Colonoscopic polypectomy
Introduction
Colorectal cancer is the second leading cause of cancer related deaths in the United States. In 2016, 67.3% of U.S. adults aged 50–75 years were up to date with their colorectal cancer screening and 25.6% had never been screened. For adults without health insurance, only 36.3% have been screened (1). The American Cancer Society currently recommends colorectal cancer screening starting at age 45 for patients who are average risk (2). Colonoscopy serves as a diagnostic and therapeutic modality for colorectal cancer screening. Endoscopic polypectomy techniques are widely employed in the everyday clinical setting. This article provides an overview of different polypectomy techniques, complications, and surveillance after polypectomy.
Classification
Polyps are classified on their gross appearance and histologic diagnosis. Histologic types of colon polyps are adenomas, sessile serrated adenomas, hyperplastic, hamartomas, and inflammatory. Adenomas account for 60–70% of polyps identified during colonoscopy (3). Adenomas are further defined by the percentage of villous component as tubular, tubulovillous, and villous. Almost 90% of polyps identified during colonoscopy are less than 10 mm with polyps in this group being divided further into <5 and 6–9 mm. Ponugoti et al. reported the largest series with polyps less than 10 mm where high grade dysplasia was found in 0.3% of polyps <5 mm and 0.8% of polyps 6–9 mm (4). Invasive cancer was not identified in any polyp less than 10 mm. Even though there is a current discussion regarding remove and discard for diminutive polyps, standard of care remains that colon polyps be resected by the safest technique once identified.
Adenoma detection rate (ADR)
The ADR is a well-recognized quality indicator for colonoscopy (5,6). The ADR is defined as the number of screening colonoscopies where at least one adenoma is removed divided by the total number of screening colonoscopies performed by the endoscopist (7). The minimum number of colonoscopies needed to calculate an accurate ADR with a tight 95% confidence interval is 500 (8). Serrated adenomas are not included in the calculation of the ADR. The American Society of Gastrointestinal Endoscopists and the American College of Gastroenterology include ADR in their quality metrics for colonoscopy with a level of evidential support of 1C (9). ADR minimal benchmarks for men and women are 30% and 20%, respectively.
ADR varies significantly between providers conferring decreased interval cancer risks and mortality for patients who have a colonoscopy performed by a provider with a high ADR. ADR is a composite measure that accounts for withdrawal time, prep quality, and technique influencing the initial surveillance recommendation after screening colonoscopy as well as the detection of interval cancers. Endoscopist variability in identifying polyps, ensuring complete removal, and surveying the entire colon account for the majority of interval or post-polypectomy cancers (10,11). Corley et al. evaluated the ADR for 223,842 patients who underwent 264,792 colonoscopies by 136 endoscopists (6). For each 1% improvement in the ADR, a 3% decrease in colorectal cancer incidence and a 5% decrease in mortality was noted.
Polypectomy techniques
Forcep polypectomy
While polypectomy techniques are variable in current practice, a standardized survey interviewing 285 practicing gastroenterologists revealed that cold forceps polypectomy is the most commonly employed technique for diminutive polyps 1–3 mm in size (12). The polyp is grasped with standard or jumbo forceps that exit the channel of a therapeutic colonoscope at the 5 to 7 o’clock position and the forceps removed with the lesion in the jaws of the forceps (13). Cold forceps polypectomy is limited to the removal of small polyps ≤5 mm and can result in an incomplete resection rate as high as 61% (13,14). Hot forceps polypectomy is similar to cold, with the utilization of electrocautery to fulgurate residual lesion on the colonic mucosa (13).
Snare polypectomy
Snare polypectomy has been established as a safe and effective technique for resection of small colorectal polyps ≤10 mm in multiple studies (12,15,16). A snare device is passed through the working channel of a colonoscope and a metal ring deployed over the polyp (13). Once the polyp, along with 2 mm of healthy colonic mucosal margin is captured, the snare is closed until the base of the polyp is cut. Any bleeding at the base of the lesion is generally self-limiting. For snare electrocautery, the snare should be closed slowly, while the polyp is pulled away from the colonic mucosa to avoid deep thermal injury (13).
The current paradigm shift in endoscopic management of small colorectal polyps favors cold snare instead of hot snare polypectomy (13). A meta-analysis of 12 studies involving 2,481 patients who had polyps less than 10 mm removed with either hot or cold snare polypectomy demonstrated no statistical difference for complete resection rate and bleeding needing treatment. The hot snare polypectomy group had significantly longer total colonoscopy and polypectomy times (16).
There is only one systematic review and meta-analysis evaluating cold snare polypectomy for polyps greater than 10 mm (17). Eight studies with 522 polyps conducted between 2014 and 2018 were included with the mean polyp size of 17.5 mm. The overall intra- and post-procedure pooled bleeding rates were 0.7% and 0.5%, respectively. For polyps >20 mm (n=132) the intra-procedure pooled bleeding rate was 1.3% and there were no post-procedure bleeds. The pooled complete resection rate for all polyps was 99.3%.
Advanced polypectomy techniques
Endoscopic mucosal resection (EMR)
EMR generally refers to the technique of submucosal injection of a polyp followed by snare polypectomy (18). Submucosal injection technique can be considered prior to the polypectomy to create a submucosal cushion, assisting in the complete resection of the lesion. Saline is widely used for the submucosal injection since it is cheap and readily available. Diluted epinephrine (concentration of 1:100,000) is often added to promote hemostasis. Indigo Carmen or methylene blue, in a 0.04% concentration, can be added as well providing definition to the borders of the polyp and identification of the submucosal plane (19). Other submucosal injectable agents include hyaluronic acid, hypertonic saline, hydroxypropyl methyl cellulose, fibrinogen and glycerol. These agents are expensive, difficult to prepare, activate an inflammatory response, and challenging to deliver (20,21).
The goal of EMR is an en bloc resection using a precise injection technique to raise the lesion into the lumen and closer to the colonoscope. If that is not achievable, the polyp should be removed in as few sections as possible. The colonoscope is manipulated such that the polyp is placed at the 5 o’clock position where the instrument exits the working channel, and the injection needle penetrates the polyp proximally and perpendicularly just 2–3 mm behind the polyp. If a polyp is located at an area of angulation or draped across a fold, inject and resect the most challenging section of the polyp first (19). A well-placed injection will lift the mucosa while an extramural injection will cause no change in the mucosa. The volume of fluid needed to adequately lift a polyp is dependent on its size. Larger polyps may be approached with a series of sequential injections and resections (19). Follow up colonoscopy is recommended within 4-6 months of the procedure and then yearly for 3 years (19).
While EMR provides a safe way of avoiding deep thermal spread to the colonic wall when using electrocautery to resect large polyps, it has several limitations (20,21). Intra-procedure bleeding occurs in 11% of EMR cases when the polyp is greater than 20 mm (22). Bleeding can be controlled by snare tip cautery and clips. The post-procedure bleeding rate for these larger polyps is 6.2–7%, usually occurs within the first 48 hours, and is associated with right-sided lesions, increasing polyp size, and technical difficulties (22,23).
The perforation rate associated with EMR is 1–2% (24). Injury to the muscularis propria can be identified by a “target sign” on the resection side of the specimen and its mirror image in the colonic resection bed (25). A target sign consists of a circle with a white circumference, mucosa, and piece of muscularis propria in the middle. Clips should be applied to close the luminal defect.
Recurrence rates remain a challenge for piecemeal resections of polyps. A systematic review and meta-analysis of 33 studies found the recurrence rate after piecemeal and en bloc resections to be 20% and 3% (26). Over 90% of recurrences occurred within 6 months leading the authors to recommend follow-up colonoscopy at 6 months.
Endoscopic submucosal dissection (ESD)
ESD was originally developed in Japan in the early 20th century for the treatment of low risk gastric cancer (27). ESD indications now include colorectal lesions greater than 20 mm that were traditionally removed piecemeal (28). The goal of ESD is to remove lateral spreading tumors/lesions en bloc to decrease recurrence. ESD provides a curative option with en bloc resection of low risk submucosal invasive cancer (defined as well- or moderately differentiated adenocarcinoma with submucosal invasion ≤1,000 µm that cannot be accomplished by EMR) (29). Identifying appropriate lesions for ESD led to the development of the Kudo, Paris, and NICE classification systems to assess the level of submucosal invasion (30-32).
Similar to EMR, ESD starts with a submucosal injection that contains pigmentation to lift and mark the mucosal layer, followed by circumferential marking of the lesion with a needle knife that is connected to electrocautery (33,34). A plastic cap is attached to the tip of the colonoscope to help retract the lesion off of the base and provide hemostasis. The dissection is performed at the submucosal plane just above the muscularis propria to prevent deep thermal injury and perforation. Change in patient position can sometimes provide traction by gravity to pull the flap of tissue away during dissection and provide a better view of the submucosal plane (33).
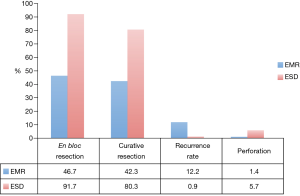
A meta-analysis including 8 studies with over 2,000 lesions compared EMR and ESD and their rates of en bloc resection, curative resection, recurrence, and adverse events [Figure 1 (35)]. It was worth noting that these studies consisted of historical cohort and case-control studies, with no randomized controlled trials. The authors concluded that ESD has a higher rate of en bloc and curative resection with a lower recurrence rate than EMR (91.7% vs. 46.7%, 80.3% vs. 42.3%, and 0.9% vs. 12.2%), but is more likely to result in perforation (5.7% vs. 1.4%) and takes 3 times longer even in experienced hands (35). The rates of delayed bleeding are similar between ESD and EMR (3.5% vs. 2%).
ESD is a technically challenging and time-consuming procedure. The higher prevalence and screening of gastric cancer in Japan allows endoscopists to first master this technique in the distal stomach, where early neoplasms are most commonly found. Gastric anatomy makes it easier to perform endoscopic interventions as compared to the angulations and folds in the colon where the endoscope is more difficult to maneuver and the muscle layer is thin (18,36). In the Western world where gastric cancer incidence is much lower, endoscopists lack the opportunity to perfect the techniques of ESD in easier locations prior to graduating to more challenging ones in the colon (27,29). For endoscopists with experience in gastric ESD, the learning curve for colon ESD ranges from 30–50 cases (37,38). For endoscopists in the United States without experience in gastric ESD using an un-tutored prevalence based approach the number of cases needed to be proficient is estimated to be 250 (39).
Fuccio et al. recommend ESD be used specifically for lesions that are highly suspicious for submucosal invasive cancers involving only Sm1 and EMR be employed for all other lateral spreading lesions (40). Their meta-analysis of 11,260 ESDs identified submucosal invasive cancers involving Sm1 in 8% of the lesions while deeper lesions were seen in 7.7% of specimens. Standard of care for patients with lesions greater than Sm1 is surgical resection. The authors estimated that 16.7 ESDs would need to be performed to prevent one surgery.
Combined laparoscopic and endoscopic resections (CLERs)
CLERs are described for difficult polyps that are not amenable to standard approaches, such as EMR or ESD. CLER encompasses a spectrum of procedures employing both laparoscopic and endoscopic techniques to improve visualization of the difficult polyp, and thus the safety and precision of the procedure. CLER offers the advantage of the ability to fully mobilize the colon laparoscopically and guide the colonoscope under direct visualization. The following table reviews single center studies with at least 30 patients demonstrating success rates from 74–96% (41) (Table 1). Length of stay is 1–2 days and recurrence rates are low. Patient selection and endoscopist/surgeon experience is essential to the successful completion of these novel procedures.
Table 1
| First author, year | Number of patients | Success rate (%) | Median size (cm) | Time (minutes) | Length of stay (days) | Recurrence N, follow-up in months |
|---|---|---|---|---|---|---|
| Franklin, 2007 (42) | 110 | 83 | 2.3 | NS | 1 | NS |
| Wilhelm, 2009 (43) | 146 | 82 | NS | 92 | 8 | 1, 31 months |
| Goh, 2014 (44) | 30 | 73 | 1.4 | 105 | 2 | 0, 20 months |
| Lee, 2013 (45) | 65 | 74 | 3.0 | 145 | 1 | 5, 65 months |
| Crawford, 2015 (46) | 30 | 96 | 4.0 | 71.5 | 2 | 1, 22 months |
NS, not-specified.
Surveillance after polypectomy
The current recommendations for surveillance colonoscopy after successful polypectomy are based on risk stratification. According to the 2012 US Multi-Society Task Force Update patients with low risk, defined as one or two small tubular adenomas, should undergo surveillance at a 5–10-year interval. Patients with high risk polyps, defined as adenoma ≥1 cm, high grade dysplasia, or villous histology, should undergo surveillance in 3 years (47). There is no clear evidence to support when to perform surveillance in patients who have undergone piecemeal polypectomy for laterally spreading tumors despite their significant recurrence rate.
Malignant polyps
Malignant polyps comprise 12% of resected polyps (48). It is essential for endoscopists to identify features of malignant polyps at the time of the index colonoscopy and manage them appropriately by marking the polypectomy site, resecting them in entirety instead of piecemeal, and referring the patient for surgical evaluation.
Differentiating between superficial (<1 mm) and deep (>1 mm) submucosal invasion is essential for management (endoscopy or surgery) of malignant polyps. Two commonly available techniques to identify submucosal invasion are narrow band imaging (NBI) and magnifying chromoendoscopy (M-CE). Systematic review and meta-analysis including 17 studies determined the sensitivity and specificity of NBI and M-CE as 74% and 84% and 98% and 97%, respectively (48). If a polyp is too large to safely remove or has deep submucosal invasion, the lesion should be marked and the patient referred for surgical resection.
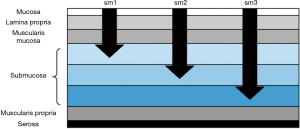
Many times malignancy is discovered when the pathology results come back. In these cases, multiple factors determine management: morphology, depth of invasion, margins, and histology (49). Morphology and depth of invasion are closely linked with the definitions of invasion and associated risk is dependent on whether the polyp is sessile or pedunculated. For sessile polyps, removed en bloc, the depth of submucosal invasion (Sm1-invasion into the upper third, Sm2-invasion into the middle third, Sm3-invasion in the lower third) determines the risk of lymph node metastases [Figure 2 (50,51)]. Invasion into the lower third of the submucosa (Sm3) is associated with a 23% rate of lymph node metastases (52).
For pedunculated polyps, removed en bloc, Haggitt levels 1–4 are used for depth of invasion. Level 1 indicates invasion of the submucosa confined to the head of the polyp, level 2 is submucosal invasion in the neck of the polyp, level 3 is submucosal invasion in the stalk, and level 4 is submucosal invasion at the base of the polyp (53). Level 4 invasion is associated with a 27% rate of lymph node metastases (54).
Margins are determined ideally in pedunculated or sessile polyps removed en bloc. Piecemeal resection makes margin determinations unreliable. For en bloc polyp resections, margins of at least 2 mm are recommended (55,56). Malignant polyp features that mandate surgical resection with appropriate segmental colectomy are lymphovascular invasion, poor differentiation, piecemeal excision, submucosal invasion >1 mm, and margins less than 1 mm (2 mm used as well) (57,58). Tumor budding, defined as a single cell or cluster of tumor cells not exceeding four, has been added by the College of American Pathologists to their colon cancer protocol (59). Tumor budding is associated with increased risk for lymph node metastasis and is included as an adverse risk factor in the National Comprehensive Cancer Network 2019 guidelines for colon and rectal cancers (59-63).
Conclusions
Endoscopic polypectomy is an important therapeutic option in the treatment of colorectal polyps. Different techniques are currently described in the literature: forceps, snare, EMR, and ESD. Endoscopists should be familiar with each technique and complication profile in determining which one to offer based on patient and lesion characteristics as well as their own skill set. Future studies are necessary in comparing the safety and efficacy of some of the novel techniques such as ESD.
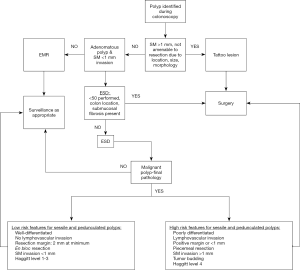
Please see Figure 3 for advanced polyp pathway.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Jeffrey M. Marks and Ryan M. Juza) for the series “Surgical Endoscopy: Essential Skills in Gastrointestinal Surgery” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2019.08.09). The series “Surgical Endoscopy: Essential Skills in Gastrointestinal Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- CDC. Colorectal cancer screening in the U.S. Behavioral risk factor surveillance system-2016. CDC, 2019 [updated February 4, 2019; cited 2019.
- American Cancer Society guideline for colorectal cancer screening, 2018. American Cancer Society, 2018. updated 2018; cited 2019.
- Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology 2008;134:1570-95. [Crossref] [PubMed]
- Ponugoti PL, Cummings OW, Rex DK. Risk of cancer in small and diminutive colorectal polyps. Dig Liver Dis 2017;49:34-7. [Crossref] [PubMed]
- Kaminski MF, Bretthauer M, Zauber AG, et al. The NordICC Study: rationale and design of a randomized trial on colonoscopy screening for colorectal cancer. Endoscopy 2012;44:695-702. [Crossref] [PubMed]
- Corley DA, Jensen CD, Marks AR, et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med 2014;370:1298-306. [Crossref] [PubMed]
- Calderwood AH, Jacobson BC. Colonoscopy quality: metrics and implementation. Gastroenterol Clin North Am 2013;42:599-618. [Crossref] [PubMed]
- Do A, Weinberg J, Kakkar A, et al. Reliability of adenoma detection rate is based on procedural volume. Gastrointest Endosc 2013;77:376-80. [Crossref] [PubMed]
- Rex DK, Schoenfeld PS, Cohen J, et al. Quality indicators for colonoscopy. Am J Gastroenterol 2015;110:72-90. [Crossref] [PubMed]
- le Clercq CM, Bouwens MW, Rondagh EJ, et al. Postcolonoscopy colorectal cancers are preventable: a population-based study. Gut 2014;63:957-63. [Crossref] [PubMed]
- Robertson DJ, Lieberman DA, Winawer SJ, et al. Colorectal cancers soon after colonoscopy: a pooled multicohort analysis. Gut 2014;63:949-56. [Crossref] [PubMed]
- Singh N, Harrison M, Rex DK. A survey of colonoscopic polypectomy practices among clinical gastroenterologists. Gastrointest Endosc 2004;60:414-8. [Crossref] [PubMed]
- Fyock CJ, Draganov PV. Colonoscopic polypectomy and associated techniques. World J Gastroenterol 2010;16:3630-7. [Crossref] [PubMed]
- Ferlitsch M, Moss A, Hassan C, et al. Colorectal polypectomy and endoscopic mucosal resection (EMR): European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy 2017;49:270-97. [Crossref] [PubMed]
- Shinozaki S, Kobayashi Y, Hayashi Y, et al. Efficacy and safety of cold versus hot snare polypectomy for resecting small colorectal polyps: Systematic review and meta-analysis. Dig Endosc 2018;30:592-9. [Crossref] [PubMed]
- Qu J, Jian H, Li L, et al. Effectiveness and safety of cold versus hot snare polypectomy: A meta-analysis. J Gastroenterol Hepatol 2019;34:49-58. [Crossref] [PubMed]
- Thoguluva Chandrasekar V, Spadaccini M, Aziz M, et al. Cold snare endoscopic resection of nonpedunculated colorectal polyps larger than 10 mm: a systematic review and pooled-analysis. Gastrointest Endosc 2019;89:929-936.e3. [Crossref] [PubMed]
- Moss A, Nalankilli K. Standardisation of polypectomy technique. Best Pract Res Clin Gastroenterol 2017;31:447-53. [Crossref] [PubMed]
- Bourke M. Endoscopic mucosal resection in the colon: A practical guide. Tech Gastrointest Endosc 2011;13:35-49. [Crossref]
- Angarita FA, Feinberg AE, Feinberg SM, et al. Management of complex polyps of the colon and rectum. Int J Colorectal Dis 2018;33:115-29. [Crossref] [PubMed]
- Ahlenstiel G, Hourigan LF, Brown G, et al. Actual endoscopic versus predicted surgical mortality for treatment of advanced mucosal neoplasia of the colon. Gastrointest Endosc 2014;80:668-76. [Crossref] [PubMed]
- Burgess NG, Bourke MJ. Endoscopic resection of colorectal lesions: The narrowing divide between East and West. Dig Endosc 2016;28:296-305. [Crossref] [PubMed]
- Metz AJ, Bourke MJ, Moss A, et al. Factors that predict bleeding following endoscopic mucosal resection of large colonic lesions. Endoscopy 2011;43:506-11. [Crossref] [PubMed]
- Holt BA, Bourke MJ. Wide field endoscopic resection for advanced colonic mucosal neoplasia: current status and future directions. Clin Gastroenterol Hepatol 2012;10:969-79. [Crossref] [PubMed]
- Swan MP, Bourke MJ, Moss A, et al. The target sign: an endoscopic marker for the resection of the muscularis propria and potential perforation during colonic endoscopic mucosal resection. Gastrointest Endosc 2011;73:79-85. [Crossref] [PubMed]
- Belderbos TD, Leenders M, Moons LM, et al. Local recurrence after endoscopic mucosal resection of nonpedunculated colorectal lesions: systematic review and meta-analysis. Endoscopy 2014;46:388-402. [Crossref] [PubMed]
- Bergman JJ. How to justify endoscopic submucosal dissection in the Western world. Endoscopy 2009;41:988-90. [Crossref] [PubMed]
- Draganov PV, Wang AY, Othman MO, et al. AGA Institute Clinical Practice Update: Endoscopic Submucosal Dissection in the United States. Clin Gastroenterol Hepatol 2019;17:16-25.e1. [Crossref] [PubMed]
- Bourke MJ, Neuhaus H, Bergman JJ. Endoscopic Submucosal Dissection: Indications and Application in Western Endoscopy Practice. Gastroenterology 2018;154:1887-1900.e5. [Crossref] [PubMed]
- Kudo S, Kashida H, Nakajima T, et al. Endoscopic diagnosis and treatment of early colorectal cancer. World J Surg 1997;21:694-701. [Crossref] [PubMed]
- The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc 2003;58:S3-43. [Crossref] [PubMed]
- Hayashi N, Tanaka S, Hewett DG, et al. Endoscopic prediction of deep submucosal invasive carcinoma: validation of the narrow-band imaging international colorectal endoscopic (NICE) classification. Gastrointest Endosc 2013;78:625-32. [Crossref] [PubMed]
- Fuccio L, Ponchon T. Colorectal endoscopic submucosal dissection (ESD). Best Pract Res Clin Gastroenterol 2017;31:473-80. [Crossref] [PubMed]
- Pettke E SA, Whelan RL. Endoscopic submucosal dissection and endoscopic mucosal resection methods for the removal of large sessile polyps. Semin Colon Rectal Surg 2017;28:17-23. [Crossref]
- Fujiya M, Tanaka K, Dokoshi T, et al. Efficacy and adverse events of EMR and endoscopic submucosal dissection for the treatment of colon neoplasms: a meta-analysis of studies comparing EMR and endoscopic submucosal dissection. Gastrointest Endosc 2015;81:583-95. [Crossref] [PubMed]
- Yamamoto S, Uedo N, Ishihara R, et al. Endoscopic submucosal dissection for early gastric cancer performed by supervised residents: assessment of feasibility and learning curve. Endoscopy 2009;41:923-8. [Crossref] [PubMed]
- Shiga H, Kuroha M, Endo K, et al. Colorectal endoscopic submucosal dissection (ESD) performed by experienced endoscopists with limited experience in gastric ESD. Int J Colorectal Dis 2015;30:1645-52. [Crossref] [PubMed]
- Jeon HH, Lee HS, Youn YH, et al. Learning curve analysis of colorectal endoscopic submucosal dissection (ESD) for laterally spreading tumors by endoscopists experienced in gastric ESD. Surg Endosc 2016;30:2422-30. [Crossref] [PubMed]
- Zhang X, Ly EK, Nithyanand S, et al. Learning Curve for Endoscopic Submucosal Dissection With an Untutored, Prevalence-Based Approach in the United States. Clin Gastroenterol Hepatol 2019. [Epub ahead of print].
- Fuccio L, Repici A, Hassan C, et al. Why attempt en bloc resection of non-pedunculated colorectal adenomas? A systematic review of the prevalence of superficial submucosal invasive cancer after endoscopic submucosal dissection. Gut 2018;67:1464-74. [Crossref] [PubMed]
- Aslani N, Alkhamesi NA, Schlachta CM. Hybrid Laparoendoscopic Approaches to Endoscopically Unresectable Colon Polyps. J Laparoendosc Adv Surg Tech A 2016;26:581-90. [Crossref] [PubMed]
- Franklin ME Jr, Leyva-Alvizo A, Abrego-Medina D, et al. Laparoscopically monitored colonoscopic polypectomy: an established form of endoluminal therapy for colorectal polyps. Surg Endosc 2007;21:1650-3. [Crossref] [PubMed]
- Wilhelm D, von Delius S, Weber L, et al. Combined laparoscopic-endoscopic resections of colorectal polyps: 10-year experience and follow-up. Surg Endosc 2009;23:688-93. [Crossref] [PubMed]
- Goh C, Burke JP, McNamara DA, et al. Endolaparoscopic removal of colonic polyps. Colorectal Dis 2014;16:271-5. [Crossref] [PubMed]
- Lee SW, Garrett KA, Shin JH, et al. Dynamic article: long-term outcomes of patients undergoing combined endolaparoscopic surgery for benign colon polyps. Dis Colon Rectum 2013;56:869-73. [Crossref] [PubMed]
- Crawford AB, Yang I, Wu RC, et al. Dynamic article: combined endoscopic-laparoscopic surgery for complex colonic polyps: postoperative outcomes and video demonstration of 3 key operative techniques. Dis Colon Rectum 2015;58:363-9. [Crossref] [PubMed]
- Lieberman DA, Rex DK, Winawer SJ, et al. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2012;143:844-57. [Crossref] [PubMed]
- Zhang QW, Teng LM, Zhang XT, et al. Narrow-band imaging in the diagnosis of deep submucosal colorectal cancers: a systematic review and meta-analysis. Endoscopy 2017;49:564-80. [Crossref] [PubMed]
- Aarons CB, Shanmugan S, Bleier JI. Management of malignant colon polyps: current status and controversies. World J Gastroenterol 2014;20:16178-83. [Crossref] [PubMed]
- Kudo S. Endoscopic mucosal resection of flat and depressed types of early colorectal cancer. Endoscopy 1993;25:455-61. [Crossref] [PubMed]
- Kikuchi R, Takano M, Takagi K, et al. Management of early invasive colorectal cancer. Risk of recurrence and clinical guidelines. Dis Colon Rectum. 1995;38:1286-95. [Crossref] [PubMed]
- Nascimbeni R, Burgart LJ, Nivatvongs S, et al. Risk of lymph node metastasis in T1 carcinoma of the colon and rectum. Dis Colon Rectum 2002;45:200-6. [Crossref] [PubMed]
- Haggitt RC, Glotzbach RE, Soffer EE, et al. Prognostic factors in colorectal carcinomas arising in adenomas: implications for lesions removed by endoscopic polypectomy. Gastroenterology 1985;89:328-36. [Crossref] [PubMed]
- Nivatvongs S, Rojanasakul A, Reiman HM, et al. The risk of lymph node metastasis in colorectal polyps with invasive adenocarcinoma. Dis Colon Rectum 1991;34:323-8. [Crossref] [PubMed]
- Netzer P, Binek J, Hammer B, et al. Significance of histologic criteria for the management of patients with malignant colorectal polyps and polypectomy. Scand J Gastroenterol 1997;32:910-6. [Crossref] [PubMed]
- Seitz U, Bohnacker S, Seewald S, et al. Is endoscopic polypectomy an adequate therapy for malignant colorectal adenomas? Presentation of 114 patients and review of the literature. Dis Colon Rectum 2004;47:1789-96; discussion 1796-7.
- Ramirez M, Schierling S, Papaconstantinou HT, et al. Management of the malignant polyp. Clin Colon Rectal Surg 2008;21:286-90. [Crossref] [PubMed]
- Butte JM, Tang P, Gonen M, et al. Rate of residual disease after complete endoscopic resection of malignant colonic polyp. Dis Colon Rectum 2012;55:122-7. [Crossref] [PubMed]
- Cho SJ, Kakar S. Tumor Budding in Colorectal Carcinoma: Translating a Morphologic Score Into Clinically Meaningful Results. Arch Pathol Lab Med 2018;142:952-7. [Crossref] [PubMed]
- Bosch SL, Teerenstra S, de Wilt JH, et al. Predicting lymph node metastasis in pT1 colorectal cancer: a systematic review of risk factors providing rationale for therapy decisions. Endoscopy 2013;45:827-34. [Crossref] [PubMed]
- Cappellesso R, Luchini C, Veronese N, et al. Tumor budding as a risk factor for nodal metastasis in pT1 colorectal cancers: a meta-analysis. Hum Pathol 2017;65:62-70. [Crossref] [PubMed]
- Beaton C, Twine CP, Williams GL, et al. Systematic review and meta-analysis of histopathological factors influencing the risk of lymph node metastasis in early colorectal cancer. Colorectal Dis 2013;15:788-97. [Crossref] [PubMed]
- NCCN. National Comprehensive Cancer Network. Colon Cancer 2019; Version 2. 2019.
Cite this article as: Lam W, Times M. Colonoscopic polypectomy. Ann Laparosc Endosc Surg 2019;4:97.