Minimally invasive surgery for submucosal benign esophageal tumors: indications, preoperative investigations, patient selection, and clinical outcomes
Introduction
Submucosal benign esophageal tumors are rare compared to malignant tumors. More recent data have shown an increased prevalence of these lesions, which likely reflects improvement in diagnosis and screening (1). Benign esophageal masses can be classified as intramural [leiomyoma, gastrointestinal stromal tumor (GIST), schwannoma], intraluminal (fibrovascular polyps, lipoma, hemangioma, granular cell tumor), and extraesophageal (duplication and cysts).
Leiomyoma, the most commonly encountered submucosal esophageal tumor, is a mesenchymal neoplasm usually originating from the muscularis propria that is composed by bundles of spindle-cells with rare mitoses. The most common anatomical location is the middle or lower third of the esophagus. The tumor mass ranges from 0.5 to 5 cm in diameter and appears encapsulated with round or oval morphology. A marked deformity of the esophageal lumen is expected to occur in patients with large, horse-shoe shaped tumors and a prevalent intra-luminal growth pattern. Typically, immunohistochemical analysis is positive for smooth muscle actin and desmin.
Leiomyomas are usually asymptomatic because of the slow growth and the capacity of the esophageal lumen to adapt to extrinsic compression. The most common symptom is dysphagia especially when the tumor growth is intraluminal. Other symptoms caused by intraluminal growth may be substernal discomfort, chest pain, regurgitation, and heartburn (2). These non-specific symptoms may result from an associated condition (hiatus hernia, gastroesophageal reflux disease). In patients with extramucosal growth, the mass can cause compression on the airways or the inferior vena cava (3). Endoluminal bleeding from large ulcerated leiomyomas is extremely rare. In such patients, the diagnosis of a GIST should rather be considered.
Natural history
The natural history of these neoplasms is favorable. In fact, small and asymptomatic submucosal masses can be managed conservatively. Patients should be reassured about the benign nature of the tumor, and endoscopic ultrasound surveillance can be proposed in order to detect any progressive change in tumor size or shape. Tumor growth over time is an indication for resection. Other indications include the appearance of symptoms, mucosal ulceration, regional node enlargement, and irregularity of the mass. A recent study (4) has estimated that leiomyoma growth during surveillance was about 0.5 mm during a mean follow-up longer than 5 years.
Diagnosis
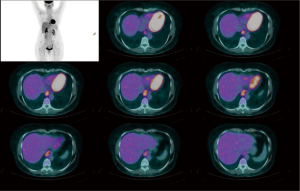
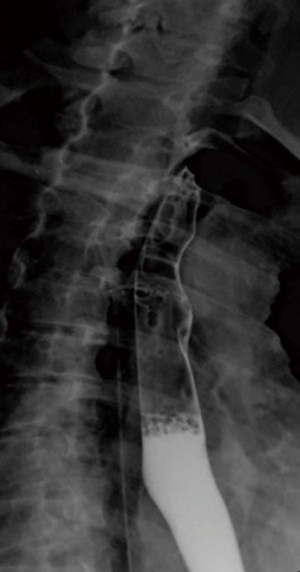
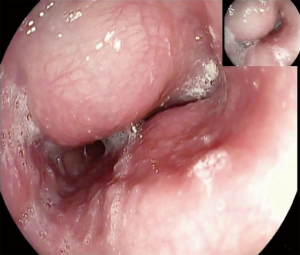
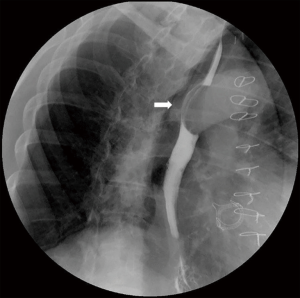
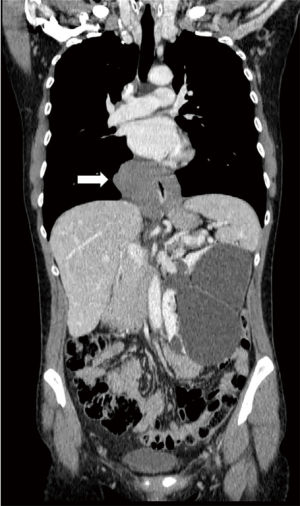
In the majority of patients, the diagnosis of leiomyoma is incidental during barium swallow study, upper gastrointestinal endoscopy, or computed tomography (CT) scan of the chest. A thorough workup is needed in order to plan the best therapeutic strategy. Endoscopic evaluation typically reveals a bulging in the esophageal lumen with an intact mucosa (Figure 1) that allows smooth passage of the scope. Mucosal biopsies should be discouraged in the majority of patients with a submucosal esophageal mass (5). The radiological features of leiomyoma on a barium swallow study are typical (Figure 2). Computed tomography of the chest is particularly useful in case of giant leiomyomas with extraluminal growth to exclude malignancy (Figure 3). The leiomyoma has a homogeneous and hypoechoic appearance at endoscopic ultrasonography (EUS), and analysis of the wall layers allows to identify the site of origin of the mass. A fine-needle aspiration biopsy can be performed if there is a suspicion of GIST or malignancy. Positron-emission tomography (PET) scans with fluorine-18-fluorideooxyglucose may occasionally cause a false positive test as in other benign conditions, especially in patients with CD34 negative lesions (6) (Figure 4).
Although extra-mucosal cysts are extremely rare, the differential diagnosis should be considered in patients with submucosal tumors. These cysts have mixed embryogenesis and can be congenital (i.e., bronchogenic, gastric, duplication, or inclusion cysts), neuroenteric, or acquired (retention cysts) (7). About 40% of extramucosal cysts are diagnosed in patients between the second and the fourth decade of age (8), and more than one third of patients are asymptomatic at presentation (9). Most common clinical presentation in adults consists of upper gastrointestinal symptoms such as gastroesophageal reflux, dysphagia, and epigastric pain.
Treent
Traditionally, open resection has been the standard of care for esophageal leiomyomas (5,10); more recently, minimally invasive techniques have become increasingly used in specialized centers (11,12). Due to the benign nature of the tumor and the presence of a relatively easy cleavage plan, the most common surgical procedure is minimally invasive enucleation and reconstruction of the muscle layer through a thoracoscopic or laparoscopic approach. For leiomyomas of the gastroesophageal junction, preservation of sphincter integrity and function is of paramount importance and special care is required to prevent postoperative gastroesophageal reflux.
Technique of leiomyoma enucleation
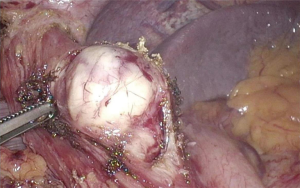
Regardless of the route of approach, the procedure consists of a myotomy extended above and below the leiomyoma with maximal preservation of the muscle fibers. Intraoperative endoscopy allows air inflation and trans-illumination during the procedure and may increase safety (13). External traction applied with a transfixing suture can facilitate identification of the cleavage plan from the mucosa and progressive enucleation of the mass. This can avoid the effect of tumor fragmentation by direct grasping. An alternative technique consists of inflating an endoscopic balloon and pushing the tumor outward to facilitate enucleation. After tumor removal, the mucosa needs to be carefully inspected to ensure integrity, and the longitudinal muscular fibers are approximated with interrupted sutures. Symptomatic pseudodiverticula have been reported in case of enucleation procedures not followed by suture of the residual muscle edges (5).
During the last three decades, minimally invasive surgery has showed clear advantages over the open approach. Thoracoscopic, laparoscopic, endoscopic, and hybrid procedures have almost substituted open surgery in referral hospitals. Laparoscopic enucleation is preferred for tumors of the distal esophagus because it provides a perfect exposure of the lower mediastinum, and allows to perform an antireflux repair (Dor or Toupet fundoplication) (Figure 5). Five ports are required, and critical steps of the operation can be performed using endoscopic assistance.
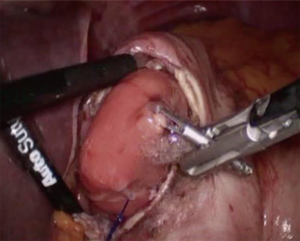
In patients with giant tumors prolapsing through the gastroesophageal junction, a transgastric approach is needed (14). Preoperative endoscopic clipping of the distal tip of the tumor and intraoperative endoscopic assistance facilitate the procedure. Endoclips are easily identified through a small anterior gastrotomy and the mass is exteriorized. Excision of the leiomyoma is performed using a linear endostapler paying attention to prevent obstruction of the esophageal outlet. After manual or stapled closure of the gastrotomy incision, an anterior fundoplication is performed to prevent gastroesophageal reflux (Figure 6).
Endoscopic submucosal dissection through retroflexed video-endoscopy has also proven safe and effective in patients with leiomyomas of the cardia (12,15). With the patient under general anesthesia and in the supine position, diluted epinephrine is injected with a sclerotherapy needle in the submucosal plane to lift the mucosa. A CO2 insufflator is preferred. The mucosal incision overlying the mass is preferably performed with the scope in a retroflexed view starting at the lower border of the lesion. The electrosurgical knife (IT-knife 2, Olympus) and the hook knife are used. The mucosal edges are closed using 3–5 metallic clips. At the end of the procedure the tumor is retrieved with a plastic endobag and extracted through the mouth. Pneumoperitoneum in the absence of perforation is a potential complication of the procedure, and is due to air/CO2 filtration through the thin posterior gastric wall at the cardia not covered by serosa layer. This complication can be treated conservatively or may require bedside percutaneous drainage (16).
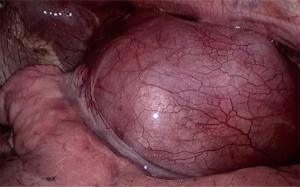
Thoracoscopic enucleation is reserved to leiomyomas of the upper and middle third of the esophagus. A 3-port thoracoscopic approach in the prone or semi-prone decubitus position provides a good exposure of the entire length of the thoracic esophagus (Figure 7) while significantly improving global oxygen delivery and decreasing pulmonary shunt (17,18). After incision of the mediastinal pleura, the leiomyoma is identified and enucleated as in the laparoscopic approach, and the muscle layer carefully approximated. For leiomyomas located in the upper thoracic esophagus, division of the arch of the azygos vein using hemolock clips or a linear stapler may be required for better exposure.
More rarely, in patients with diffuse leiomyomatosis of the gastroesophageal junction or giant masses not eligible for enucleation because of large intraoperative mucosal injuries or the inability to reapproximate residual muscle edges, transthoracic or transhiatal video-assisted esophagectomy and gastric conduit reconstruction may be indicated (19).
Management of extra-mucosal cysts
Patients who are asymptomatic can be surveilled with EUS or CT-scan. Conversely, the presence of dysphagia, respiratory symptoms, weight loss, increase in cyst size, and the suspicion of malignancy are indications for surgical therapy. Cyst aspiration has been proposed in patients unfit for surgery, but thoracoscopic or laparoscopic resection remain the preferred treent in expert centers (20). The basic tenets of surgical therapy are the same described for leiomyoma, but stapling is preferred in case of dense adhesions to the esophageal wall.
Results of leiomyoma enucleation
In our institution, during the period 2002–2017, 35 patients were considered eligible for surgery and underwent leiomyoma enucleation through thoracoscopy (n=15), laparoscopy (n=15), and endoscopy (n=5). No conversion to open surgery and no esophagectomies were necessary in this cohort of patients which include individuals with giant (up to 12 cm) tumors. Overall morbidity rate was 14.3% and there was no mortality. Postoperative wound pain was treated with paracetamol and/or ketorolac as needed. Routine gastrografin swallow study was performed on postoperative day 1. Patients were allowed to drink and to remain on a soft diet until the first follow-up visit 1 month later. In all patients, histopathology confirmed the diagnosis of benign leiomyoma. The median diameter of the mass was 7.5 cm. Immunohistochemistry resulted positive for smooth-muscle actin and desmin, and negative for CD117 and CD34. The median follow-up was 49 months and all patients were followed for at least 1 year. No recurrences of leiomyoma were documented at endoscopy and/or barium swallow study (Figure 8). At the latest follow-up, the SF-36 scores were unchanged compared to baseline. However, reflux symptoms and PPI use occurred more frequently after endoscopic treent (12).
Conclusions
The approach to submucosal benign esophageal tumors has changed significantly compared to the previous decades. The advent of minimally invasive techniques has probably decreased the threshold for surgery due to lesser morbidity and greater patient comfort. Today, minimally invasive enucleation of leiomyoma should be carried out according to tumor location and morphology. Laparoscopy and thoracoscopy should represent the initial approach even in patients who present with large masses at unfavorable locations. Compared to the endoscopic approach, laparoscopic leiomyoma enucleation has the potential to eliminate dependency from proton-pump inhibitors. In the future, third-space endoscopy is likely to play a more important role in patients with submucosal tumors.
Acknowledgments
Work supported by AIRES (Associazione Italiana Ricerca ESofago).
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Muhammed Ashraf Memon and Abe Fingerhut) for the series “Laparoendoscopic Surgery for Benign Oesophagogastric Conditions” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2019.06.13). The series “Laparoendoscopic Surgery for Benign Oesophagogastric Conditions” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rice TW. Benign esophageal tumors: esophagoscopy and endoscopic esophageal ultrasound. Semin Thorac Cardiovasc Surg 2003;15:20-6. [Crossref] [PubMed]
- Choong CK, Meyers BF. Benign esophageal tumors: introduction, incidence, and clinical features. Semin Thorac Cardiovasc Surg 2003;15:3-8. [Crossref] [PubMed]
- Lovece A, Milito P, Asti E, et al. Giant esophageal leiomyoma causing severe hypertension. BMJ Case Rep 2016;2016: [Crossref] [PubMed]
- Codipilly DC, Fang H, Alexander JA, et al. Subepithelial esophageal tumors: a single-center review of resected and surveilled lesions. Gastrointest Endosc 2018;87:370-7. [Crossref] [PubMed]
- Bonavina L, Segalin A, Rosati R, et al. Surgical therapy of esophageal leiomyoma. J Am Coll Surg 1995;181:257-62. [PubMed]
- Depypere L, Coosemans W, Nafteux P. Fluorine-18-fluorodeoxyglucose uptake in a benign oesophageal leiomyoma: a potential pitfall in diagnosis. Interact Cardiovasc Thorac Surg 2012;14:234-6. [Crossref] [PubMed]
- Arbona JL, Fazzi GF, Mayoral J. Congenital esophageal cysts: case report and review of the literature. Am J Gastroenterol 1984;79:177-82. [PubMed]
- Posthlethwait RW, Lowe JE. Benign tumors and cysts of the esophagus. In: Zuidema GD, Orringer MB, editors. Shackelford’s Surgery of the Alimentary Tract. Philadelphia: WB Saunders, 1996;1:369-86.
- Cioffi U, Bonavina L, De Simone M, et al. Presentation and surgical management of bronchogenic and esophageal duplication cysts in adults. Chest 1998;113:1492-96. [Crossref] [PubMed]
- Kent M, d’Amato T, Nordman C, et al. Minimally invasive resection of benign esophageal tumors. J Thorac Cardiovasc Surg 2007;134:176-81. [Crossref] [PubMed]
- Shin S, Choi YS, Shim YM, et al. Enucleation of esophageal submucosal tumors: a single institution's experience. Ann Thorac Surg 2014;97:454-9. [Crossref] [PubMed]
- Milito P, Asti E, Aiolfi A, et al. Clinical outcomes of minimally invasive enucleation of leiomyoma of the esophagus and esophagogastric junction. J Gastrointest Surg 2019; [Epub ahead of print]. [Crossref] [PubMed]
- Jeon HW, Choi MG, Lim CH, et al. Intraoperative esophagoscopy provides accuracy and safety in video-assisted thoracoscopic enucleation of benign esophageal submucosal tumors. Dis Esophagus 2015;28:437-41. [Crossref] [PubMed]
- Xu X, Chen K, Zhou W, et al. Laparoscopic transgastric resection of gastric submucosal tumors located near the esophagogastric junction. J Gastrointest Surg 2013;17:1570-5. [Crossref] [PubMed]
- Park YS, Park SW, Kim TI, et al. Endoscopic enucleation of upper GI submucosal tumor by using an insulated-tip electrosurgical knife. Gastrointest Endosc 2004;59:409-15. [Crossref] [PubMed]
- Siboni S, Bona D, Abate E, et al. Tension pneumoperitoneum following endoscopic submucosal dissection of leiomyoma of the cardia. Endoscopy 2010;42:E152 [Crossref] [PubMed]
- Palanivelu C, Rangarajan M, Senthilkumar R, et al. Combined thoracoscopic and endoscopic management of mid-esophageal benign lesions: use of the prone patient position. Surg Endosc 2008;22:250-4. [Crossref] [PubMed]
- Bonavina L, Laface L, Abate E, et al. Comparison of ventilation and cardiovascular parameters between prone thoracoscopic and Ivor Lewis esophagectomy. Updates Surg 2012;64:81-5. [Crossref] [PubMed]
- Lee LS, Singhal S, Brinster CJ, et al. Current management of esophageal leiomyoma. J Am Coll Surg 2004;198:136-46. [Crossref] [PubMed]
- Noguchi T, Hashimoto T, Takeno S, et al. Laparoscopic resection of esophageal duplication cyst in an adult. Dis Esophagus 2003;16:148-50. [Crossref] [PubMed]
Cite this article as: Asti E, Siboni S, Sironi A, Barbieri L, Bonavina L. Minimally invasive surgery for submucosal benign esophageal tumors: indications, preoperative investigations, patient selection, and clinical outcomes. Ann Laparosc Endosc Surg 2019;4:84.