Updates in laparoscopic surgery for perforated peptic ulcer disease: state of the art and future perspectives
Introduction
Every year, peptic ulcer disease (PUD) affects about 4 million people globally (1). According to different series, the prevalence of perforation in patients with PUD ranges between 2–14% (2-4). Perforation is a life-threatening complication of PUD, and patients with a perforated peptic ulcer (PPU) often show up with diffuse peritonitis and generalized sepsis, conditions that carry high risks for morbidity and mortality (5). The prevalence of PUD has decreased in recent decades, but this has not been followed by a similar reduction in the complications from peptic ulcers. The reduction in PUD can be partially attributed to the onset of H. Pylori eradication therapy and the extensive use of proton pump inhibitors (PPIs). Despite the introduction of these therapies, the incidence of PPU has remained constant in many parts of the world (6,7), occurring in about 2–14% of peptic ulcers; it continues to be both the second most frequent cause of hollow viscus perforation requiring urgent surgery and the most frequent indication for gastric emergency surgery (8-10). The reasons for the discrepant incidences of PUD and PPU has yet to be identified. Known risk factors for PPU are nonsteroidal anti-inflammatory drug (NSAID) use, H. Pylori, smoking, steroid use, physiological stress (4), fasting, cocaine use, and chemotherapy with bevacizumab (6). The etiology of PPU is different in developing countries, where patients are typically young, male, and smokers, whereas in developed countries, the etiology is usually linked to NSAID or steroid use in older and more frail patients (4).
The management of PPU is primarily surgical, and several repair techniques have been described.
In 1989, the first laparoscopic repair of a duodenal ulcer was described by Mouret et al. (11).
Since then, many papers comparing the laparoscopic and open approaches have been published.
This literature review aims to survey and discuss the most recent and ongoing clinical applications of laparoscopic surgery for PPU, including its indications, different repair techniques, and outcomes.
Clinical features
PPU often presents with non-specific abdominal symptoms which makes early diagnosis somewhat difficult. Typical symptoms include the sudden onset of severe abdominal pain, nausea and vomiting and pyrexia. When a perforation occurs, gastric juice and air enter the peritoneal space and lead to chemical peritonitis, which leads to severe abdominal pain and tachycardia. Sudden-onset abdominal pain, tachycardia, and abdominal rigidity are the typical triad of PPU (4). Fewer than two-thirds of patients present with frank peritonitis, and in some cases this phenomenon may result in a delay in the diagnosis of PPU (12). Early intervention is crucial since each hour from the onset of symptoms reduces the chances of survival by 2.4% (13,14).
Diagnosis
Radiology
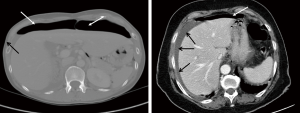
An erect chest X-ray is commonly performed in patients with acute upper abdominal pain suspected of perforation (4). However, a contrast-enhanced computed tomography is the best diagnostic modality with an accuracy of 98% (15). Furthermore, CT scans can rule out other acute abdominal conditions such as acute cholecystitis, acute pancreatitis, acute appendicitis and acute mesenteric ischemia to name but a few. Some of these acute abdominal conditions may not require surgical intervention at least in the initial phase. In resource-poor healthcare facilities, an erect chest X-ray is extremely useful in detecting free air under the diaphragm confirming visceral perforation (4). Peptic ulcer perforation can also generate detectable sonographic signs, such as pneumoperitoneum; free intraperitoneal air tends to accumulate around the liver, duodenum, and stomach, local thickening of the gastroduodenal wall containing an echogenic focus or line, the presence of localized extraluminal gas and fluid. However, sonography does not typically play a role in either first-line investigation or management workup of PPU (16) (Figure 1).
Laboratory
Laboratory tests such as full blood count, urea and electrolytes, C reactive protein, serum amylase and lipase and venous or arterial blood gases, are performed in PPU patients in order to rule out other diagnoses, to assess the function of other systems and lastly to gauge the acuteness of surgical condition. For instance, elevated serum amylase and lipase levels are suggestive of acute pancreatitis (17,18). Similarly, white blood cell count and C-reactive protein are usually raised in most acute abdominal conditions due to inflammation or infection and they are non-specific in nature (17). Impaired renal function, increased lactate levels, and metabolic acidosis can be secondary to a number of acute abdominal conditions and reflect hypotension, inadequate perfusion, systemic inflammatory response syndrome (SIRS) and/or pre-renal injury but are not specific to PPU (7). These signs suggest hypovolemic shock and sepsis.
Material and methods
An extensive literature search was performed for articles available in the PubMed database. The terms used for the search were “gastric/duodenal/peptic ulcer”, “perforation/perforated”, “laparoscopy/laparoscopic”, and “repair/surgery”. Only articles written in English that used adult patients were considered.
Laparoscopic approach
For the majority of its treatment history, laparotomy and the direct closure of the perforation with interrupted sutures and an omental pedicle plug (Cellan-Jones) has been the primary approach to PPU (7). However, in the last two and half decades, the minimal access approach has emerged as a viable option. Despite these solutions, not every patient is suitable for laparoscopy and thus patient selection for laparoscopic surgery is crucial (19).
Patient selection
Guidelines about which patients with PPU are suitable for the minimally invasive approach have yet to be defined. However, patients with shock at admission, late presentation (24 hours after the onset of symptoms), older than 70 years, American Society of Anesthesiologists (ASA) grade III or IV, and a high Boey score, are considered high-risk patients, and should undergo laparotomy (20). In a multicenter retrospective study, Mirabella et al. demonstrated the relationship between a patient’s Boey score and morbidity and mortality (Table 1). In their study, patients with Boey scores from 0 to 2 had a morbidity and mortality of 4.7% and 0.8%, respectively, while those with a Boey score of 3 had a morbidity and mortality of 21.4% and 10.7%, respectively (21). These authors therefore selected not to use laparoscopy in high-risk Boey’s score patients. They however suggested that it will be interesting to evaluate the usefulness of a minimally invasive technique in high risk patients in large randomized controlled trials. Teoh et al. (22) undertook a retrospective analysis of 373 patients undergoing surgery for PPU with laparoscopic first approach (LFA). Twenty-five point two percent of the patients had a Boey score of ≥2. These authors showed that there was a significant increase in the number of operations performed yearly by the LFA. The authors concluded that the adoption of LFA in patients with PPU was associated with acceptable rates of mortality and morbidity. The approach could also be selectively adopted in patients with Boey score ≥2 provided their ASA grading is low and they are hemodynamically stable (22).
Table 1
| Risk score | Mortality (OR) | Morbidity (OR) |
|---|---|---|
| 0 | 1% | 11% |
| 1 | 2.4% | 2.9% |
| 2 | 3.5% | 4.3% |
| 3 | 7.7% | 4.9% |
Boey score factors: (I) concomitant severe medical illness; (II) preoperative shock (persistent hypotension: systolic blood pressure <90 mmHg, mean arterial pressure <60 mmHg, or a reduction in systolic blood pressure >40 mmHg from baseline); (III) duration of perforation >24 hours (duration of the perforation was determined as the time interval between the onset of severe acute abdominal pain, and arrival time at the hospital). Score: 0–3 (each factor scores 1 point if positive). Adapted from Lohsiriwat V, Prapasrivorakul S, Lohsiriwat D. Perforated peptic ulcer: clinical presentation, surgical outcomes, and the accuracy of the Boey scoring system in predicting postoperative morbidity and mortality. World J Surg 2009;33:80-5. PPU, perforated peptic ulcer; OR, odds ratio.
Surgical technique
There is no agreement as to how to execute the laparoscopic repair of PPU, as it depends on operator preference. Bertleff et al. reviewed 29 studies and reported that 44% of operators chose to stand between the patient’s legs, while 33% preferred to perform the procedure standing on the patient’s left side. Furthermore, the number, placement, and size of trocars varied among surgeons (2). A nasogastric tube was used in 96% of cases, and a drain was left in 79% of cases even though there is no evidence that a drain has any impact on the incidence of post-operative intra-abdominal collections (23).
Also, the ideal surgical technique for laparoscopic repair of perforation remains undefined; laparoscopic repair techniques mirror techniques of open surgery, but it has been reported to require greater operating time. To obviate this problem, some studies of direct suture without omental patching have been published that indicate a significantly shortened duration of surgery. Avoiding omentoplasty could reduce the duration of surgeries but may be the cause of a higher rate of leakage or duodenal stricture (24,25). A recent study by Wang et al. compared the efficacy of a sutureless onlay omental patch with a sutured patch after direct closure of the perforation in 43 patients. The operating time was significantly shorter in the sutureless onlay omental patch group. There was no statistically significant difference in the outcome between sutureless versus sutured omental patch repair. This work indicates that both techniques might be effective and safe for laparoscopic repair of PPU (26). A sutureless technique involving a gelatin sponge plug and fibrin glue sealing has also been described (27). However, it has not been widely adopted due to a high rate of repair site leak (20).
Peritoneal lavage
Peritoneal lavage is one of the key steps in the surgical treatment of PPU (8), but it can also lengthen the operating time. There is no consensus concerning the amount of fluid to be used for irrigation.
In a review from Bertleff et al. that included 29 studies, lavage has been completed with 2 to 6 liters of warm saline solution, but lavage with up to 10 liters have also been reported (2). Siow et al. described a focused sequential manner to perform peritoneal lavage that involved quadrant-to-quadrant washing with tilting of the patient and position switching between the surgeon and the camera driver. The fluid was then aspirated until the recesses were clean of debris. In this case series, there were no postoperative intra-abdominal abscesses or collections (28).
Conversion
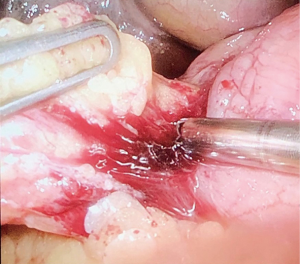
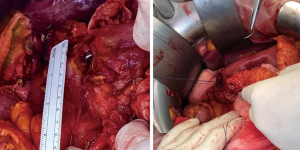
The potential reasons for conversion to open surgery include the difficulty in localizing the perforation site for anatomical reasons, i.e., perforation located in a region other than the duodenal anterior wall, large perforations (described by some authors as 6 mm diameter or larger, and by others as over 10 mm) (Figures 2,3), peritoneal adhesions from previous surgeries, and ulcers with fragile edges (20,21,29,30), Mannheim peritonitis index >21, and generalized peritonitis (31). A recent Danish study of 726 patients undergoing surgery for perforated PUD reported a laparoscopy rate of 32.8%, with 24.5% converted from laparoscopic to open (32). However, the UK study has shown a much smaller conversion rate in their cohort of patients; 13.1% of patients underwent surgical repair of their perforated ulcer via a laparoscopic approach, out of which 6.9% were converted to open approach. The authors believed that under reporting may be cause of this low conversion rate (33). A recent systematic review of 32 studies however confirmed a conversion rate of only 4.9% (34) which may suggest that (I) laparoscopic surgical training in the complex gastrointestinal surgery is becoming common place; (II) better surgical equipment and instruments may have a role to play and (III) better senior supervision in these acute surgical cases may have become more routine and lastly (IV) surgeons are gaining more experience in emergency laparoscopic techniques.
Perioperative morbidity and mortality
A PPU is related to significant postoperative morbidity and mortality regardless of whether the surgery is laparoscopic or open (35). The estimated post-operative mortality ranges from 1.3% to 20%, with the 30-day mortality rate reaching 20% and the 90-day mortality rate reaching up to 30% (13,18,36-40). Risk factors for mortality are no different for either an open or laparoscopic approach and include shock at admission, co-morbidities, resection surgery, female, elderly patients, a delay presentation of more than 24 hours, metabolic acidosis, acute renal failure, hypoalbuminemia, being underweight and smokers.
The reported surgical complications after laparoscopic repair of PPU include surgical site infection (SSI), intra-abdominal collection/abscess, wound dehiscence, enterocutaneous fistulas, peritonitis, ileus, pneumonia and incisional hernias (4,21,41). A meta-analysis by Tan et al. stated that laparoscopic repair had a lower overall complication rate than an open repair for PPU, but this difference did not achieve statistical significance (P>0.05) (41). When subgroup analysis of complications was performed, the laparoscopic approach had a similar incidence of repair site leakage, intra-abdominal collections, postoperative ileus, urinary tract infection, and pneumonia. However, the SSI rate was lower in the laparoscopy group (P<0.005) (41).
The latest meta-analysis assessing eight randomized controlled trials comparing the laparoscopic to open approach in patients with PPU has shown slightly lower mortality in the laparoscopy group (1.6%) compared to an open repair (4.2%). Reoperations rates were higher in the laparoscopy group: 4.2% compared to 1.8% in the open group; rates of repair site leaks were 3.7% in the laparoscopy group versus 1.7% in the open group; the intra-abdominal abscess rate was 4.4% in the laparoscopy group versus 3.3% in the open group; and reoperation rates for intra-abdominal collections were 1.2% in both the laparoscopy and open groups. Statistically significant differences in the study included reduced early (first 24 hours) postoperative abdominal pain and fewer wound infections in laparoscopic repair (42). The main reasons for reoperation after the laparoscopic repair were suture site leak or reperforation. Previous studies have shown a suture leak rate of 7% with laparoscopic repair; however, more recent studies have demonstrated that this can be substantially reduced to around 2.18%, however it is still three times higher than an open repair according to a recent systematic review (30,34,43). Surgeons’ inexperience and the steep learning curve involved may be possible explanations; indeed, more recent investigations have shown that progress in a surgeon’s surgical technique and experience could improve this outcome (29,44).
Conclusions
Perforation occurs in 2–14% of peptic ulcer patients, and it remains the second most common cause of visceral perforation that requires urgent surgery. The diagnosis should be based on high index of suspicion which requires good clinical history and examination complimented by blood tests and radiological studies, of which contrast-enhanced abdominal CT scan represents the most reliable diagnostic tool in hemodynamically stable patients. Early intervention (<24 hours from symptom onset) is crucial for a good outcome. A literature review of recent meta-analyses suggests the slight advantage of the laparoscopic approach over open surgery in terms of postoperative pain and length of hospital stay, but these results are biased towards the selection of younger patients, ASA risk scores I–II, small perforations (<10 mm), and access to the Emergency Department within 24 hours from the onset of symptoms. No studies have evaluated laparoscopic repair in high-risk patients, such as those with a Boey score of 3, duration of symptoms >24 hours, patient ≥70 years, and ASA 3. High-risk patients should indeed be treated with open surgery, especially those who are hemodynamically unstable (20). An LFA could also be selectively adopted for patients with a Boey score ≥2, provided that the ASA score was <3, and the patient was hemodynamically stable (22). A sutureless omental patch can reduce operative time but may increase the risk of leak rate and reoperation. The best current and most up-to-date evidence suggests that there is no difference in postoperative mortality when comparing the open to the laparoscopic approach in patients with PPU. The advantages of laparoscopy in terms of postoperative pain and wound infection rate may support a minimally invasive approach as the treatment of choice where it is situationally appropriate. Outcomes in subpopulations including elderly, frail, and high-risk patients need further investigation and multicenter randomized controlled trials.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Laparoscopic and Endoscopic Surgery for the series “Laparoendoscopic Surgery for Benign Oesophagogastric Conditions”. The article did not undergo external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2019.11.03). The series “Laparoendoscopic Surgery for Benign Oesophagogastric Conditions” was commissioned by the editorial office without any funding or sponsorship. MAM served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Annals of Laparoscopic and Endoscopic Surgery from Jun 2019 to May 2021. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zelickson MS, Bronder CM, Johnson BL, et al. Helicobacter pylori is not the predominant etiology for peptic ulcers requiring operation. Am Surg 2011;77:1054-60. [PubMed]
- Bertleff MJ, Lange JF. Laparoscopic correction of perforated peptic ulcer: first choice? A review of literature. Surg Endosc 2010;24:1231-9. [Crossref] [PubMed]
- Lau JY, Sung J, Hill C, et al. Systematic review of the epidemiology of complicated peptic ulcer disease: incidence, recurrence, risk factors and mortality. Digestion 2011;84:102-13. [Crossref] [PubMed]
- Chung KT, Shelat VG. Perforated peptic ulcer - an update. World J Gastrointest Surg 2017;9:1. [Crossref] [PubMed]
- Bas G, Eryilmaz R, Okan I, et al. Risk Factors of Morbidity and Mortality in Patients with Perforated Peptic Ulcer. Acta Chir Belg 2008;108:424-7. [Crossref] [PubMed]
- Wysocki A, Budzyński P, Kulawik J, et al. Changes in the Localization of Perforated Peptic Ulcer and its Relation to Gender and Age of the Patients throughout the Last 45 Years. World J Surg 2011;35:811-6. [Crossref] [PubMed]
- Søreide K, Thorsen K, Harrison EM, et al. Perforated peptic ulcer. Lancet 2015;386:1288-98. [Crossref] [PubMed]
- Lagoo S, McMahon RL, Kakihara M, et al. The sixth decision regarding perforated duodenal ulcer. JSLS 2002;6:359-68. [PubMed]
- Thorsen K, Søreide JA, Søreide K. Scoring systems for outcome prediction in patients with perforated peptic ulcer. Scand J Trauma Resusc Emerg Med 2013;21:25. [Crossref] [PubMed]
- Sanabria A, Villegas MI, Morales Uribe CH. Laparoscopic repair for perforated peptic ulcer disease. Cochrane Database Syst Rev 2013;CD004778 [PubMed]
- Mouret P, François Y, Vignal J, et al. Laparoscopic treatment of perforated peptic ulcer. Br J Surg 1990;77:1006. [Crossref] [PubMed]
- Hirschowitz BI, Simmons J, Mohnen J. Clinical outcome using lansoprazole in acid hypersecretors with and without Zollinger-Ellison syndrome: a 13-year prospective study. Clin Gastroenterol Hepatol 2005;3:39-48. [Crossref] [PubMed]
- Boey J, Choi SK, Poon A, et al. Risk stratification in perforated duodenal ulcers. A prospective validation of predictive factors. Ann Surg 1987;205:22-6. [Crossref] [PubMed]
- Buck DL, Vester-Andersen M, Møller MH. Surgical delay is a critical determinant of survival in perforated peptic ulcer. Br J Surg 2013;100:1045-9. [Crossref] [PubMed]
- Kim HC, Yang DM, Kim SW, et al. Gastrointestinal tract perforation: evaluation of MDCT according to perforation site and elapsed time. Eur Radiol 2014;24:1386-93. [Crossref] [PubMed]
- Kuzmich S, Harvey CJ, Fascia DT, et al. Perforated Pyloroduodenal Peptic Ulcer and Sonography. AJR Am J Roentgenol 2012;199:W587-94 [Crossref] [PubMed]
- Fakhry SM, Watts DD, Luchette FA, et al. Current diagnostic approaches lack sensitivity in the diagnosis of perforated blunt small bowel injury: analysis from 275,557 trauma admissions from the EAST multi-institutional HVI trial. J. Trauma 2003;54:295-306. [Crossref] [PubMed]
- Søreide K, Thorsen K, Soreide JA. Strategies to improve the outcome of emergency surgery for perforated peptic ulcer. Br J Surg 2014;101:e51-64. [Crossref] [PubMed]
- Lau H. Laparoscopic repair of perforated peptic ulcer: a meta-analysis. Surg Endosc 2004;18:1013-21. [Crossref] [PubMed]
- Lee FY, Leung KL, Lai BS, et al. Predicting mortality and morbidity of patients operated on for perforated peptic ulcers. Arch Surg 2001;136:90-4. [Crossref] [PubMed]
- Mirabella A, Fiorentini T, Tutino R, et al. Laparoscopy is an available alternative to open surgery in the treatment of perforated peptic ulcers: A retrospective multicenter study. BMC Surg 2018;18:78. [Crossref] [PubMed]
- Teoh AY, Chiu PW, Kok AS, et al. The selective use of laparoscopic repair is safe in high-risk patients suffering from perforated peptic ulcer. World J Surg 2015;39:740-5. [Crossref] [PubMed]
- Lunevicius R, Morkevicius M. Management Strategies, Early Results, Benefits, and Risk Factors of Laparoscopic Repair of Perforated Peptic Ulcer. World J Surg 2005;29:1299-310. [Crossref] [PubMed]
- Lau WY. Perforated peptic ulcer: open versus laparoscopic repair. Asian J Surg 2002;25:267-9. [Crossref] [PubMed]
- Siu WT, Chau CH, Law BK, et al. Routine use of laparoscopic repair for perforated peptic ulcer. Br J Surg 2004;91:481-4. [Crossref] [PubMed]
- Wang YC, Hsieh CH, Lo HC, et al. Sutureless onlay omental patch for the laparoscopic repair of perforated peptic ulcers. World J Surg 2014;38:1917-21. [Crossref] [PubMed]
- Lau WY, Leung KL, Kwong KH, et al. A Randomized Study Comparing Laparoscopic Versus Open Repair of Perforated Peptic Ulcer Using Suture or Sutureless Technique. Ann Surg 1996;224:131-8. [Crossref] [PubMed]
- Siow SL, Mahendran HA. Laparoscopic repair of perforated peptic ulcers: the sutured omental patch and focused sequential lavage technique. Surg Laparosc Endosc Percutan Tech 2014;24:134-9. [Crossref] [PubMed]
- Di Saverio S, Bassi M, Smerieri N, et al. Diagnosis and treatment of perforated or bleeding peptic ulcers: 2013 WSES position paper. World J Emerg Surg 2014;9:45. [Crossref] [PubMed]
- Lunevicius R, Morkevicius M. Comparison of laparoscopic versus open repair for perforated duodenal ulcers. Surg Endosc 2005;19:1565-71. [Crossref] [PubMed]
- Muller MK, Wrann S, Widmer J, et al. Perforated Peptic Ulcer Repair: Factors Predicting Conversion in Laparoscopy and Postoperative Septic Complications. World J Surg 2016;40:2186-93. [Crossref] [PubMed]
- Wilhelmsen M, Møller MH, Rosenstock S. Surgical complications after open and laparoscopic surgery for perforated peptic ulcer in a nationwide cohort. Br J Surg 2015;102:382-7. [Crossref] [PubMed]
- Byrne BE, Bassett M, Rogers CA, et al. Short-term outcomes after emergency surgery for complicated peptic ulcer disease from the UK National Emergency Laparotomy Audit: a cohort study. BMJ Open 2018;8:e023721 [Crossref] [PubMed]
- Varcus F, Paun I, Duta C, et al. Laparoscopic repair of perforated peptic ulcer. Minerva Chir 2018;73:188-93. [PubMed]
- Lunevicius R, Morkevicius M. Systematic review comparing laparoscopic and open repair for perforated peptic ulcer. Br J Surg 2005;92:1195-207. [Crossref] [PubMed]
- Hermansson M, Stael von Holstein C, Zilling T. Surgical approach and prognostic factors after peptic ulcer perforation. Eur J Surg 1999;165:566-72. [Crossref] [PubMed]
- Rajesh V, Chandra SS, Smile SR. Risk factors predicting operative mortality in perforated peptic ulcer disease. Trop Gastroenterol 2003;24:148-50. [PubMed]
- Buck DL, Moller MH. Danish Clinical Register of Emergency, S. Influence of body mass index on mortality after surgery for perforated peptic ulcer. Br J Surg 2014;101:993-9. [Crossref] [PubMed]
- Noguiera C, Silva AS, Santos JN, et al. Perforated peptic ulcer: main factors of morbidity and mortality. World J Surg 2003;27:782-7. [Crossref] [PubMed]
- Kocer B, Surmeli S, Solak C, et al. Factors affecting mortality and morbidity in patients with peptic ulcer perforation. J Gastroenterol Hepatol 2007;22:565-70. [Crossref] [PubMed]
- Tan S, Wu G, Zhuang Q, et al. Laparoscopic versus open repair for perforated peptic ulcer: A meta analysis of randomized controlled trials. Int J Surg 2016;33 Pt A:124-32.
- Cirocchi R, Soreide K, Di Saverio S, et al. Meta-analysis of perioperative outcomes of acute laparoscopic versus open repair of perforated gastroduodenal ulcers. J Trauma Acute Care Surg 2018;85:417-25. [Crossref] [PubMed]
- Bhogal RH, Athwal R, Durkin D, et al. Comparison Between Open and Laparoscopic Repair of Perforated Peptic Ulcer Disease. World J Surg 2008;32:2371-4. [Crossref] [PubMed]
- Agresta F, Ansaloni L, Baiocchi GL, et al. Laparoscopic approach to acute abdomen from the Consensus Development Conference of the Società Italiana di Chirurgia Endoscopica e nuove tecnologie (SICE), Associazione Chirurghi Ospedalieri Italiani (ACOI), Società Italiana di Chirurgia (SIC), Società Italiana di Chirurgia d'Urgenza e del Trauma (SICUT), Società Italiana di Chirurgia nell'Ospedalità Privata (SICOP), and the European Association for Endoscopic Surgery (EAES). Surg Endosc 2012;26:2134-64. [Crossref] [PubMed]
Cite this article as: Pansa A, Kurihara H, Memon MA. Updates in laparoscopic surgery for perforated peptic ulcer disease: state of the art and future perspectives. Ann Laparosc Endosc Surg 2020;5:5.