A current review of robotic colorectal surgery
Minimally invasive colorectal surgery: the framework and history
Over the last 30 years, colorectal surgery has seen significant advancement in minimally invasive surgery (MIS) techniques. The earliest case reports of laparoscopic colon resection were published in in the early 1990’s (1-3) and laparoscopic volume has steadily increased over the last 30 years to represent 40–50% of all colorectal resection for both benign and malignant disease (4,5). After Heald’s landmark description of the “holy plane” for total mesorectal excision (TME) for rectal cancer (6), early adopters of laparoscopy pioneered the laparoscopic approach to rectal resection (7,8). Since then, several landmark studies have confirmed equivalent, non-inferior or improved short and long term perioperative and oncologic outcomes of laparoscopy when compared to traditional open resection (9-15). Laparoscopic MIS techniques have shown to result in earlier return of bowel function, earlier initiation of diet, shorter index hospitalization length of stay, decreased postoperative pain, and improved cosmesis (10,16). With advancements in laparoscopic platforms, instruments, technical skill and early training during surgical residency, laparoscopy has become, in many instances, the default surgical approach for many surgeons within the abdominal cavity.
In the dawn of the new millennium, robotic surgery made its first legitimate appearance as a novel technique in minimally invasive abdominal surgery, and in July, 2,000 robotic surgery was FDA-approved for abdominal, general surgery procedures (17). The use of robotics in pelvic surgery, especially in a nerve-sparing manner within the narrow pelvis, was championed early-on by our urologic and gynecologic colleagues (18,19). In 2009 85% of all robotic cases were limited to urologic and gynecologic procedures (20) and by 2012, 75% of all radical prostatectomies were performed robotically (21).
The first robotic colorectal series was published in 2002 (22), treating benign colon and sigmoid pathologies. Soon thereafter, various national and international groups began publishing their own data comparing robotic and laparoscopic colorectal surgery (23-25). Not surprisingly, these were non-randomized, retrospective, singe institution experiences which did not differentiate benign and malignant pathology with small sample sizes.
In 2006, the first case-series describing robotic, oncologic low anterior resections (LAR) with TME was published (26). The authors reported no significant differences in perioperative clinical outcomes or pathologic assessment within their small sample size when compared to conventional laparoscopic LAR with TME. Despite the paucity of data at the time, the technical advantages of the robotic platform over laparoscopy, especially within in the narrow pelvis, were often cited by proponents of the new instrumentation, including the seven degrees of movement with micro-wristed instruments, magnified 3-D view with lack of hand-tremor, a natural hand-eye target axis and ergonomic comfort (27-29).
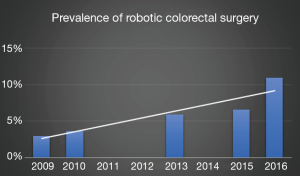
From 2010 to 2012 the prevalence of robotics within U.S. hospital systems rose from 20% to 27% according to data compiled from the American Hospital Association (30), with the highest density of robotic platforms centralized in metropolitan, teaching hospitals that provide inpatient, oncologic surgery. There has been a steady annual increase in robotic colorectal surgery to almost 11% of all colorectal resections in 2016 (31) (Figure 1).
Concomitantly, marketing and advertising for robotics increased steeply with 86% of 400 randomly selected U.S. hospitals that offered robotic surgery publishing statements of clinical superiority of robotics hospital websites (35). When surveyed on the perceived benefits of robotic surgery over laparoscopy, patients, administrators and non-surgeon providers demonstrated several assumed conclusions (and misconceptions) as to the benefits (perioperative outcomes, oncologic outcomes, etc.) of robotic surgery which were largely based on an expected correlation to a new and advanced technology (36).
The MIS learning curve: from laparoscopy to robotics
With robotic colorectal surgery increasing year by year (30,33,37) and with its increasing popularity, new and experienced surgeons alike must face a steep learning curve—the process of becoming proficient, safe and independent—with this relatively novel surgical technique. Facing this robotic learning curve, however, is not an unfamiliar process for the practicing minimally invasive surgeon who also faced a learning curve when developing their laparoscopic skills. Tekkis et al. evaluated the learning curve for laparoscopic left-sided and right-sided resections using multidimensional rubric that included operative time, conversion to open surgery, readmission rates and postoperative complications (38). The authors calculated that 55 cases were necessary to gain proficiency for right-sided resections while left-sided resections necessitated 62 cases. Park et al. using similar multidimensional methods, found the learning curve for proficient laparoscopic rectal resection is 90 cases (39).
The available knowledge on the robotic learning curve, however, should be approached with an a priori understanding that many first-time robotics users have a foundation in minimally invasive and laparoscopic techniques. Since 2008, General Surgery trainees have been required to demonstrate competence in fundamental laparoscopy by successfully completing the Fundamental of Laparoscopic Surgery (FLS) course and are required to complete 100 basic- and 75 complex-laparoscopic cases as a prerequisite to sit for the American Board of Surgery (ABS) certifying exam (40,41). Additionally, with the aforementioned technical improvements in the robotic platform, such as 3-D visualization, degrees of freedom of wristed instruments, etc. the proposed advantages to robotics should conceivably offer a more rapidly advanced skill-progression to the surgeon with a strong MIS foundation (28). However, even with these ergonomic and technical advantages and advances, there continue to be real limitations to immediate adoption, including a loss of haptic feedback, limited range of motion of the robotic arms, inability to reposition the surgical bed while docked (although the option/upgrade is available) and continued need for skilled (and trained) bedside assistance that a new user must overcome.
Demographic data on current robotics users has shown that younger surgeons (0–10 years out from training), board certified colorectal surgeons and high-volume surgeons are more likely to be performing robotic surgery (42). With colorectal surgery fellows expressing increased need for more robotic experience within their training curriculum (43), understanding the expected learning curve is an important aspect of developing new training curricula. Jimenez-Rodriguez et al. performed a meta-analysis of the currently available, reliable data pertaining to the learning curve of robotic rectal cancer surgery and calculated the mean number of cases required for surgeon to be classified as an expert in robotic to be 39 (44), which is notably less than the aforementioned laparoscopic learning curve numbers (38,39).
Robotic colorectal surgery: data to date
After just a decade, available data on robotics in colorectal surgery has increased exponentially. We now have multi-institutional, national and international database analyses, systematic reviews (45) and meta analyses evaluating robotic outcomes. Queries of the National Inpatient Sample (NIS) (30,33), National Cancer Database (NCDB) (37), and ACS/NSQIP (34) data sets have demonstrated that robotic surgery results in similar or lower postoperative lengths of stay, equal perioperative morbidity, similar anastomotic leak rates, similar postoperative ileus and a decreased conversion to open surgery in both colonic and rectal resections when compared to laparoscopy. Furthermore, pathologically, there were no reported differences in lymph node (LN) retrieval or positive surgical margins. The most consistently reported limitations of robotics, however, were an increase in operative time and overall cost when compared to laparoscopy—items to be discussed later. These large analyses have demonstrated that robotic colorectal surgery is, at the least, a safe and feasible alternative to laparoscopy.
As previously noted, many of the technical advantages of robotic surgery have been highlighted while working in the pelvis, and, as such, rectal surgery has been thoroughly evaluated as a primary utilization for robotics in colorectal surgery. In 2017 the long awaited Robotic vs. Laparoscopic Resection for Rectal Cancer (ROLARR) trial was published (46), an international (10 countries), multicenter (29 sites), randomized controlled trial (RCT) assessing primary endpoint of conversion to laparotomy for performing part of the TME, and is the largest study of its kind to date. The study was designed to exclude surgeons who were still on their learning curve, and, as such, only experienced surgeons in laparoscopy (mean of at least 90 prior cases) and robotics (mean of at least 50) were included. Secondary endpoints included evaluation of circumferential radial margin, postoperative complications, mortality, bladder and sexual dysfunction, and pathologic assessment of the TME. There were no reported differences in conversion rates or other secondary end points, but robotic surgery did result in longer operative time. These authors concluded that robotic resection does not confer an advantage in rectal cancer, and given the increased cost with near equivalent outcomes, robotic surgery for rectal cancer is not justified.
ROLARR’s results were met with mixed responses, especially within the robotics community. Soliman notes in an editorial (47), that the ROLARR trial was underpowered based on results from the CLASICC trial (16) and the study only utilized the da Vinci Si robot. When looking closer, the data show there were clear advantages favoring robotics to prevent conversion in obese patients, patients with low tumors, and males. These are the patients where one would expect the most benefit. He also notes that surgeons with fewer robotic case experience had equivalent outcomes to surgeons with 2–3 times the laparoscopic case experience and that circumferential radial margin (CRM) positive rates in the robotic arm (5.1%) was less than those reported laparoscopically in COLOR II (10%) (14), CLASICC (7%) (16), AlaCaRT (7%) (15) and the Z6501 (12%) (9) trials.
In a 2018 meta-analysis of five randomized controlled trials, including ROLARR, Prete et al. assessed robotic versus laparoscopic resection for rectal cancer (48). The authors found no significant differences in TME grade, CRM positivity rates, LN harvest, postoperative leak, mortality, or complication rates. There, again, was a decreased rate of open conversion in the robotics arm, however, a significant increase in operative time in robotic resections across all five trials.
Oncologically speaking, the decreased conversion rates are of great importance for short and long-term outcomes for several reasons. Conversion from a MIS to open resection results in increased postoperative complication rates, overall episodic costs, delays in time to chemotherapy completion which can ultimately affects oncologic outcomes, specifically, decreasing 5-year disease fee survival and trends towards higher recurrence rates (34,49-51).
While arguments favoring robotic approaches for pelvic surgery and rectal resection seem to be clear, the utility of robotic surgery on colonic resections, specifically right-sided resections, remain unknown. Ma et al., in 2019, published a systematic review and meta-analysis of 13 trials, mostly retrospective in design, comparing robotic and laparoscopic right-sided resections (52). The pooled data found that robotic resections were associated with significantly longer operative times and higher costs, but a decreased postoperative length of stay, and, again, lower conversion rates. Authors of the only included RCT from 2012 included in this analysis concluded in their study that the robotic right colectomy was feasible when compared to the laparoscopic approach but provided no benefit to the costs of the surgery (53).
There is new data emerging, however, on the utility of robotics on performing a right sided complete mesocolic excision (CME) with central venous ligation as initially descried in the open setting by Hohenberger et al. (54). A modified CME done robotically has been shown in retrospective series to have equivalent oncologic outcomes with negligible conversion rates when compared to laparoscopy (55-57). Authors highlight that the delicate vascular dissection is improved with the utility of the robot when compared to laparoscopy. Nonetheless, with a higher morbidity and complication rate, it’s unclear if any small oncologic survival benefit derived from the resection approach is of value (58), regardless of approach.
Cost analysis
To date, there is only one mainstream distributor producing commercially available robot platforms within the United States. Da Vinci (Intuitive Surgical Corp, Sunnyvale, Ca) robots have dominated the market and represent the operating platform for all the aforementioned studies. In 2006, Intuitive launched its “S” system and most of the data we have available to us today have been based on studies and experiences on that console and its variations. Compared to newer, advanced models within the da Vinci family, the S and Si systems were limited in their performance capacity. Specifically, the configuration and maneuverability of its operative arms limited access to several abdominal quadrants and often required a hybrid approach with laparoscopy and/or repositioning the robot to achieve adequate dissection and exposure. For left sided colorectal resections, for example, the splenic flexure mobilization and vessel (inferior mesenteric artery) dissection and division were often performed laparoscopically while the TME was dissected robotically or the robot was repositioned mid procedure to complete these steps (59,60).
In 2014, da Vinci launched the Xi platform, their fourth-generation design. The Xi Touts new advances including smaller arms with wider range of motion with patient clearing joints allowing for multi-quadrant access, longer instruments and easier docking and undocking capacity (28) dies comparing the Xi and Si platforms in colorectal surgery demonstrated shorter console times with equivalent quality and oncologic outcome measures (60,61).
When laparoscopic surgery was first being evaluated for cost effectiveness, supporters of its use note that any additional costs incurred in the operating room were the result of increased OR time or instrumentation costs which were ultimately justified by hospitalization savings with decreased length of stay, readmission rates and complication rates (16,62). As surgeons became more proficient with laparoscopy and had plateaued on their learning curve, the operative times decreased, and laparoscopy became increasingly cost-effective (63).
With perioperative clinical outcome data being thus far equivalent between robotic and -laparoscopic surgery, the question of cost-efficacy with the robotic platform is often cited as a significant limitation and disadvantage (46,53). There are data demonstrating decreased postoperative length of stay following robotic colorectal surgery and other data demonstrating a 2–3 fold decreased conversion rates when compared to laparoscopy which both have direct effects on cost (33,34,42,49).
The “cost” of a robot is a common discussion-point, but understanding the nuances of the robotic “price-tag” is critical when trying to place a value on its use. According to the 2017 Intuitive Surgical Annual Report along with data collected from the American Hospital Association there were approximately 4,400 da Vinci systems worldwide in 2017, with nearly 2,800 of the 5,500 (51%) United States hospitals having a robotic presence (up from 27% in 2012). There were an estimated 644,000 robotic procedures performed in the United States the same year with largest growing market (and second largest standing market behind gynecology) being within the general surgery population which includes colorectal surgery. The average sales price of a da Vinci robot in 2017 was $1.47 million with an annual service contract ranging from $80,000 to $170,000.
Using these data, Feldstein et al. (64) note that cost of ownership of a da Vinci robot can be calculated by the summation of the cost of initial equipment acquisition, variable case cost by service or procedure (supplies, staff time), and the maintenance cost. The authors calculated that the average robot in the United States performs 424 cases annually and estimate that the average fixed cost per use of the robot is $948. The weighted average variable cost per procedure was $8,025 [ranging from $3,325 (cholecystectomy) to $16,986 (rectal resection)]. Based on their calculations of 14 high-volume robotic hospitals (academic, community and rural) the total cost for a single colon resection was $11,258 and for a rectal resection was $17,970. These data have been validated within randomized controlled trials which demonstrated significantly increased costs for robotic vs. laparoscopic colonic resection, even after insurance patient payments (52,53).
Conclusions
There has been remarkable advancement in MIS techniques in colorectal surgery in recent decades. However, in a newly published population analysis of 191,000 patients undergoing colorectal surgery from the Medicare Provider Analysis and Review from 2010 to 2016, nearly half (46%) were still performed open (32). The current body of high-quality data available (database/population analysis, randomized control trials, meta-analysis, and systematic review) confirms that minimally invasive techniques are superior to an open approach but equal to one-another with regards to perioperative outcomes. The primary differentiating variable between laparoscopy and robots, in the hands of seasoned users, at this moment, is cost. As a younger generation of surgeons enter the work-force who are trained to be technically proficient and efficient in minimally invasive approaches, and as technology and the market-share for robotics continues to grow and diversify, costs will improve. As such, the surgeon will be able to use his or her discretion to determine optimal approach.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Steven D. Schwaitzberg and Rafael Perez) for the series “Advances in Robotic Surgery” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: The series “Advances in Robotic Surgery” was commissioned by the editorial office without any funding or sponsorship. MR Albert: Applied Medical: consultant, stock options, education; Stryker Endoscopy: consultant; Conmed Surgical: consultant; Human Extensions: consultant, stock options; Cooper Surgical: consultant, honorarium. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jacobs M, Verdeja JC, Goldstein HS. Minimally invasive colon resection (laparoscopic colectomy). Surg Laparosc Endosc 1991;1:144-50. [PubMed]
- Monson JR, Darzi A, Carey PD, et al. Prospective evaluation of laparoscopic-assisted colectomy in an unselected group of patients. Lancet 1992;340:831-3. [Crossref] [PubMed]
- Falk PM, Beart RW Jr, Wexner SD, et al. Laparoscopic colectomy: a critical appraisal. Dis Colon Rectum 1993;36:28-34. [Crossref] [PubMed]
- Simorov A, Shaligram A, Shostrom V, et al. Laparoscopic colon resection trends in utilization and rate of conversion to open procedure: a national database review of academic medical centers. Ann Surg 2012;256:462-8. [Crossref] [PubMed]
- Fox J, Gross CP, Longo W, et al. Laparoscopic colectomy for the treatment of cancer has been widely adopted in the United States. Dis Colon Rectum 2012;55:501-8. [Crossref] [PubMed]
- Heald RJ. The 'Holy Plane' of rectal surgery. J R Soc Med 1988;81:503-8. [Crossref] [PubMed]
- Hartley JE, Mehigan BJ, Qureshi AE, et al. Total mesorectal excision: assessment of the laparoscopic approach. Dis Colon Rectum 2001;44:315-21. [Crossref] [PubMed]
- Darzi A, Lewis C, Menzies-Gow N, et al. Laparoscopic abdominoperineal excision of the rectum. Surg Endosc 1995;9:414-7. [Crossref] [PubMed]
- Fleshman J, Branda ME, Sargent DJ, et al. Disease-free Survival and Local Recurrence for Laparoscopic Resection Compared With Open Resection of Stage II to III Rectal Cancer: Follow-up Results of the ACOSOG Z6051 Randomized Controlled Trial. Ann Surg 2019;269:589-95. [Crossref] [PubMed]
- Kang SB, Park JW, Jeong SY, et al. Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): short-term outcomes of an open-label randomised controlled trial. Lancet Oncol 2010;11:637-45. [Crossref] [PubMed]
- Weeks JC, Nelson H, Gelber S, et al. Short-term quality-of-life outcomes following laparoscopic-assisted colectomy vs open colectomy for colon cancer: a randomized trial. Jama 2002;287:321-8. [Crossref] [PubMed]
- Bonjer HJ, Hop WC, Nelson H, et al. Laparoscopically assisted vs open colectomy for colon cancer: a meta-analysis. Arch Surg 2007;142:298-303. [Crossref] [PubMed]
- Jackson TD, Kaplan GG, Arena G, et al. Laparoscopic versus open resection for colorectal cancer: a metaanalysis of oncologic outcomes. J Am Coll Surg 2007;204:439-46. [Crossref] [PubMed]
- van der Pas MH, Haglind E, Cuesta MA, et al. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol 2013;14:210-8. [Crossref] [PubMed]
- Stevenson AR, Solomon MJ, Lumley JW, et al. Effect of Laparoscopic-Assisted Resection vs Open Resection on Pathological Outcomes in Rectal Cancer: The ALaCaRT Randomized Clinical Trial. Jama 2015;314:1356-63. [Crossref] [PubMed]
- Guillou PJ, Quirke P, Thorpe H, et al. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet 2005;365:1718-26. [Crossref] [PubMed]
- Administration USFaD. 510(k) Premarket Notification. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K990144
- Pasticier G, Rietbergen JB, Guillonneau B, et al. Robotically assisted laparoscopic radical prostatectomy: feasibility study in men. Eur Urol 2001;40:70-4. [Crossref] [PubMed]
- Elliott DS, Chow GK, Gettman M. Current status of robotics in female urology and gynecology. World J Urol 2006;24:188-92. [Crossref] [PubMed]
- Anderson JE, Chang DC, Parsons JK, et al. The first national examination of outcomes and trends in robotic surgery in the United States. J Am Coll Surg 2012;215:107-14; discussion 114-06.
- Pearce SM, Pariser JJ, Karrison T, et al. Comparison of Perioperative and Early Oncologic Outcomes between Open and Robotic Assisted Laparoscopic Prostatectomy in a Contemporary Population Based Cohort. J Urol 2016;196:76-81. [Crossref] [PubMed]
- Weber PA, Merola S, Wasielewski A, et al. Telerobotic-assisted laparoscopic right and sigmoid colectomies for benign disease. Dis Colon Rectum 2002;45:1689-94; discussion 1695-86.
- Hashizume M, Shimada M, Tomikawa M, et al. Early experiences of endoscopic procedures in general surgery assisted by a computer-enhanced surgical system. Surg Endosc 2002;16:1187-91. [Crossref] [PubMed]
- Vibert E, Denet C, Gayet B. Major digestive surgery using a remote-controlled robot: the next revolution. Arch Surg 2003;138:1002-6. [Crossref] [PubMed]
- Delaney CP, Lynch AC, Senagore AJ, et al. Comparison of robotically performed and traditional laparoscopic colorectal surgery. Dis Colon Rectum 2003;46:1633-9. [Crossref] [PubMed]
- Pigazzi A, Ellenhorn JD, Ballantyne GH, et al. Robotic-assisted laparoscopic low anterior resection with total mesorectal excision for rectal cancer. Surg Endosc 2006;20:1521-5. [Crossref] [PubMed]
- Hance J, Rockall T, Darzi A. Robotics in colorectal surgery. Dig Surg 2004;21:339-43. [Crossref] [PubMed]
- Ngu JC, Tsang CB, Koh DC. The da Vinci Xi: a review of its capabilities, versatility, and potential role in robotic colorectal surgery. Robot Surg 2017;4:77-85. [Crossref] [PubMed]
- Lanfranco AR, Castellanos AE, Desai JP, et al. Robotic surgery: a current perspective. Ann Surg 2004;239:14-21. [Crossref] [PubMed]
- Schootman M, Hendren S, Ratnapradipa K, et al. Adoption of Robotic Technology for Treating Colorectal Cancer. Dis Colon Rectum 2016;59:1011-8. [Crossref] [PubMed]
- Damle A, Damle RN, Flahive JM, et al. Diffusion of technology: Trends in robotic-assisted colorectal surgery. Am J Surg 2017;214:820-4. [Crossref] [PubMed]
- Sheetz KH, Norton EC, Dimick JB, et al. Perioperative Outcomes and Trends in the Use of Robotic Colectomy for Medicare Beneficiaries From 2010 Through 2016. JAMA Surg 2019; [Epub ahead of print]. [Crossref] [PubMed]
- Halabi WJ, Kang CY, Jafari MD, et al. Robotic-assisted colorectal surgery in the United States: a nationwide analysis of trends and outcomes. World J Surg 2013;37:2782-90. [Crossref] [PubMed]
- Bhama AR, Obias V, Welch KB, et al. A comparison of laparoscopic and robotic colorectal surgery outcomes using the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) database. Surg Endosc 2016;30:1576-84. [Crossref] [PubMed]
- Jin LX, Ibrahim AM, Newman NA, et al. Robotic surgery claims on United States hospital websites. J Healthc Qual 2011;33:48-52. [Crossref] [PubMed]
- Ahmad A, Ahmad ZF, Carleton JD, et al. Robotic surgery: current perceptions and the clinical evidence. Surg Endosc 2017;31:255-63. [Crossref] [PubMed]
- Mirkin KA, Kulaylat AS, Hollenbeak CS, et al. Robotic versus laparoscopic colectomy for stage I-III colon cancer: oncologic and long-term survival outcomes. Surg Endosc 2018;32:2894-901. [Crossref] [PubMed]
- Tekkis PP, Senagore AJ, Delaney CP, et al. Evaluation of the learning curve in laparoscopic colorectal surgery: comparison of right-sided and left-sided resections. Ann Surg 2005;242:83-91. [Crossref] [PubMed]
- Park IJ, Choi GS, Lim KH, et al. Multidimensional analysis of the learning curve for laparoscopic resection in rectal cancer. J Gastrointest Surg 2009;13:275-81. [Crossref] [PubMed]
- Tsuda S. Fundamentals of Laparoscopic Surgery. SAGES. Available online: https://www.sages.org/wiki/fundamentals-laparoscopic-surgery/
- ACGME. Case Long Information - Surgery Minimums. Available online: https://www.acgme.org/Specialties/Case-Log-Information/pfcatid/24/Surgery
- Justiniano CF, Becerra AZ, Xu Z, et al. A Population-Based Study of 90-Day Hospital Cost and Utilization Associated With Robotic Surgery in Colon and Rectal Cancer. J Surg Res 2020;245:136-44. [Crossref] [PubMed]
- Saraidaridis JT, Read TE, Marcello PW, et al. What do Young Colorectal Surgeons Value From Their CRS Residency Training? J Surg Educ 2019;76:720-6. [Crossref] [PubMed]
- Jiménez-Rodríguez RM, Rubio-Dorado-Manzanares M, Díaz-Pavón JM, et al. Learning curve in robotic rectal cancer surgery: current state of affairs. Int J Colorectal Dis 2016;31:1807-15. [Crossref] [PubMed]
- Kim CW, Kim CH, Baik SH. Outcomes of robotic-assisted colorectal surgery compared with laparoscopic and open surgery: a systematic review. J Gastrointest Surg 2014;18:816-30. [Crossref] [PubMed]
- Jayne D, Pigazzi A, Marshall H, et al. Effect of Robotic-Assisted vs Conventional Laparoscopic Surgery on Risk of Conversion to Open Laparotomy Among Patients Undergoing Resection for Rectal Cancer: The ROLARR Randomized Clinical Trial. Jama 2017;318:1569-80. [Crossref] [PubMed]
- Soliman M. ROLARR - The Real Story. Available online: https://www.linkedin.com/pulse/rolarr-real-story-mark-soliman-md-facs-fascrs
- Prete FP, Pezzolla A, Prete F, et al. Robotic Versus Laparoscopic Minimally Invasive Surgery for Rectal Cancer: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Ann Surg 2018;267:1034-46. [Crossref] [PubMed]
- Cleary RK, Mullard AJ, Ferraro J, et al. The cost of conversion in robotic and laparoscopic colorectal surgery. Surg Endosc 2018;32:1515-24. [Crossref] [PubMed]
- Keller DS, Senagore AJ, Fitch K, et al. A new perspective on the value of minimally invasive colorectal surgery-payer, provider, and patient benefits. Surg Endosc 2017;31:2846-53. [Crossref] [PubMed]
- Rottoli M, Bona S, Rosati R, et al. Laparoscopic rectal resection for cancer: effects of conversion on short-term outcome and survival. Ann Surg Oncol 2009;16:1279-86. [Crossref] [PubMed]
- Ma S, Chen Y, Guo T, et al. Short-term outcomes of robotic-assisted right colectomy compared with laparoscopic surgery: A systematic review and meta-analysis. Asian J Surg 2019;42:589-98. [Crossref] [PubMed]
- Park JS, Choi GS, Park SY, et al. Randomized clinical trial of robot-assisted versus standard laparoscopic right colectomy. Br J Surg 2012;99:1219-26. [Crossref] [PubMed]
- Hohenberger W, Weber K, Matzel K, et al. Standardized surgery for colonic cancer: complete mesocolic excision and central ligation--technical notes and outcome. Colorectal Dis 2009;11:354-64; discussion 364-55.
- Cho MS, Baek SJ, Hur H, et al. Modified complete mesocolic excision with central vascular ligation for the treatment of right-sided colon cancer: long-term outcomes and prognostic factors. Ann Surg 2015;261:708-15. [Crossref] [PubMed]
- Spinoglio G, Marano A, Bianchi PP, et al. Robotic Right Colectomy with Modified Complete Mesocolic Excision: Long-Term Oncologic Outcomes. Ann Surg Oncol 2016;23:684-91. [Crossref] [PubMed]
- Bae SU, Yang SY, Min BS. Totally robotic modified complete mesocolic excision and central vascular ligation for right-sided colon cancer: technical feasibility and mid-term oncologic outcomes. Int J Colorectal Dis 2019;34:471-9. [Crossref] [PubMed]
- Wang C, Gao Z, Shen K, et al. Safety, quality and effect of complete mesocolic excision vs non-complete mesocolic excision in patients with colon cancer: a systemic review and meta-analysis. Colorectal Dis 2017;19:962-72. [Crossref] [PubMed]
- Koh DC, Tsang CB, Kim SH. A new application of the four-arm standard da Vinci(R) surgical system: totally robotic-assisted left-sided colon or rectal resection. Surg Endosc 2011;25:1945-52. [Crossref] [PubMed]
- Ozben V, Cengiz TB, Atasoy D, et al. Is da Vinci Xi Better than da Vinci Si in Robotic Rectal Cancer Surgery? Comparison of the 2 Generations of da Vinci Systems. Surg Laparosc Endosc Percutan Tech 2016;26:417-23. [Crossref] [PubMed]
- Protyniak B, Jorden J, Farmer R. Multiquadrant robotic colorectal surgery: the da Vinci Xi vs Si comparison. J Robot Surg 2018;12:67-74. [Crossref] [PubMed]
- Alkhamesi NA, Martin J, Schlachta CM. Cost-efficiency of laparoscopic versus open colon surgery in a tertiary care center. Surg Endosc 2011;25:3597-604. [Crossref] [PubMed]
- Rashidi L, Neighorn C, Bastawrous A. Outcome comparisons between high-volume robotic and laparoscopic surgeons in a large healthcare system. Am J Surg 2017;213:901-5. [Crossref] [PubMed]
- Feldstein J, Schwander B, Roberts M, et al. Cost of ownership assessment for a da Vinci robot based on US real-world data. Int J Med Robot 2019;15:e2023 [Crossref] [PubMed]
Cite this article as: Wright JP, Albert MR. A current review of robotic colorectal surgery. Ann Laparosc Endosc Surg 2020;5:9.