s), pathologists, etc.] likely account for much of the variance observed. While the subgroup analysis of the post-learning curve data is a better comparison, it is underpowered to categorically confirm our finding that the two platforms are equivalent. We are reassured by the similar “good TME” rates observed in both cohorts when compared to established benchmarks from the previously published RCTs comparing laparoscopic and open TME (21,37,38).
In TaTME, intraoperative injuries rarely observed in conventional TME surgery have been described: urethral injury (13) and neurovascular bundle of Walsh damage (12). In this study, there were no major intraoperative injuries. The incidence of other complications were similar to the established benchmarks for TME surgery and not significantly different between groups (39).
Other variables analysed included operative time, conversion rate, type of reconstruction, extraction site and length of stay. Those may depend on different factors other than the type of platform utilized in surgery. Conversion rate is also variable depending on the judgement of the operating surgeon as well as site of extraction and perhaps type of reconstruction. LOS might be affected by cultural and social reasons or surgeon’s preference, despite implementation of ERAS protocols.
This study has limitations that we recognize. There were numerous differences in the baseline characteristics of the groups. We used IPTW techniques to apply sample weights to each patient this is a well stablished methodology resulting in “an artificial population in which baseline covariates are independent of treatment status” (22) in order to account for some of these measurable differences. However, there are likely many immeasurable differences in surgical practice and patient selection between the two groups. Also we acknowledge that having 1 surgeon in the TAMIS group, limits generalizability, however these results are similar to those from authors who have reported excellent outcomes with the flexible platform (25,27). The procedures were performed at two high volume centers by specialized colorectal surgeons which can also limit the ability to generalize the results.
We conclude that short term outcomes of TaTME appear to be safe and adequate regardless of the platform chosen by the surgeon/institution. While we did demonstrate a difference in the short-term oncologic outcomes between the platforms, we feel that these differences should be evaluated in a larger, multicenter study. Most importantly, we demonstrate that surgeons using both platforms can achieve similar short-term oncologic results to those considered the current benchmark for rectal cancer surgery with acceptable complication rates.
Currently, there are other devices, such as robotic platforms that are continuously evolving in an attempt to improve access to the pelvis, however limitations still exist regarding superiority of any technology (21,37,40).
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2020.01.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Approval of the study protocol from the local ethics review board (ERB) was obtained at both institutions. Written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Heald RJ, Husband EM, Ryall RDH. The mesorectum in rectal cancer surgery—the clue to pelvic recurrence? Br J Surg 1982;69:613-6. [Crossref] [PubMed]
- Buess G, Theiss R, Hutterer F, et al. Transanal endoscopic surgery of the rectum - testing a new method in animal experiments. Leber Magen Darm 1983;13:73-7. [PubMed]
- Sylla P, Rattner DW, Delgado S, et al. NOTES transanal rectal cancer resection using transanal endoscopic microsurgery and laparoscopic assistance. Surg Endosc 2010;24:1205-10. [Crossref] [PubMed]
- Atallah S, Albert M, Larach S. Transanal minimally invasive surgery: a giant leap forward. Surg Endosc 2010;24:2200-5. [Crossref] [PubMed]
- Lee L, Edwards K, Hunter IA, et al. Quality of Local Excision for Rectal Neoplasms Using Transanal Endoscopic Microsurgery Versus Transanal Minimally Invasive Surgery. Dis Colon Rectum 2017;60:928-35. [Crossref] [PubMed]
- O’Neill CH, Platz J, Moore JS, et al. Transanal Endoscopic Microsurgery for Early Rectal Cancer. Dis Colon Rectum 2017;60:152-60. [Crossref] [PubMed]
- Penna M, Hompes R, Arnold S, et al. Incidence and Risk Factors for Anastomotic Failure in 1594 Patients Treated by Transanal Total Mesorectal Excision: Results From the International TaTME Registry. Ann Surg. 2019;269:700-11. [Crossref] [PubMed]
- Penna M, Hompes R, Arnold S, et al. Transanal Total Mesorectal Excision. Ann Surg 2017;266:111-7. [Crossref] [PubMed]
- Westwood DA, Cuda TJ, Hamilton AER, et al. Transanal total mesorectal excision for rectal cancer: state of the art. Tech Coloproctol 2018;22:649-55. [Crossref] [PubMed]
- van Oostendorp SE, Koedam TWA, Sietses C, et al. Transanal total mesorectal excision compared to laparoscopic TME for mid and low rectal cancer—current evidence. Ann Laparosc Endosc Surg 2018;3:41-1. [Crossref]
- Ma B, Gao P, Song Y, et al. Transanal total mesorectal excision (TaTME) for rectal cancer: a systematic review and meta-analysis of oncological and perioperative outcomes compared with laparoscopic total mesorectal excision. BMC Cancer 2016;16:380. [Crossref] [PubMed]
- Atallah S, Albert M. The neurovascular bundle of Walsh and other anatomic considerations crucial in preventing urethral injury in males undergoing transanal total mesorectal excision. Tech Coloproctol 2016;20:411-2. [Crossref] [PubMed]
- Kneist W, Stelzner S, Aigner F, et al. Urethral injury in body donor TaTME training. Coloproctology 2017;39:179-83. [Crossref]
- Complications of minimal invasive surgery for rectal cancer - a systematic review. Available online: https://zenodo.org/record/1040866#.WygfwRJKh24
- Brown C, Raval MJ, Phang PT, et al. The surgical defect after transanal endoscopic microsurgery: open versus closed management. Surg Endosc 2017;31:1078-82. [Crossref] [PubMed]
- Caycedo-Marulanda A, Jiang HY, Kohtakangas EL. Transanal minimally invasive surgery for benign large rectal polyps and early malignant rectal cancers: experience and outcomes from the first Canadian centre to adopt the technique. Can J Surg 2017;60:416-23. [Crossref] [PubMed]
- Gad Z, Gareer W, El Hossieny H, et al. Optimal timing of surgery after neoadjuvant chemo-radiation therapy in locally advanced rectal cancer. Eur J Surg Oncol 2016;42:S87. [Crossref]
- Sloothaak DAM, Geijsen DE, van Leersum NJ, et al. Optimal time interval between neoadjuvant chemoradiotherapy and surgery for rectal cancer. Br J Surg 2013;100:933-9. [Crossref] [PubMed]
- Caycedo-Marulanda A, Ma G, Ashamalla S. Single-Team TaTME. In: Atallah S, editor. Transanal Minimally Invasive Surgery (TAMIS) and Transanal Total Mesorectal Excision (taTME). Springer, 2019:229-43.
- Nagtegaal ID, van de Velde CJH, van der Worp E, et al. Macroscopic Evaluation of Rectal Cancer Resection Specimen: Clinical Significance of the Pathologist in Quality Control. J Clin Oncol 2002;20:1729-34. [Crossref] [PubMed]
- Fleshman J, Branda M, Sargent DJ, et al. Effect of Laparoscopic-Assisted Resection vs Open Resection of Stage II or III Rectal Cancer on Pathologic Outcomes: The ACOSOG Z6051 Randomized Clinical Trial. JAMA 2015;314:1346-55. [Crossref] [PubMed]
- Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med 2015;34:3661-79. [Crossref] [PubMed]
- Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res 2011;46:399-424. [Crossref] [PubMed]
- Normand ST, Landrum MB, Guadagnoli E, et al. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: A matched analysis using propensity scores. J Clin Epidemiol 2001;54:387-98. [Crossref] [PubMed]
- Koedam TWA, Veltcamp Helbach M, van de Ven PM, et al. Transanal total mesorectal excision for rectal cancer: evaluation of the learning curve. Tech Coloproctol 2018;22:279-87. [Crossref] [PubMed]
- Kawada K, Sakai Y. Can we predict surgical difficulty of rectal surgery? Ann Laparosc Endosc Surg 2018;3:44. [Crossref]
- Lacy AM, Tasende MM, Delgado S, et al. Transanal Total Mesorectal Excision for Rectal Cancer: Outcomes after 140 Patients. J Am Coll Surg 2015;221:415-23. [Crossref] [PubMed]
- Perdawood SK, Khefagie Al GAA. Transanal vs. laparoscopic total mesorectal excision for rectal cancer: initial experience from Denmark. Colorectal Dis 2016;18:51-8. [Crossref] [PubMed]
- Abbott SC, Stevenson ARL, Bell SW, et al. An assessment of an Australasian pathway for the introduction of transanal total mesorectal excision (TaTME). Colorectal Dis 2018;20:O1-O6. [Crossref] [PubMed]
- Caycedo-Marulanda A, Ma G, Jiang HY. Transanal total mesorectal excision (taTME) in a single-surgeon setting: refinements of the technique during the learning phase. Tech Coloproctol 2018;22:433-43. [Crossref] [PubMed]
- Mege D, Bridoux V, Maggiori L, et al. What is the best tool for transanal endoscopic microsurgery (TEM)? A case-matched study in 74 patients comparing a standard platform and a disposable material. Int J Colorectal Dis 2017;32:1041-5. [Crossref] [PubMed]
- Kim MJ, Park JW, Ha HK, et al. Initial experience of transanal total mesorectal excision with rigid or flexible transanal platforms in cadavers. Surg Endosc 2016;30:1640-7. [Crossref] [PubMed]
- Birbeck KF, Macklin CP, Tiffin NJ, et al. Rates of Circumferential Resection Margin Involvement Vary Between Surgeons and Predict Outcomes in Rectal Cancer Surgery. Ann Surg 2002;235:449-57. [Crossref] [PubMed]
- Quirke P, Steele R, Monson J, et al. Effect of the plane of surgery achieved on local recurrence in patients with operable rectal cancer: a prospective study using data from the MRC CR07 and NCIC-CTG CO16 randomised clinical trial. Lancet 2009;373:821-8. [Crossref] [PubMed]
- García-Granero E, Faiz O, Muñoz E, et al. Macroscopic assessment of mesorectal excision in rectal cancer. Cancer 2009;115:3400-11. [Crossref] [PubMed]
- Song SB, Wu GJ, Pan HD, et al. The quality of total mesorectal excision specimen: A review of its macroscopic assessment and prognostic significance. Chronic Dis Transl Med 2018;4:51-8. [Crossref] [PubMed]
- Stevenson ARL, Solomon MJ, Lumley JW, et al. Effect of Laparoscopic-Assisted Resection vs. Open Resection on Pathological Outcomes in Rectal Cancer. JAMA 2015;314:1356-63. [Crossref] [PubMed]
- van der Pas MH, Haglind E, Cuesta MA, et al. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol 2013;14:210-8. [Crossref] [PubMed]
- Park EJ, Baik SH, Kang J, et al. The Impact of Postoperative Complications on Long-term Oncologic Outcomes After Laparoscopic Low Anterior Resection for Rectal Cancer. Medicine 2016;95:e3271-9. [Crossref] [PubMed]
- Jayne D, Pigazzi A, Marshall H, et al. Effect of Robotic-Assisted vs. Conventional Laparoscopic Surgery on Risk of Conversion to Open Laparotomy Among Patients Undergoing Resection for Rectal Cancer. JAMA 2017;318:1569-80. [Crossref] [PubMed]
Cite this article as: Caycedo-Marulanda A, Karimuddin A, Caswell J, Raval M, Conlon M, Phang T, Brown C. Important outcomes for transanal total mesorectal excision in a Canadian population after using transanal minimally invasive surgery (flexible) or transanal endoscopic microsurgery (rigid) platforms. Ann Laparosc Endosc Surg 2020;5:12.


