Current techniques for combined endoscopic and laparoscopic surgery (CELS)
Introduction
Widespread adoption of risk-targeted colorectal cancer screening has been successful in reducing the overall mortality from the disease. Screening has also precipitated an increase in the detection of large and complex polyps, many of which cannot be managed by simple endoscopic polypectomy. Frequently described factors include large polyp size, inaccessible location on a luminal fold or flexure and proximity to the appendiceal orifice or ileocecal valve. Complex polyps may be pedunculated or sessile. These patients are referred for surgical management, which classically has meant undergoing segmental colon resection. While the number of colectomies performed annually for cancer has decreased, there are now 28,000 colectomies performed every year in the United States for benign lesions, which represents a substantial increase from 5.9 per 100,000 patients in 2000 to 9.4 per 100,000 patients in 2014 (1). Segmental colon resections for benign polyps are similarly reported at persistently high rates in Europe (2). Minimally invasive approaches and enhanced recovery protocols have substantially reduced the trauma, complication rate and overall cost associated with colon resection, but patients undergoing colectomy continue to bear significant perioperative and long-term morbidity.
Innovation has fueled the development of combined endoscopic and laparoscopic surgery (CELS) techniques for removal of complex polyps without colon resection (3-5). The safety and effectiveness of these procedures has been established in multiple retrospective series and systematic reviews demonstrating low complication rates and high rates of successful resection without colectomy (6-9). Perhaps not surprisingly at this point in the adoption of CELS techniques, there have been few studies that are able to evaluate long-term outcomes in CELS patients. Those that exist in the literature demonstrate reassuring data for those who may have concerns about adopting CELS techniques due to oncologic concerns. In these series there were no cases of malignant lesions developing in patients with completely resected histopathologically benign polyps (6,10).
In the current era of increased scrutiny over healthcare costs, adoption of CELS holds great promise from an economic perspective, both for patients as well as for broader healthcare systems. Most patients undergoing CELS can be discharged on the day of surgery, with a small minority possessing significant comorbidities or concerning intraoperative findings admitted for observation until the first postoperative day. Therefore, the slightly higher equipment cost of CELS procedures, is far surpassed by the substantial savings realized from reduced inpatient hospital utilization (11,12). An analysis identified the total cost per CELS procedure at $6,554 which compared favorably to $12,585 for laparoscopic segmental resection and $18,216 for open resection (13).
CELS is indicated for management of benign appearing polyps that cannot be removed by endoscopic polypectomy alone. Lesions with concerning appearance including irregular contours, central depression or ulceration, and those that fail to lift with submucosal injection, or those with irregular vascular or pit patterns on narrow-band imaging are suspicious for invasive malignancy and are not appropriate for CELS in most cases.
Patient selection
Patient selection is perhaps the most critical factor when undertaking a CELS procedure. These techniques forego the traditional oncological resection and therefore should not be performed in patients with known malignancy, a known polyposis syndrome or lesions with high risk features. Lesions found to exhibit high grade dysplasia but absent any other concerning features may be amenable to a CELS approach. In addition to the standard history and physical exam, particular attention should be paid to family history of colorectal cancer and inflammatory bowel disease. It is necessary to review the referring colonoscopy report with relevant images and pathology consistent with a benign lesion. We strongly recommend in-office evaluation of left sided lesions by flexible endoscopy to verify polyp location, size and the absence of concerning characteristics. As an important component of the surgical informed consent, patients must be counseled that should the lesion be found intraoperatively to be inaccessible or unresectable by CELS, or if features concerning for malignancy are identified, the operating surgeon will proceed with an oncologic colon resection. Furthermore, if a successfully removed polyp is found on pathologic evaluation to contain malignancy, this may necessitate a subsequent operation for formal resection.
Morbid obesity and history of prior abdominal surgeries are not contraindications for CELS procedures, however such factors can make the procedure more challenging. The selection of laparoscopic trocar size and sites will need to be adjusted to maintain triangulation at the target colon segment, and bariatric instruments employed in patients with super-morbid obesity.
Techniques
Laparoscopic-assisted polypectomy
Laparoscopic-assisted polypectomy was first described in 1993 as a method for complete excision of moderate-sized sessile polyps that avoided colon resection in select patients (3). The technique is applicable for complex polyps located throughout the colon proximal to the peritoneal reflection. As such, it has become the most widely employed CELS technique.
Patients are necessarily placed in a modified lithotomy position to allow simultaneous operation by the endoscopist and abdominal surgeon. The endoscopist will start by performing an intraoperative colonoscopy to identify the target lesion and confirm that it is amenable to CELS resection. The lesion should be examined to confirm the absence of concerning features such as hardness, fold convergence, expansile growth, and depression or ulceration. The size and location of the lesion should additionally be assessed, and more advanced techniques or formal resection considered if the lesion is not removable by laparoscopic-assisted polypectomy. CO2 insufflation is preferred when performing CELS procedures for its more rapid absorption. Colon distention is minimized, which facilitates simultaneous laparoscopic and endoscopic visualization (14).
Abdominal access proceeds by standard technique and laparoscopic trocars are placed following establishment of pneumoperitoneum. Insertion of a laparoscope allows identification of the target lesion, either by tattoo identification or endoscopic transillumination of the colon wall. Two 5 or 3 mm working trocar sites are selected to triangulate the identified target lesion.
Employing laparoscopic assistance overcomes the challenge of inaccessible polyp location as a barrier to endoscopic resection. The abdominal surgeon can reposition the colon wall to expose lesions located along the backside of folds. Polyps located behind flexures or kinks from scarring are more difficult and will not respond to simple manipulation of the colon wall, but laparoscopic mobilization of the corresponding segment of colon to straighten out the tissue exposes the previously hidden polyp for resection. Each polyp’s particular location determines the extent to which external manipulation and colon mobilization are required. The ability to externally manipulate the colon becomes a marked technical advantage for CELS when compared to purely endoscopic approaches.
An endoscopic injection needle is inserted through the working channel of the colonoscope and submucosal injection is employed to lift the polyp off the muscular layer of the colon wall. We typically utilize a dilute solution of 50% indigo carmine or methylene blue and either saline or albumin to mark the location of the lesion as well as raise the mucosal based polyp. Failure to create a broad smooth cushion while injecting likely indicates needle insertion into a deeper layer of the colon wall. Simply adjust by steadily pulling back the injection needle while continuing to inject to find the correct plane and visualize the desired effect. It may be necessary to repeat injection during the procedure if the established lift has dissipated.
A laparoscopic instrument is utilized to position and deliver the polyp for removal by endoscopic electrosurgical snare polypectomy. The surgeon can monitor the serosal side of the polypectomy site for thermal injury created by the procedure under direct visualization. When there is concern for weakness or injury, the affected area can immediately be reinforced or repaired with a laparoscopic imbricating suture. Large or complex lesions may need to be removed in several piecemeal snare excisions. In such cases, we recommend cautery fulguration of the edges of the resection margin. The snare, closed rather than open, may be employed for endoscopic fulguration of wound edges. This may help prevent polyp recurrence at the same site. Do not lose track of the specimens prior to collection. Lost specimen fragments risk incomplete pathologic evaluation. Small specimens (<5 mm) or those excised in a piecemeal fashion can be removed by colonoscopic suction with an in-line a specimen trap. Larger specimens are better collected with a Roth net.
A lesion that does not elevate with submucosal injection may indicate the presence of an occult invasive tumor. Use caution and evaluate for additional concerning signs as mentioned previously. When there is concern for an invasive lesion, formal laparoscopic colectomy should be performed. Following endoscopic removal, we recommend examining the polyp prior to sending it to pathology. Hold the specimen between the thumb and index fingers, roll between them and asses for hard or firm components. Hard and firm lesions or those with concerning gross findings should raise suspicion for occult malignancy for which segmental colectomy is the recommended treatment options. If the polyp truly appears benign, and there is a history of prior biopsy at the same site, the failure to lift may be the result of scarring from the previous biopsies. In such cases, the decision to proceed with endoscopic removal is at the discretion of the operating surgeon. The incidence of cancer found in benign-appearing lesions after CELS resection is low (~2%) (6). In such cases, patients are able to undergo a subsequent oncologic resection as appropriate. Routine frozen sections are not necessary. In select cases where intraoperative clinical findings increase suspicion for malignancy, frozen section may serve as an adjunct.
In order to enhance the capability of CELS to address increasingly large and complex lesions, additional CELS techniques have been developed. These include laparoscopic-assisted colon wall excision or partial cecectomy and full-thickness CELS (15). As surgeons continue to gain experience and comfort with CELS, the use of these advanced techniques will grow.
Full thickness CELS
Laparoscopic-assisted snare polypectomy dramatically expanded the capability of surgeons to remove difficult polyps endoscopically. However there remain many lesions for which it is inadequate, including large serrated adenomas and polyps with significant scarring as a result of prior biopsies. Full thickness CELS is an advanced technique to address such lesions (16).
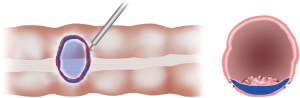
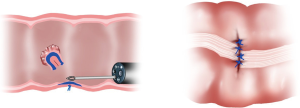
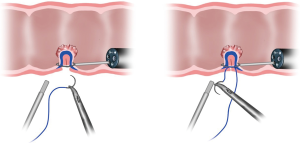
Endoscopic identification and inspection of the polyp is once again the first step. Submucosal dilute dye injection is utilized, as described in the prior section, to elevate and mark the polyp (Figure 1). Once the entire area of the lesion is elevated with dye, the circumference of the resection area is marked on the serosal surface laparoscopically using cautery. The seromuscular layer is then carefully divided along the marked circumference of the marked resection (Figure 2). Excessively deep dissection at this point results in violation of the mucosal layer of the colon wall and a full thickness perforation. After complete seromuscular division, a laparoscopic bowel grasper is used to invaginate the resection area containing the polyp into the colon lumen. The flat and adherent lesion now protrudes into the lumen and can be encircled with an endoscopic polypectomy snare (Figure 3). Without dividing the mucosa, the snare is gently closed, which pulls together the edges of the serosal dissection (Figure 4). Laparoscopic suture closure of the seromuscular defect is performed with a running 3-0 vicryl suture. The endoscopist completes the snare polypectomy by applying energy to the snare (Figure 5). The resected polyp is collected in a Roth net and removed from the colon.
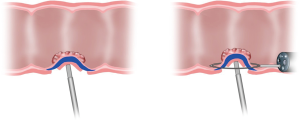
We recommend performing a final leak test by submerging the resection site in sterile saline and endoscopically insufflating the colon with CO2 under laparoscopic visualization prior to completion of the procedure. An additional layer of imbricating sutures can be placed to repair the defect if there is an identified air leak.
Full thickness laparoscopic endoscopic excision (FLEX)
Complex polyps located in the right side of the colon (cecum to the transverse colon) can be particularly challenging to resect endoscopically. They are often located near the ileocecal valve or involving the appendiceal orifice. Laparoscopic stapled wall excision or partial cecectomy performed under colonoscopy guidance facilitates complete full-thickness excision, while protecting the aforementioned structures from damage (17).
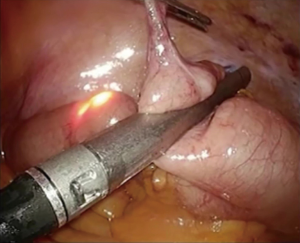
As for the previously described techniques, the polyp is first identified endoscopically and confirmed to lack concerning features. Laparoscopic ports are placed, including a 12 mm trocar to accommodate a laparoscopic linear cutting stapler and 5 mm suprapubic. Trendelenburg positioning of the operating table with the right side elevated to allow gravity to assist in retraction is recommended. The cecum is mobilized by division of the peritoneum and lateral attachments to enable optimal staple line placement and avoid excessive narrowing of the lumen. Ascending colon lesions will require additional cephalad mobilization though usually not all the way to the hepatic flexure.
While the surgeon positions the laparoscopic stapler, the endoscopist will confirm by direct visualization that the entire lesion is included in the resection. The colonoscope is positioned within the terminal ileum as a mechanical barrier while the surgeon closes the stapler (Figure 6). Analogous to the function of a Bougie, the scope ensures there is no ileocecal valve narrowing or stricture created during resection of complex lesions in close proximity to the terminal ileum.
Endoluminal surgical intervention (ELSI)
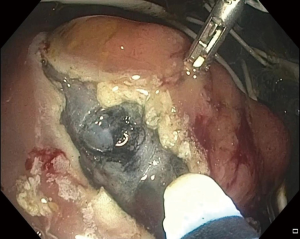
In recent years advanced endoscopic techniques have been increasingly utilized for resection of colon polyps. Interventional endoscopists have developed facility with endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) in the upper gastrointestinal tract and have applied the same techniques to management of complex colon polyps (18). New technologies, early in their adoption, expand upon these techniques and allow surgeons to apply the fundamentals of exposure, traction and counter-traction to ELSI (19). There are currently two device leaders in the space. The ORISETM Tissue Retractor System (Boston Scientific Corp) is an over the scope device with a deployable cage that establishes a stable working environment at the site of a polyp. The device has two channels that accommodate independently controlled articulating tissue grasping arms for retraction during endoluminal resection by ESD. The articulating arms grasp and retract the specimen, providing more optimal counter-traction, and exposing the correct safe dissection plane during ESD (Figure 7). Use of the platform currently is limited to the left colon and rectosigmoid due to the length of the device. The DiLumenTM (Lumendi, Ltd.), like the ORISE, is inserted over a flexible endoscope and establishes a stable working platform to facilitate endoluminal resections. This is accomplished by deploying a pair of inflatable balloons proximal and distal to the target lesion. Investigations of these devices are ongoing but the limited data available demonstrated that use of the ELSI platform significantly reduced the operative time for the procedure (20,21).
We are early on the adoption curve for ELSI. The technology, devices, and indications for use are likely to expand. ELSI is a complementary skill set to the described CELS techniques and has the potential to become an important component of colon-preserving management of complex colon polyps.
Post-operative management
Most CELS operations can safely be performed as outpatient procedures. In our current practice, patients that undergo CELS full-thickness excision or colonoscopy-assisted laparoscopic partial cecectomy are routinely admitted overnight for observation. Additionally, cases during which a full or partial thickness colon injury is identified and repaired intraoperatively merit a short hospital stay to ensure there are no post-operative complications.
Patients should be counseled on the importance of surveillance colonoscopy following CELS procedures. There is a reported 10% overall incidence rate of subsequent polyps over 10 years (6). We recommend a follow-up colonoscopy at 3 months, followed by a surveillance interval determined by the final pathology of the resected lesion.
CELS procedures have a low overall complication rate, reported between 4–13% in multiple series (22-24). Post-operative ileus and wound complications are the most commonly reported. Continuous laparoscopic visualization during CELS procedures permits immediate intra-operative detection and suture repair of colon perforation or partial thickness injury. In pertinent series, surgeons have reported placing reinforcing sutures for partial-thickness injuries in 10–43% of laparoscopic assisted polypectomy cases (25). Additionally, an intra-operative air leak test can be employed by using CO2 colonoscope insufflation and submerging any area of concern with laparoscopic irrigation fluid.
Post-polypectomy bleeding has not been reported with significant incidence in the available series of CELS patients, likely because the majority of bleeds are detected and managed during the procedure. Immediate bleeding from polypectomy sites can be controlled using the polypectomy snare to deliver electrocautery. Alternative options include injection of epinephrine or placement of endoscopic hemostatic clips based on surgeon preference. Delayed bleeding may occur up to a month after the procedure and is managed with resuscitation followed by repeat endoscopy for localization and hemostatic interventions (26).
Conclusions
Combined endoscopic and laparoscopic surgical techniques facilitate successful removal of challenging and complex polyps without the need for colectomy. These procedures are demonstrated to be safe and effective. Since the initial description over two decades ago, endoscopic and laparoscopic techniques have evolved to address ever more complex lesions throughout colon and rectum. Increased adoption of CELS will allow a greater number of patients to avoid the substantial morbidity of colectomy with lower cost and shorter recovery. Endoluminal surgical platforms are a developing technology. Suitable application and efficacy remains to be defined, but such technologies represent the next evolution in the surgeon’s armamentarium for colon-preserving surgery.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Michael J Stamos and Mehraneh Dorna Jafari) for the series “Laparoscopic Colon Surgery” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2019.05.07). The series “Laparoscopic Colon Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Peery AF, Cools KS, Strassle PD, et al. Increasing Rates of Surgery for Patients With Nonmalignant Colorectal Polyps in the United States. Gastroenterology 2018;154:1352-60.e3. [Crossref] [PubMed]
- Bronzwaer MES, Koens L, Bemelman WA, et al. Volume of surgery for benign colorectal polyps in the last 11 years. Gastrointest Endosc 2018;87:552-61.e1. [Crossref] [PubMed]
- Beck DE, Karulf RE. Laparoscopic-assisted full-thickness endoscopic polypectomy. Dis Colon Rectum 1993;36:693-5. [Crossref] [PubMed]
- Franklin ME, Díaz-E JA, Abrego D, et al. Laparoscopic-assisted colonoscopic polypectomy: the Texas Endosurgery Institute experience. Dis Colon Rectum 2000;43:1246-9. [Crossref] [PubMed]
- Wood JJ, Lord AC, Wheeler JM, et al. Laparo-endoscopic resection for extensive and inaccessible colorectal polyps: a feasible and safe procedure. Ann R Coll Surg Engl 2011;93:241-5. [Crossref] [PubMed]
- Lee SW, Garrett KA, Shin JH, et al. Dynamic article: long-term outcomes of patients undergoing combined endolaparoscopic surgery for benign colon polyps. Dis Colon Rectum 2013;56:869-73. [Crossref] [PubMed]
- Wilhelm D, von Delius S, Weber L, et al. Combined laparoscopic-endoscopic resections of colorectal polyps: 10-year experience and follow-up. Surg Endosc 2009;23:688-93. [Crossref] [PubMed]
- Lascarides C, Buscaglia JM, Denoya PI, et al. Laparoscopic right colectomy vs laparoscopic-assisted colonoscopic polypectomy for endoscopically unresectable polyps: a randomized controlled trial. Colorectal Dis 2016;18:1050-6. [Crossref] [PubMed]
- Nakajima K, Sharma SK, Lee SW, et al. Avoiding colorectal resection for polyps: is CELS the best method? Surg Endosc 2016;30:807-18. [Crossref] [PubMed]
- Franklin ME, Portillo G. Laparoscopic monitored colonoscopic polypectomy: long-term follow-up. World J Surg 2009;33:1306-9. [Crossref] [PubMed]
- Jayaram A, Barr N, Plummer R, et al. Combined endo-laparoscopic surgery (CELS) for benign colon polyps: a single institution cost analysis. Surg Endosc 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Gamaleldin M, Benlice C, Delaney CP, et al. Management of the colorectal polyp referred for resection: A case-matched comparison of advanced endoscopic surgery and laparoscopic colectomy. Surgery 2018;163:522-7. [Crossref] [PubMed]
- Sharma S, Xing J, Nakajima K, et al. Combined endo-laparoscopic surgery is significantly less costly than traditional surgery. J Am Coll Surg 2015;221:S29. [Crossref]
- Nakajima K, Lee SW, Sonoda T, et al. Intraoperative carbon dioxide colonoscopy: a safe insufflation alternative for locating colonic lesions during laparoscopic surgery. Surg Endosc 2005;19:321-5. [Crossref] [PubMed]
- Garrett KA, Lee SW. Combined Endoscopic and Laparoscopic Surgery. Clin Colon Rectal Surg 2015;28:140-5. [Crossref] [PubMed]
- Lin AY, O'Mahoney PR, Milsom JW, et al. Full-Thickness Excision for Benign Colon Polyps Using Combined Endoscopic Laparoscopic Surgery. Dis Colon Rectum 2016;59:16-21. [Crossref] [PubMed]
- Noren ER, Cologne KG, Lee SW. Endoscopically Guided Laparoscopic Partial Cecectomy For Management Of Benign Cecal Polyps. Diseases of the Colon & Rectum 2018;61:e313-4.
- Nishiyama H, Isomoto H, Yamaguchi N, et al. Endoscopic submucosal dissection for colorectal epithelial neoplasms. Dis Colon Rectum 2010;53:161-8. [Crossref] [PubMed]
- Yang D, Jawaid S, Perbtani YB, et al. Endoscopic submucosal dissection of a large rectal lesion by use of a novel tissue retractor system. VideoGIE 2018;4:37-9. [Crossref] [PubMed]
- Sharma S, Momose K, Hara H, et al. Facilitating endoscopic submucosal dissection: double balloon endolumenal platform significantly improves dissection time compared with conventional technique (with video). Surg Endosc 2019;33:315-21. [Crossref] [PubMed]
- Kantsevoy SV, Bitner M, Piskun G. New endoscopic platform for endoluminal en bloc tissue resection in the gastrointestinal tract (with videos). Surg Endosc 2016;30:3145-51. [Crossref] [PubMed]
- Crawford AB, Yang I, Wu RC, et al. Dynamic article: combined endoscopic-laparoscopic surgery for complex colonic polyps: postoperative outcomes and video demonstration of 3 key operative techniques. Dis Colon Rectum 2015;58:363-9. [Crossref] [PubMed]
- Goh C, Burke JP, McNamara DA, et al. Endolaparoscopic removal of colonic polyps. Colorectal Dis 2014;16:271-5. [Crossref] [PubMed]
- Gorgun E, Benlice C, Abbas MA, et al. Experience in colon sparing surgery in North America: advanced endoscopic approaches for complex colorectal lesions. Surg Endosc 2018;32:3114-21. [Crossref] [PubMed]
- Yan J, Trencheva K, Lee SW, et al. Treatment for right colon polyps not removable using standard colonoscopy: combined laparoscopic-colonoscopic approach. Dis Colon Rectum 2011;54:753-8. [Crossref] [PubMed]
- Lowenfeld L, Saur NM, Bleier JIS. How to avoid and treat endoscopic complications. Semin Colon Rectal Surg 2017;28:41-6. [Crossref]
Cite this article as: Noren ER, Wickham C, Lee SW. Current techniques for combined endoscopic and laparoscopic surgery (CELS). Ann Laparosc Endosc Surg 2019;4:77.