Laparoscopic surgery for gastroesophageal reflux disease: Nissen, Toupet or anterior fundoplication
Introduction
Gastroesophageal reflux disease (GERD) is defined as a chronic condition which develops when the reflux of stomach contents causes troublesome symptoms and/or complications (1). GERD is one of the most common gastrointestinal disorders worldwide; the estimated prevalence of patients experiencing at least weekly heartburn or regurgitation in the Western world ranges from 10% to 30% (2). However, the real prevalence of the disease is probably higher than reported, because patients with milder symptoms use over-the-counter remedies instead of consulting health care providers.
The goals of GERD treatment are the control of symptoms, prevention of GERD complications and improvement in patients’ health-related quality of life. GERD treatment relies on lifestyle and diet modification, acid-suppressive medical therapy and laparoscopic anti-reflux surgery (LARS). The American College of Gastroenterology recommends lifestyle interventions, such as smoking cessation, head of the bed elevation, avoidance of potentially triggering foods and weight loss as the first therapeutic approaches for GERD (3). However, the level of evidence is low, and to date there is no evidence to support these modifications as primary GERD treatment (4).
Proton Pump Inhibitors (PPI) are the mainstay of medical therapy for GERD. These medications are the most effective medical treatment for GERD, relieving heartburn and healing erosive esophagitis in the majority of patients. However, they have several drawbacks. First, long-term continuative PPI therapy is needed to maintain healing of esophagitis over time. Secondly, PPI are less effective in patients with regurgitation, and response rates to PPI are even lower in patients with atypical symptoms (5). Finally, the long-term use of PPI is associated with higher risks of community-acquired infections, hip fractures and osteoporosis (6-8).
In a metanalysis of seven randomized controlled trials (RCTs) comparing medical therapy to LARS for GERD, Rickenbacher et al. found that surgery was more effective than medical treatment in improving symptoms and health-related quality of life in the short to medium follow-up (9). In addition, several studies confirmed these results also in the long-term follow-up (10-13).
Preoperative assessment of GERD
Clinical history
The clinical diagnosis of GERD is based on the presence of typical symptoms such as heartburn and regurgitation, and on a favorable response to a trial of empiric medical therapy with PPI, the so-called “PPI trial” (3). However, many studies demonstrated that a GERD diagnosis based on symptoms alone was not reliable. Several other diseases, such as irritable bowel syndrome, gallstone disease and coronary artery disease could be misdiagnosed as GERD (14-16). For instance, Patti et al. performed functional esophageal tests on 822 consecutive patients with a clinical diagnosis of GERD and found that 30% of patients had normal reflux scores. Moreover, the incidence of heartburn and regurgitation, and the use of acid-reducing medications were similar between patients with or without pathologic reflux (14).
Also, in a metanalysis of 12 studies, Numans et al. showed that PPI trial, compared to 24-hour pH monitoring, had a sensitivity of 78% but a specificity of only 54% (17). Therefore, clinical diagnosis of GERD based on symptoms and response to PPI trial is not sufficient before considering LARS, and objective documentation of GERD is required.
Endoscopy
It is generally performed at the clinical presentation in case of persistent GERD symptoms despite appropriate medical therapy or in cases with warning signs (dysphagia, unintentional weight loss, hematemesis) (3,18). The presence of erosive esophagitis, peptic strictures, or Barrett’s esophagus (BE) is diagnostic for GERD with a specificity of 95% (19). However, up to one-quarter of patients with abnormal reflux does not present any mucosal damage at endoscopy (20,21). Upper endoscopy is performed in all patients before LARS, in order to exclude other diagnoses such as esophageal cancer and eosinophilic esophagitis.
Esophageal manometry
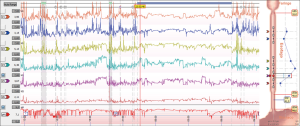
Esophageal manometry, performed with conventional water-perfused catheters or more recently with a high-resolution solid-state catheter, is of limited importance in the primary diagnosis of GERD, but its strength lies in the clinical evaluation of oesophageal motility (Figure 1). It provides information regarding the functions of the lower esophageal sphincter (LES) and the esophageal body motility. Manometric findings of defective LES can be seen in up to 60% of GERD patients, and the degree of impaired esophageal motility seems to increase with the severity of erosive esophagitis (22).
The main purpose of preoperative esophageal manometry is to rule out the presence of primary esophageal motility disorders, such as achalasia, that would be contraindications to LARS. Moreover, esophageal manometry is required to correctly place the esophageal pH monitoring probe (5 cm above the LES) (23).
Ambulatory 24-hour impedance-pH monitoring
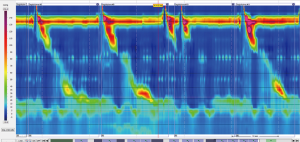
Ambulatory 24-hour impedance-pH monitoring is considered the gold standard for diagnosing GERD because it quantifies the number of reflux episodes and their correlation with symptoms, using the symptom index (SI) or the symptom association probability (SAP) (24,25) (Figure 2). It allows the characterization of the refluxate based on pH (into acid, weakly acidic and weakly alkaline reflux) and type of the refluxate (liquid, gaseous, mixed).
The current most common indications for this test are: patients with typical symptoms refractory to PPI therapy, patients with atypical symptoms and evaluation of endoscopy-negative patients with GERD symptoms (26).
Barium swallow
It is not a diagnostic test for GERD because it has low diagnostic sensitivity and specificity (respectively 40% and 85%) (27). However, it provides information about the length and diameter of the esophagus, and regarding the presence and size of a hiatal hernia. In the case of findings of large hiatal hernia, additional investigations such as computed tomography or magnetic resonance can be performed preoperatively for more accurate sizing (28).
Indications
When the diagnosis of GERD is objectively confirmed, indications to LARS are: (I) patients with typical symptoms and successful medical management; (II) patients with inadequate symptom control despite adequate medical therapy; (III) patients with GERD complications (i.e., BE, peptic strictures); (IV) patients with extra-esophageal symptoms that are related to GERD (29).
Patients with typical symptoms and successful response to PPI are the best candidates to LARS. Patients might choose to undergo surgery despite adequate symptom control for several reasons, including the need of taking lifelong medications, especially in younger patients, poor compliance or the presence of side effects associated with medical treatment, and the cost of prolonged medical therapy.
Historically, LARS was advocated mainly for GERD patients with failed medical therapy. During the last decades, however, indications to LARS have evolved (30). Campos et al. performed a multivariate analysis on 199 consecutive patients undergoing laparoscopic 360° fundoplication (LTF). Three preoperative factors were statistically significant predictors of success after LARS: abnormal pH monitoring score, the presence of typical symptoms, and the favorable response to acid suppressive therapy (31). Comparable results were reported by Davis et al. in a review of 13 RCT assessing outcomes of LARS. They concluded that the presence of a large hiatal hernia, lack of response to preoperative acid-reducing medications, the presence of atypical symptoms and overweight were associated with significantly lower success rates after laparoscopic fundoplication (32).
A careful selection of patients is mandatory in case of symptoms unresponsive to PPI before considering LARS. Traditionally, failure of medical therapy was attributed to the presence of residual reflux, either acid or non-acidic (33). More recently, the Rome IV classification recognized two separate functional esophageal disorders, functional heartburn and reflux hypersensitivity, that overlap with GERD and are considered to be responsible for the lack of response to treatment despite adequate medical therapy in the majority of PPI non-responder patients (5,34,35). Understanding the reasons beneath medical failure is essential to address patients to specific individualized treatment properly.
The role of LARS in patients with BE is controversial because the evidence is very limited. Only a few small retrospective studies have been published to date regarding the outcomes of laparoscopic fundoplication in patients with BE, showing a good symptom control rate (36-39). Also, these studies showed a not negligible rate of BE regression, ranging from14 to 52%, after LARS (40). In their recently published guidelines, the British Society of Gastroenterology concluded that LARS was not superior to acid-reducing medications in the prevention of BE neoplastic progression; surgery can be offered to highly symptomatic BE patients with poor response to PPI (41).
Surgical treatment for GERD
Laparoscopic fundoplication is the procedure of choice for the surgical treatment of GERD. The surgical procedure results in an increased LES tone and reduced backflow of gastric contents to the esophagus.
The following technical steps in performing a laparoscopic fundoplication have been standardized: (I) opening of the phreno-esophageal ligament, with preservation of the anterior vagus nerve; (II) dissection of both crura; (III) mobilization of the distal esophagus to allow at least 3 cm of esophagus in the abdominal cavity; (IV) eventual short gastric vessel division; (V) posterior crural repair; (VI) wrap creation (42).
Laparoscopic 360° fundoplication (LTF)
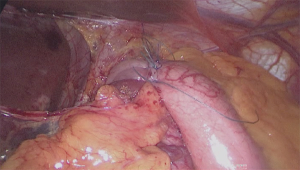
LTF is the standard procedure for the surgical treatment of GERD. A 360° short and floppy wrap is created by bringing the right and left portion of the gastric fundus around the distal esophagus (42) (Figure 3). Compared to conventional (open) 360° fundoplication, the laparoscopic approach allows similar perioperative and long-term outcomes with lower postoperative morbidity and mortality rates (43-47). Several studies demonstrated that LTF is highly effective in reflux control over time, relieving heartburn and regurgitation in about 80–90% of patients at 10 and 20 years follow-up (46,48-51).
Two major causes of patients’ dissatisfaction with LTF are dysphagia and gas-bloat syndrome. Transient dysphagia is frequently experienced by patients submitted to LTF in the early postoperative period, while long-term dysphagia is rare.
In order to reduce the incidence of these side-effects, several potential technical factors were evaluated. The use of a calibration bougie while performing the fundoplication was associated with decreased postoperative dysphagia rate (52). Routine short gastric vessel division was suggested by some authors to achieve a tension-free wrap. However, several RCTs showed no differences in postoperative dysphagia rate for patients submitted to routine short gastric vessels division during LTF (53-55). Therefore selective short gastric vessels division is required only in selected cases when the gastric fundus cannot be wrapped around the esophagus without tension (29).
Several preoperative patient characteristics were evaluated to determine whether they could predict the incidence of postoperative side effects. The presence of ineffective preoperative esophageal motility was investigated as a risk factor for the development of postoperative dysphagia. However, multiple RCTs and meta-analysis showed similar rates of dysphagia following 360° and partial fundoplication either with normal or defective esophageal peristalsis (56-58). To date, there is no evidence of clinical benefits in tailoring the fundoplication depending on manometric parameters (29). No correlation also was found between preoperative impedance-pH monitoring parameters and outcomes after LARS (59,60). Finally, the presence of preoperative delayed gastric emptying seemed to be correlated with worst functional and clinical results after LTF compared to patients with normal gastric function, probably due to lower gastric compliance to proximal gastric distension (61). However, further studies are needed to confirm the results.
Laparoscopic 360° vs. partial posterior fundoplication
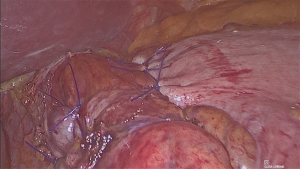
Laparoscopic partial posterior fundoplication (LPPF) is considered an alternative to LTF in the treatment of GERD (Figure 4). Several RCTs were conducted to investigate the differences between LTF and LPPF. These studies showed minimal differences in clinical outcomes between the two procedures, that could not allow for providing definitive answers (56,62-64).
Broeders et al. performed a systematic review and meta-analysis on 7 RCTs comparing LTF (404 patients) and LPPF (388 patients). Perioperative outcomes and satisfaction with the intervention were similar for both procedures. There were no differences between the two procedures in the presence of subjective reflux recurrence, in the percentage of recurrent pathological exposure, and in the presence of esophagitis. Dysphagia and gas-related symptoms, as well as surgical reinterventions, were more frequent in the LTF group compared to LPPF (65). This study, however, had some major limitations due to the limited methodological quality and power of the included RCTs, and to the heterogeneity in technical issues performing the procedures, such as the length of the wraps, and in outcomes definition. Moreover, the follow-up ranged from 12 to 60 months. Longer follow-up data are needed to confirm these results, especially because several non-randomized studies reported inferior long-term reflux control of LPPF compared to LTF (22,66). Finally, Mardani et al. published the long-term results of an RCT comparing open 360° versus partial posterior fundoplication, showing equally well-controlled reflux, while the differences in side effects equalized at 10 years after surgery (67).
Laparoscopic 360° vs. partial anterior fundoplication
Watson et al. performed a double-blind RCT in 1999 comparing 53 LTF and 54 laparoscopic partial anterior fundoplication (LPAF). Perioperative outcomes were similar in both groups (68). LPAF patients had a significantly lower incidence of dysphagia for solid food, inability to belch and increased satisfaction rate compared to LTF both at short- and long-term follow-up (68-70). However, at 14-year follow-up, patients submitted to LPAF had less effective reflux control, with an increased number of reflux episodes detected ad 24-hour pH monitoring and lower LES resting pressure at esophageal manometry compared to LTF (71).
Similar results, showing an increased rate of symptom recurrence after LPAF compared to LTF were also published by others (72-76). For instance, Baigrie et al. performed an RCT comparing LTF (84 patients) and LPAF (79 patients). The two surgical procedures provided comparable results in terms of symptom control, dysphagia and satisfaction rates both at short and long-term follow-up (77). However, at 12 years follow-up, the use of acid-reducing medications was higher in LPAF patients compared to LTF (8% vs. 29%) (78).
Conclusions
GERD is one of the most frequent upper gastrointestinal disorders worldwide. Objective GERD diagnosis with endoscopy and 24-hour impedance-pH monitoring is needed before considering LARS. Further diagnostic investigations such as esophageal manometry and barium swallow provide useful information to establish correct surgical indications.
A careful preoperative selection of patients is the first critical step to reduce the risk of side effects and failure of LARS.
According to the available evidence, LTF is the surgical procedure of choice for GERD treatment. Laparoscopic partial fundoplication, either anterior or posterior, is associated with a lower incidence of dysphagia and gas bloat syndrome, but the effects on reflux control over time are questionable. The limited quality of the studies and the lack of long-term data cannot allow drawing definitive conclusions. Therefore, the choice of the type of wrap (partial or 360°) depends on the single surgeon’s experiences and preferences.
Further efforts are needed to identify preoperative prognostic factors, selecting GERD patient subgroups that could benefit from a different type of fundoplication, in order to individualize GERD treatment depending on each patient characteristics.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Muhammed Ashraf Memon and Abe Fingerhut) for the series “Laparoendoscopic Surgery for Benign Oesophagogastric Conditions” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2019.06.14). The series “Laparoendoscopic Surgery for Benign Oesophagogastric Conditions” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Vakil N, van Zanten SV, Kahrilas P, et al. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol 2006;101:1900-20; quiz 1943.
- El-Serag HB, Sweet S, Winchester CC, et al. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut 2014;63:871-80. [Crossref] [PubMed]
- Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol 2013;108:308-28; quiz 329. [Crossref] [PubMed]
- Vemulapalli R. Diet and lifestyle modifications in the management of gastroesophageal reflux disease. Nutr Clin Pract 2008;23:293-8. [Crossref] [PubMed]
- Gyawali CP, Fass R. Management of Gastroesophageal Reflux Disease. Gastroenterology 2018;154:302-18. [Crossref] [PubMed]
- Bavishi C, Dupont HL. Systematic review: the use of proton pump inhibitors and increased susceptibility to enteric infection. Aliment Pharmacol Ther 2011;34:1269-81. [Crossref] [PubMed]
- Johnstone J, Nerenberg K, Loeb M. Meta-analysis: proton pump inhibitor use and the risk of community-acquired pneumonia. Aliment Pharmacol Ther 2010;31:1165-77. [Crossref] [PubMed]
- Ngamruengphong S, Leontiadis GI, Radhi S, et al. Proton pump inhibitors and risk of fracture: a systematic review and meta-analysis of observational studies. Am J Gastroenterol 2011;106:1209-18; quiz 1219. [Crossref] [PubMed]
- Rickenbacher N, Kötter T, Kochen MM, et al. Fundoplication versus medical management of gastroesophageal reflux disease: systematic review and meta-analysis. Surg Endosc 2014;28:143-55. [Crossref] [PubMed]
- Galmiche JP, Hatlebakk J, Attwood S, et al. Laparoscopic antireflux surgery vs esomeprazole treatment for chronic GERD: the LOTUS randomized clinical trial. JAMA 2011;305:1969-77. [Crossref] [PubMed]
- Mehta S, Bennett J, Mahon D, et al. Prospective trial of laparoscopic nissen fundoplication versus proton pump inhibitor therapy for gastroesophageal reflux disease: Seven-year follow-up. J Gastrointest Surg 2006;10:1312-6; discussion 1316-7. [Crossref] [PubMed]
- Anvari M, Allen C, Marshall J, et al. A randomized controlled trial of laparoscopic Nissen fundoplication versus proton pump inhibitors for the treatment of patients with chronic gastroesophageal reflux disease (GERD): 3-year outcomes. Surg Endosc 2011;25:2547-54. [Crossref] [PubMed]
- Grant AM, Cotton SC, Boachie C, et al. Minimal access surgery compared with medical management for gastro-oesophageal reflux disease: five year follow-up of a randomised controlled trial (REFLUX). BMJ 2013;346:f1908. [Crossref] [PubMed]
- Patti MG, Diener U, Tamburini A, et al. Role of esophageal function tests in diagnosis of gastroesophageal reflux disease. Dig Dis Sci 2001;46:597-602. [Crossref] [PubMed]
- Moayyedi P, Talley NJ, Fennerty MB, et al. Can the clinical history distinguish between organic and functional dyspepsia? JAMA 2006;295:1566-76. [Crossref] [PubMed]
- Dent J, Vakil N, Jones R, et al. Accuracy of the diagnosis of GORD by questionnaire, physicians and a trial of proton pump inhibitor treatment: the Diamond Study. Gut 2010;59:714-21. [Crossref] [PubMed]
- Numans ME, Lau J, de Wit NJ, et al. Short-term treatment with proton-pump inhibitors as a test for gastroesophageal reflux disease: a meta-analysis of diagnostic test characteristics. Ann Intern Med 2004;140:518-27. [Crossref] [PubMed]
- ASGE Standards of Practice Committee. The role of endoscopy in the management of GERD. Gastrointest Endosc 2015;81:1305-10. [Crossref] [PubMed]
- Richter JE. Diagnostic tests for gastroesophageal reflux disease. Am J Med Sci 2003;326:300-8. [Crossref] [PubMed]
- Zagari RM, Fuccio L, Wallander MA, et al. Gastro-oesophageal reflux symptoms, oesophagitis and Barrett’s oesophagus in the general population: the Loiano-Monghidoro study. Gut 2008;57:1354-9. [Crossref] [PubMed]
- Ronkainen J, Aro P, Storskrubb T, et al. High prevalence of gastroesophageal reflux symptoms and esophagitis with or without symptoms in the general adult Swedish population: a Kalixanda study report. Scand J Gastroenterol 2005;40:275-85. [Crossref] [PubMed]
- Jobe BA, Richter JE, Hoppo T, et al. Preoperative diagnostic workup before antireflux surgery: an evidence and experience-based consensus of the Esophageal Diagnostic Advisory Panel. J Am Coll Surg 2013;217:586-97. [Crossref] [PubMed]
- Fuchs KH, Babic B, Breithaupt W, et al. EAES recommendations for the management of gastroesophageal reflux disease. Surg Endosc 2014;28:1753-73. [Crossref] [PubMed]
- Wiener GJ, Richter JE, Copper JB, et al. The symptom index: a clinically important parameter of ambulatory 24-hour esophageal pH monitoring. Am J Gastroenterol. 1988;83:358-61. [PubMed]
- Weusten BL, Roelofs JM, Akkermans LM, et al. The symptom-association probability: an improved method for symptom analysis of 24-hour esophageal pH data. Gastroenterology 1994;107:1741-5. [Crossref] [PubMed]
- Hirano I, Richter JEPractice Parameters Committee of the American College of Gastroenterology. ACG practice guidelines: esophageal reflux testing. Am J Gastroenterol 2007;102:668-85. [Crossref] [PubMed]
- Streets CG, DeMeester TR. Ambulatory 24-hour esophageal pH monitoring: why, when, and what to do. J Clin Gastroenterol 2003;37:14-22. [Crossref] [PubMed]
- Memon MA. Hiatal Hernia Surgery: An Evidence Based Approach. Springer International Publishing; 2018.
- Stefanidis D, Hope WW, Kohn GP, et al. Guidelines for surgical treatment of gastroesophageal reflux disease. Surg Endosc 2010;24:2647-69. [Crossref] [PubMed]
- Yadlapati R, Vaezi MF, Vela MF, et al. Management options for patients with GERD and persistent symptoms on proton pump inhibitors: recommendations from an expert panel. Am J Gastroenterol 2018;113:980-6. [Crossref] [PubMed]
- Campos GM, Peters JH, DeMeester TR, et al. Multivariate analysis of factors predicting outcome after laparoscopic Nissen fundoplication. J Gastrointest Surg 1999;3:292-300. [Crossref] [PubMed]
- Davis CS, Baldea A, Johns JR, et al. The evolution and long-term results of laparoscopic antireflux surgery for the treatment of gastroesophageal reflux disease. JSLS 2010;14:332-41. [Crossref] [PubMed]
- Mainie I, Tutuian R, Shay S, et al. Acid and non-acid reflux in patients with persistent symptoms despite acid suppressive therapy: a multicentre study using combined ambulatory impedance-pH monitoring. Gut 2006;55:1398-402. [Crossref] [PubMed]
- Aziz Q, Fass R, Gyawali CP, et al. Functional Esophageal Disorders. Gastroenterology 2016; [Epub ahead of print]. [Crossref] [PubMed]
- Roman S, Keefer L, Imam H, et al. Majority of symptoms in esophageal reflux PPI non-responders are not related to reflux. Neurogastroenterol Motil 2015;27:1667-74. [Crossref] [PubMed]
- Hofstetter WL, Peters JH, DeMeester TR, et al. Long-term outcome of antireflux surgery in patients with Barrett’s esophagus. Ann Surg 2001;234:532-8; discussion 538-9. [Crossref] [PubMed]
- Morrow E, Bushyhead D, Wassenaar E, et al. The impact of laparoscopic anti-reflux surgery in patients with Barrett’s esophagus. Surg Endosc 2014;28:3279-84. [Crossref] [PubMed]
- Desai KM, Soper NJ, Frisella MM, et al. Efficacy of laparoscopic antireflux surgery in patients with Barrett’s esophagus. Am J Surg 2003;186:652-9. [Crossref] [PubMed]
- Bowers SP, Mattar SG, Smith CD, et al. Clinical and histologic follow-up after antireflux surgery for Barrett’s esophagus. J Gastrointest Surg 2002;6:532-8; discussion 539. [Crossref] [PubMed]
- Herbella FAM, Schlottmann F, Patti MG. Antireflux Surgery and Barrett’s Esophagus: Myth or Reality? World J Surg 2018;42:1798-802. [Crossref] [PubMed]
- Fitzgerald RC, di Pietro M, Ragunath K, et al. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett’s oesophagus. Gut 2014;63:7-42. [Crossref] [PubMed]
- Attwood SEA, Lundell L, Ell C, et al. Standardization of surgical technique in antireflux surgery: the LOTUS Trial experience. World J Surg 2008;32:995-8. [Crossref] [PubMed]
- Schlottmann F, Strassle PD, Patti MG. Comparative Analysis of Perioperative Outcomes and Costs Between Laparoscopic and Open Antireflux Surgery. J Am Coll Surg 2017;224:327-33. [Crossref] [PubMed]
- Oor JE, Roks DJ, Broeders JA, et al. Seventeen-year Outcome of a Randomized Clinical Trial Comparing Laparoscopic and Conventional Nissen Fundoplication: A Plea for Patient Counseling and Clarification. Ann Surg 2017;266:23-8. [Crossref] [PubMed]
- Salminen P, Hurme S, Ovaska J. Fifteen-year outcome of laparoscopic and open Nissen fundoplication: a randomized clinical trial. Ann Thorac Surg 2012;93:228-33. [Crossref] [PubMed]
- Broeders JA, Rijnhart-de Jong HG, Draaisma WA, et al. Ten-year outcome of laparoscopic and conventional nissen fundoplication: randomized clinical trial. Ann Surg 2009;250:698-706. [Crossref] [PubMed]
- Peters MJ, Mukhtar A, Yunus RM, et al. Meta-analysis of randomized clinical trials comparing open and laparoscopic anti-reflux surgery. Am J Gastroenterol 2009;104:1548-61; quiz 1547. [PubMed]
- Ross SB, Gal S, Teta AF, et al. Late results after laparoscopic fundoplication denote durable symptomatic relief of gastroesophageal reflux disease. Am J Surg 2013;206:47-51. [Crossref] [PubMed]
- Engström C, Cai W, Irvine T, et al. Twenty years of experience with laparoscopic antireflux surgery. Br J Surg 2012;99:1415-21. [Crossref] [PubMed]
- Robinson B, Dunst CM, Cassera MA, et al. 20 years later: laparoscopic fundoplication durability. Surg Endosc 2015;29:2520-4. [Crossref] [PubMed]
- Dallemagne B, Perretta S. Twenty years of laparoscopic fundoplication for GERD. World J Surg 2011;35:1428-35. [Crossref] [PubMed]
- Patterson EJ, Herron DM, Hansen PD, et al. Effect of an esophageal bougie on the incidence of dysphagia following nissen fundoplication: a prospective, blinded, randomized clinical trial. Arch Surg 2000;135:1055-61; discussion 1061-2. [Crossref] [PubMed]
- Yang H, Watson DI, Lally CJ, et al. Randomized trial of division versus nondivision of the short gastric vessels during laparoscopic Nissen fundoplication: 10-year outcomes. Ann Surg 2008;247:38-42. [Crossref] [PubMed]
- Engström C, Blomqvist A, Dalenbäck J, et al. Mechanical consequences of short gastric vessel division at the time of laparoscopic total fundoplication. J Gastrointest Surg 2004;8:442-7. [Crossref] [PubMed]
- O’Boyle CJ, Watson DI, Jamieson GG, et al. Division of short gastric vessels at laparoscopic nissen fundoplication: a prospective double-blind randomized trial with 5-year follow-up. Ann Surg 2002;235:165-70. [Crossref] [PubMed]
- Strate U, Emmermann A, Fibbe C, et al. Laparoscopic fundoplication: Nissen versus Toupet two-year outcome of a prospective randomized study of 200 patients regarding preoperative esophageal motility. Surg Endosc 2008;22:21-30. [Crossref] [PubMed]
- Booth MI, Stratford J, Jones L, et al. Randomized clinical trial of laparoscopic total (Nissen) versus posterior partial (Toupet) fundoplication for gastro-oesophageal reflux disease based on preoperative oesophageal manometry. Br J Surg 2008;95:57-63. [Crossref] [PubMed]
- Hajibandeh S, Hajibandeh S, Pugh M, et al. Impact of Toupet Versus Nissen Fundoplication on Dysphagia in Patients With Gastroesophageal Reflux Disease and Associated Preoperative Esophageal Dysmotility: A Systematic Review and Meta-Analysis. Surg Innov 2018. [Epub ahead of print].
- Desjardin M, Luc G, Collet D, et al. 24-hour pH-impedance monitoring on therapy to select patients with refractory reflux symptoms for antireflux surgery. A single center retrospective study. Neurogastroenterol Motil 2016;28:146-52. [Crossref] [PubMed]
- Wayman J, Myers JC, Jamieson GG. Preoperative gastric emptying and patterns of reflux as predictors of outcome after laparoscopic fundoplication. Br J Surg 2007;94:592-8. [Crossref] [PubMed]
- Rebecchi F, Allaix ME, Giaccone C, et al. Gastric emptying as a prognostic factor for long-term results of total laparoscopic fundoplication for weakly acidic or mixed reflux. Ann Surg 2013;258:831-6; discussion 836-7. [Crossref] [PubMed]
- Chrysos E, Tsiaoussis J, Zoras OJ, et al. Laparoscopic surgery for gastroesophageal reflux disease patients with impaired esophageal peristalsis: total or partial fundoplication? J Am Coll Surg 2003;197:8-15. [Crossref] [PubMed]
- Guérin E, Bétroune K, Closset J, et al. Nissen versus Toupet fundoplication: results of a randomized and multicenter trial. Surg Endosc 2007;21:1985-90. [Crossref] [PubMed]
- Mickevičius A, Endzinas Ž, Kiudelis M, et al. Influence of wrap length on the effectiveness of Nissen and Toupet fundoplications: 5-year results of prospective, randomized study. Surg Endosc 2013;27:986-91. [Crossref] [PubMed]
- Broeders JA, Mauritz FA, Ahmed Ali U, et al. Systematic review and meta-analysis of laparoscopic Nissen (posterior total) versus Toupet (posterior partial) fundoplication for gastro-oesophageal reflux disease. Br J Surg 2010;97:1318-30. [Crossref] [PubMed]
- Patti MG, Robinson T, Galvani C, et al. Total fundoplication is superior to partial fundoplication even when esophageal peristalsis is weak. J Am Coll Surg 2004;198:863-9; discussion 869-70. [Crossref] [PubMed]
- Mardani J, Lundell L, Engström C. Total or posterior partial fundoplication in the treatment of GERD: results of a randomized trial after 2 decades of follow-up. Ann Surg 2011;253:875-8. [Crossref] [PubMed]
- Watson DI, Jamieson GG, Pike GK, et al. Prospective randomized double-blind trial between laparoscopic Nissen fundoplication and anterior partial fundoplication. Br J Surg 1999;86:123-30. [Crossref] [PubMed]
- Ludemann R, Watson DI, Jamieson GG, et al. Five-year follow-up of a randomized clinical trial of laparoscopic total versus anterior 180 degrees fundoplication. Br J Surg 2005;92:240-3. [Crossref] [PubMed]
- Cai W, Watson DI, Lally CJ, et al. Ten-year clinical outcome of a prospective randomized clinical trial of laparoscopic Nissen versus anterior 180(degrees) partial fundoplication. Br J Surg 2008;95:1501-5. [Crossref] [PubMed]
- Broeders JA, Broeders EA, Watson DI, et al. Objective outcomes 14 years after laparoscopic anterior 180-degree partial versus nissen fundoplication: results from a randomized trial. Ann Surg 2013;258:233-9. [Crossref] [PubMed]
- Watson DI, Jamieson GG, Lally C, et al. Multicenter, prospective, double-blind, randomized trial of laparoscopic nissen vs anterior 90 degrees partial fundoplication. Arch Surg 2004;139:1160-7. [Crossref] [PubMed]
- Nijjar RS, Watson DI, Jamieson GG, et al. Five-year follow-up of a multicenter, double-blind randomized clinical trial of laparoscopic Nissen vs anterior 90 degrees partial fundoplication. Arch Surg 2010;145:552-7. [Crossref] [PubMed]
- Spence GM, Watson DI, Jamiesion GG, et al. Single center prospective randomized trial of laparoscopic Nissen versus anterior 90 degrees fundoplication. J Gastrointest Surg 2006;10:698-705. [Crossref] [PubMed]
- Watson DI, Devitt PG, Smith L, et al. Anterior 90° partial vs Nissen fundoplication--5 year follow-up of a single-centre randomised trial. J Gastrointest Surg 2012;16:1653-8. [Crossref] [PubMed]
- Memon MA, Subramanya MS, Hossain MB, et al. Laparoscopic anterior versus posterior fundoplication for gastro-esophageal reflux disease: a meta-analysis and systematic review. World J Surg 2015;39:981-96. [Crossref] [PubMed]
- Baigrie RJ, Cullis SNR, Ndhluni AJ, et al. Randomized double-blind trial of laparoscopic Nissen fundoplication versus anterior partial fundoplication. Br J Surg 2005;92:819-23. [Crossref] [PubMed]
- Roks DJ, Broeders JA, Baigrie RJ. Long-term symptom control of gastro-oesophageal reflux disease 12 years after laparoscopic Nissen or 180° anterior partial fundoplication in a randomized clinical trial. Br J Surg 2017;104:852-6. [Crossref] [PubMed]
Cite this article as: Morino M, Ugliono E, Allaix ME, Rebecchi F. Laparoscopic surgery for gastroesophageal reflux disease: Nissen, Toupet or anterior fundoplication. Ann Laparosc Endosc Surg 2019;4:83.