Fundamental use of surgical energy during endoscopic therapies
Introduction
Knowledge of the principles of safe use of surgical energy is a critical element in the practice of any endoscopist. Even endoscopists who have mainly diagnostic practices will find themselves utilizing surgical energy during procedures, such as when performing polypectomy with a “hot” snare or a “hot” biopsy forceps. Education around safe use of surgical energy has largely been driven by the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) Fundamental Use of Surgical Energy (FUSE) curriculum (1).
We review the basic principles of electrosurgical energy as taught by the FUSE curriculum and then provide endoscopy-specific applications of these principles.
Basic principles of electrosurgical energy
While electrosurgical energy is used multiple times a day in operating rooms and endoscopy suites across the world, the physical properties underlying surgical energy are often poorly understood and can lead to inappropriate or even unsafe use of energy in procedures. Perhaps the most common term used in referring to electrosurgical energy is “cauterization”. The term cauterize specifically refers to burning of flesh through passive transfer of heat or caustic substances (2) while electrosurgery refers to “the use of radiofrequency alternating current to raise intracellular temperature in order to achieve vaporization or the combination of desiccation and protein coagulation.” (1).
An important concept of electrosurgery is the understanding of active and dispersive electrodes. While all electrosurgery is “bipolar” in nature (i.e., uses two poles), instruments that are used in procedures place each electrode in different locations. Monopolar instruments use a dispersive electrode pad (colloquially referred to as a “grounding pad”) that is often attached to the patient’s thigh or hip while the active electrode is held in the surgeon’s hand. Bipolar instruments have both electrodes within one instrument under the control of the surgeon’s hand so that current can flow between the two tips without the need for a large dispersive electrode attached separately to the patient.
It is also important to note that the effects of electrosurgery on tissue are occurring first at the cellular level before macroscopic changes to the tissue are visibly seen. While some surgeons may refer to nearly any act of using electrosurgery as “cauterizing” or “bovie-ing” tissue, there are specific actions occurring at the cellular level depending on the type and amount of current being applied. Vaporization occurs when the intracellular temperature rapidly elevates to 100 °C, generating steam that causes cellular rupture through cell expansion. Desiccation refers to dehydration of cells through a thermally damaged cell wall (45–100 °C). Carbonization refers to the breakdown of molecules to sugars and the creation of an eschar. Coagulation or “white coagulation” occurs from protein denaturation (50–100 °C) and is useful to obtain hemostasis through vessel coaptation. Fulguration or “black coagulation” results in superficial protein coagulation through carbonization and organic molecular breakdown (>200 °C) via modulated high voltage waveforms typically produced by the “coagulation” output of an electrosurgical generator and a “no touch” technique. It is most effective for superficial coagulation of superficial bleeding.
As previously defined, electrosurgery utilizes alternating current for its effects. Thus, the frequency and amplitude (i.e., the waveform) of the current being delivered has direct implications on the resulting effect of the energy on the tissue. The most common electrosurgical generators have an option that is typically labeled “cut” and an option labeled “coag”. The “cut” option delivers a continuous, low-amplitude current while the “coag” option delivers current that is high-amplitude but interrupted. In fact, during “coag” delivery, the generator is delivering current only about 6% of the time. The “cut” setting is often used to achieve vaporization of cells while “coag” is effective at achieving fulguration.
We believe it is also important for endoscopists to understand the concept of current density. Current (measured in amperes) refers to the flow of electrons while impedance or resistance (R) refers to the degree to which the circuit resists electron flow. Current density is defined as the amount of current over the area in which that current is applied (measured in amps/cm2). It is inversely proportional to resistance and directly proportional to the applied power. Tissue heating can be described as current density squared. Therefore, the same current over a large area has less of an effect than when applied over a small area.
For additional detail on basic principles of electrosurgical energy—such as how current can be diverted to create inadvertent injury during minimally invasive surgery—we encourage readers to complete the free FUSE curriculum provided by SAGES at http://www.fusedidactic.org.
Electrosurgical instrumentation and their applications in endoscopy
There are a variety of electrosurgical instruments available to endoscopists to allow for a multitude of procedures. As technology has improved, the increasing availability of endoscopic instruments has led to the development of more complex endoscopic procedures such as endoscopic submucosal dissection (ESD) and per oral endoscopic myotomy (POEM). All of the electrosurgical instruments available to endoscopists adhere to the basic principles of electrosurgery summarized above.
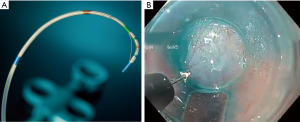
The most common instruments utilized during endoscopy are monopolar instruments that require placement of a dispersive electrode on the patient’s body. Perhaps the two most common instruments are the polypectomy snare and the biopsy forceps. Both of these instruments can be used “cold” without the application of current or “hot”, where the instrument is attached to an electrosurgical generator, at the discretion of the endoscopist. Monopolar instruments for more specific procedures include sphincterotomes for endoscopic retrograde cholangiopancreatography (ERCP) and electrosurgical knives for POEM or ESD (Figure 1A,B). With each of the aforementioned devices, the endoscopist can apply a variable amount of pressure to the tissue while delivering current to alter the depth of tissue affected. Thus, one must take particular care to avoid perforation (either immediate or delayed) from thermal injury.
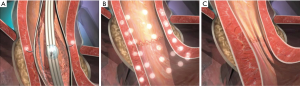
The monopolar radial balloon electrode array, also known as the Stretta catheter (Restech, Houston, TX, USA), is a specific type of monopolar instrument that is used for treatment of gastroesophageal reflux disease (GERD). The radial catheter serves as the active electrode while a dispersive electrode is placed on the patient’s back (typically between the shoulder blades). The radial balloon electrode array applies electrosurgical energy in a radial fashion to the muscularis propria of the lower esophageal sphincter and the gastric cardia (Figure 2). This leads the tissue to thicken through collagen deposition with the goal of improving the function of the lower esophageal sphincter (Figure 3) (3). The device contains additional sensors within the radial catheter array that assesses the temperature of the surrounding mucosa and stops the delivery of current when the mucosal temperature exceeds 47 degrees Celsius (4).
Argon beam plasma coagulation (APC) is a unique type of monopolar instrument that utilizes ionized argon gas as a conductive (i.e., low impedance) medium between the active electrode and the target tissue. The argon gas that is ejected from the monopolar handpiece allows for electrical current to arc from the active electrode to the tissue up to 1 cm away. APC is effective in the management of large, superficial areas of bleeding (e.g., gastric antral vascular ectasia or GAVE) and in areas where perforation can more easily occur after thermal injury (e.g., the cecum) (5). Of note, because APC works via instillation of ionized gas, suction should be used intermittently with application to avoid over-distension of the lumen.
Bipolar instruments transmit current across two active electrodes that are in very close proximity to one another. Bipolar instruments are designed primarily to achieve hemostasis through desiccation and coagulation and are not as effective as monopolar cautery in achieving vaporization (i.e., cutting of tissue). In endoscopy, the multipolar electrocoagulation probe (MPEC) is the most commonly used bipolar instrument. The MPEC probe can be used not just to desiccate tissue but also to achieve coagulation through coaptation of vessel walls. The probe can be pressed against the base of a bleeding ulcer or vessel until bleeding stops, suggesting that the walls of the bleeding vessel are suitably compressed and coapted. Administration of current through the MPEC probe then allows the vessel walls to coagulate together to seal the vessel lumen (3).
Radiofrequency (RF) ablation via the RF electrode array delivers energy for indications such as ablation of intestinal metaplasia (i.e., Barrett’s disease) in the lower esophagus, esophageal squamous cell dysplasia, GAVE, and malignant biliary strictures. The balloon array allows for energy to be delivered circumferentially while focal electrodes allow for more directed energy delivery to specific sites. Both devices allow for delivery of energy that typically extends to the muscularis mucosa. More recent developments in RF ablation technology now also allow for RF ablation of malignant biliary strictures. For these devices, it is recommended that a 1-minute resting period be observed before retrieving or moving the catheter tip as adhesion of tissue to the heated electrode could cause inadvertent damage to tissue (3).
Complications from use of electrosurgery in endoscopy
The most common complication of electrosurgical energy is thermal injury or burn. This can occur through a number of mechanisms depending on the type of electrosurgical energy being used; but with any kind of electrosurgery, one must always consider the potential damage that can occur from the active electrode. Inadvertent activation of the active electrode or ultrasonic instrument can injure tissue in direct contact with the active electrode while direct extension injuries occur when another portion of tissue is in contact with the target tissue (e.g., two structures immediately adjacent to one another or adhered to one another). The “mushroom effect” of thermal injury occurs when energy is applied for prolonged periods of time to the same area of tissue. As the tissue desiccates, its impedance increases, and current flows to surrounding tissues that offer lower resistance. This can result in unanticipated thermal damage to tissues that are immediately surrounding the target tissue (6). Breaks in the insulation can cause current to escape and burn tissue unintentionally. These breaks are often tiny holes that can lead to high current density and current transmission at the escape site.
A common site of thermal injury is at the site of the dispersive electrode. The dispersive electrode’s large area acts to reduce current density (i.e., current is applied over a larger area) and limit the risk of injury. Partial detachment of the dispersive electrode can cause burns at the site as the dispersive area is reduced, leading to increased current density at the partial site of attachment. Therefore, it is important to ensure that the area of the body to which the dispersive electrode is applied is relatively clean (including relatively free of hair) and flat to allow optimal contact between the patient’s skin and the dispersive electrode.
In endoscopy, perhaps the most noted complications of electrosurgical energy use are hemorrhage and perforation. The overall rate of perforation in colonoscopy is estimated to be less than 0.1% (7); however, the rate of perforation with colonoscopy is estimated to increase four-fold when polypectomy is performed (8). Not surprisingly, the risk of thermal injury is directly proportional to the intensity and duration of the applied current. It is important to remember that the thickness of the muscular layer of the bowel lumen varies with the stomach and rectum having the thickest muscular layer and the duodenum and cecum/ascending colon having the thinnest, thus increasing risk of perforation from thermal injury. In addition, the current density increases within a polypectomy snare as the snare is tightened and the stalk of the polyp decreases in diameter, further increasing the risk for thermal injury and perforation.
Post-polypectomy syndrome refers to the development of abdominal pain in a patient after undergoing polypectomy and is caused by localized, transmural inflammation without perforation at the site of polypectomy. Management of post-polypectomy syndrome is supportive, and patients should be observed with bowel rest, antibiotics, and adequate fluid resuscitation and maintenance. Principles of safe electrosurgical energy usage discussed throughout this paper can help to prevent transmural injury to the bowel that can lead to post-polypectomy syndrome.
The small working area at the tip of the endoscope and close proximity of multiple channels (e.g., lens and camera cable, light, working channel) create the possibility of inadvertent stray energy transfer within the endoscope itself and to surrounding tissue. A study by Jones et al. [2017] systematically investigated the effects of electrosurgical energy on temperature of tissue along the endoscope in an anesthetized pig. The authors found that during use of monopolar biopsy forceps, tissue near the tip of the endoscope (but outside the view of the endoscopist) reached a peak of 62–64 degrees Celsius, exceeding the 60-degree Celsius temperature at which cellular necrosis begins. The authors recommended the following techniques to reduce the risk of stray energy transfer during endoscopy: (I) reduce power setting from 60 to 30 W, (II) use a low-voltage mode (e.g., “cut”) instead of a high-voltage mode (e.g., “coag”), (III) use an impedance monitored generator that will automatically reduce power output when tissue resistance is not detected (9).
Techniques to avoid electrosurgical complications
Performance of therapeutic endoscopy can provide a safe, minimally invasive approach to the management of many different disease processes. A few techniques, in combination with proper training and supervision by an experienced endoscopist, can help to further support safe use of electrosurgical energy during endoscopy. These techniques are by no means comprehensive and should be used in conjunction with the basic principles on safe use of electrosurgical energy.
Colonoscopy
Evidence is mixed around which settings are best to achieve hemostasis during or after polypectomy. Using only low voltage continuous current (e.g., “cut”) is associated with a greater risk of bleeding overall while higher voltage modulated current (e.g., “coag”) is associated with greater risk of delayed (i.e., 2–8 days post-polypectomy) bleeding (3). While bleeding is often controlled using electrosurgical energy, delayed bleeding can be seen from thermocoagulation-induced ulceration of tissue (10). Therefore, some endoscopists favor first applying modulated high-voltage current until the tissue blanches, then applying low-voltage continuous current. When performing a polypectomy during colonoscopy, a saline lift or submucosal injection allows for the lifting of the mucosa away from the rest of the bowel wall to minimize the risk of a full thickness injury while also serving as a heat sink to absorb excess thermal energy (3).
ERCP
Pull sphincterotomes for ERCP utilize a wire to allow for a straight-line sphincterotomy. When performing sphincterotomy, using a low voltage, continuous current (“cut”), there is an increased risk of bleeding as well as a risk of inadvertently creating an incision that extends through the wall of the duodenum, given the relative thinness of the duodenal wall. The FUSE manual recommends using a blended current waveform or intermittent applications of continuous, low voltage current, with the latter technique offering greater control and precision (3).
Endoscopic myotomy
POEM and per oral endoscopic pyloromyotomy are endoscopic myotomy procedures used for the treatment of achalasia and gastroparesis, respectively. Both procedures involve first incising the mucosa of either the esophagus or the stomach to create a submucosal tunnel through which a myotomy can be performed to release either the lower esophageal sphincter or the pylorus. Similar to polypectomy, saline lift via injection into the submucosa allows for the lifting of the mucosa away from the muscularis to minimize the risk of a full thickness injury (Figure 4). Indigo carmine or methylene blue are often mixed with saline to allow for better visualization of the submucosal space. The monopolar triangle-tip electrosurgical knife with modulated, high-voltage current (up to 50 W) has been favored by Dr. Inoue in his published accounts of POEM as it allows for careful dissection of the circular muscle fibers, leaving the longitudinal fibers relatively intact (11).
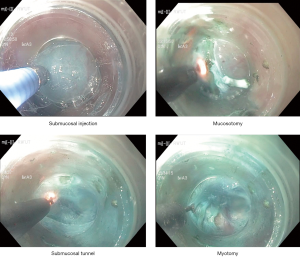
Endoscopic submucosal dissection (ESD) and endoscopic mucosal resection (EMR)
For both EMR and ESD, saline lift is a key component of the technique. However, a drawback of using saline alone is its short time to dissipation (i.e., saline only provides a lift for 1–2 minutes before dissolving into the surrounding tissue). For this reason, more viscous agents such as glycerin or hyaluronic acid are mixed with saline to provide a higher level of elevation and to prolong the duration of elevation. Maintaining direct vision of the electrosurgical knife is of utmost importance to ensure that accidental full thickness injury does not occur.
Future directions in safe use of electrosurgical energy in endoscopy
As endoscopic techniques and technology continue to improve, we will likely see more complex procedures being performed endoscopically. Interesting new developments on the horizon could further improve the safety profile of utilizing electrosurgical energy. For example, Brzezinski et al. have developed haptic feedback technology to allow surgeons to receive a tactile signal when current is flowing into tissue rather than activation without contact to tissue—an occurrence that can increase the risk of stray energy transfer (12). Furthermore, development of endoscopic ultrasonic dissectors and lasers will also necessitate education around safe use of such products in the endoscopic environment.
Conclusions
Safe use of electrosurgical energy is a key component of any endoscopist’s practice. An understanding of the principles of electrosurgery energy and their application to the unique environment of endoscopic surgery can ensure safe procedures for patients undergoing endoscopy.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Jeffrey M. Marks and Ryan M. Juza) for the series “Surgical Endoscopy: Essential Skills in Gastrointestinal Surgery” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: The series “Surgical Endoscopy: Essential Skills in Gastrointestinal Surgery” was commissioned by the editorial office without any funding or sponsorship. D Hashimoto is a consultant for Johnson & Johnson Institute and Worrell, Inc. O Meireles is a consultant for Olympus Corporation. Both Drs. Hashimoto and Meireles have received research grant support from Olympus Corporation. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Munro MG. Fundamentals of Electrosurgery Part I: Principles of Radiofrequency Energy for Surgery. In: Feldman L, Fuchshuber PR, Jones DB, editors. The SAGES manual on the fundamental use of surgical energy (FUSE). Berlin: Springer, 2012:15-59.
-
Merriam-Webster Dictionary 2019 . “cauterize”. - Dunkin BJ, Lyons CD. Electrosurgical Energy in Gastrointestinal Endoscopy. In: Feldman L, Fuchshuber P, Jones DB, editors. The SAGES Manual on the Fundamental Use of Surgical Energy (FUSE). New York, NY: Springer New York, 2012:107-21.
- Triadafilopoulos G. Stretta: a valuable endoscopic treatment modality for gastroesophageal reflux disease. World J Gastroenterol 2014;20:7730-8. [Crossref] [PubMed]
- Tariq N, Van Eps J, Dunkin BJ. Endoscopy and Endoscopic Intervention. In: Zinner MJ, Ashley SW Jr Hines OJ. editors. Maingot’s Abdominal Operations. 13th edition. New York: McGraw-Hill Education, 2018.
- Brunt LM. Fundamentals of Electrosurgery Part II: Thermal Injury Mechanisms and Prevention. In: Feldman L, Fuchshuber P, Jones DB, editors. The SAGES Manual on the Fundamental Use of Surgical Energy (FUSE). New York, NY: Springer New York, 2012:61-79.
- Ko CW, Dominitz JA. Complications of colonoscopy: magnitude and management. Gastrointest Endosc Clin N Am 2010;20:659-71. [Crossref] [PubMed]
- Rabeneck L, Paszat LF, Hilsden RJ, et al. Bleeding and perforation after outpatient colonoscopy and their risk factors in usual clinical practice. Gastroenterology 2008;135:1899-906, 906.e1.
- Jones EL, Madani A, Overbey DM, et al. Stray energy transfer during endoscopy. Surg Endosc 2017;31:3946-51. [Crossref] [PubMed]
- Levy I, Gralnek IM. Complications of diagnostic colonoscopy, upper endoscopy, and enteroscopy. Best Pract Res Clin Gastroenterol 2016;30:705-18. [Crossref] [PubMed]
- Inoue H, Minami H, Kobayashi Y, et al. Peroral endoscopic myotomy (POEM) for esophageal achalasia. Endoscopy 2010;42:265-71. [Crossref] [PubMed]
- Brzezinski A, Kuchenbecker KJ, Gomez ED, et al. Electrocautery tactile feedback systems and methods. Google Patents 2019. Available online: https://patents.google.com/patent/US20160081738
Cite this article as: Hashimoto DA, Meireles OR. Fundamental use of surgical energy during endoscopic therapies. Ann Laparosc Endosc Surg 2019;4:79.