What is the best surgical option for the resection of transverse colon cancer?
Introduction
Over 1.8 million new colorectal cancer (CRC) cases and 881,000 deaths were estimated to occur worldwide in 2018, when CRC was the third most commonly diagnosed cancer (10.2%) and the second leading cause of cancer death (9.2%) (1). In Europe, there was an estimation of 500,000 new cases and 243,000 deaths from CRC in 2018 (2). Transverse colon cancer (TCC) represent less than 10% of all CRCs (3,4). Anatomical peculiarities of the transverse colon are consequences of its central position between the foregut and midgut. Due to close relationship between the transverse mesocolon and the proximal superior mesenteric artery (SMA) and vein, also with foregut structures (i.e., greater omentum, the lesser sac, pancreas) TCC may spread to the lymph nodes of the proximal SMA and vein, to the greater omentum, and to the lower border of the pancreas (5-7). Based on the abovementioned considerations, TCC resections should associate the excision of the entire transverse mesocolon. However, surgical expertise is required to perform, either by laparoscopy or open surgery, high middle colic artery (MCA) dissection with a complete transverse mesocolic excision (8-10). These challenging aspects limited the widespread of the laparoscopic (LAP) approach in TCC and prevented the inclusion of this tumors in most of the previous randomized controlled trials.
The debate persists whether a subtotal colectomy (STC) or extended right colectomy (ERC) may achieve more complete mesocolic excision associated with higher lymph node harvesting, greater distance between the tumor and the central vascular tie, and ultimately, higher disease-free survival (DFS) and overall survival (OS) compared to more conservative approach, such as transverse colectomy (TC) (9,11). Left colectomies (LC) have been also used for distal transverse tumors near the splenic flexure and splenic flexure cancer (SFC) (12).The aim of the present narrative review is to describe the indications, outcomes, limitations and advantages of STC, ERC, LC, and TC for TCC in order to identify possible trends in the current literature suggesting which is the best treatment option in both elective and emergency settings.
Materials and methods
A literature search was conducted to identify studies focusing on the surgical treatment of TCC. The following databases were screened: Medline (through PubMed), Scopus, Embase, Cochrane Library, and Google Scholar, without date restrictions. A specific research equation was used for each database, using the following keywords and/or MeSH Terms: transverse colon cancer, splenic flexure carcinoma, open surgery, laparoscopic, robotic, surgical procedures, operative surgical procedures, extended right colectomy, segmental colectomy, subtotal colectomy, transverse colectomy, postoperative complications, mortality, survival, outcomes. Reference lists of pertinent articles were crosschecked to identify potential additional articles. Only articles written in English were considered and analyzed to identify the main endpoints of the surgical treatments for TCC. The selected studies are summarized in Table 1.
Table 1
| 1st author and year, study period, number of patients | Country | Surgical indications | Study design | Surgical procedure (n) | Surgical approach | cTNMa |
>12 LN harvested (%) | R0 resections |
Complication rate (%) | Anastomotic leakage (%) | Mortality (%) | Survival outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Studies on different procedures for TCC resection | ||||||||||||
| Leijssen et al. 2018, 2004–2014, n=103 | USA | Mid-TCC | PSM case-control | TC =38 vs. EC =65 (ERC =48 + ELC =17) | Open and laparoscopy | I =32; |
TC =84.2; |
TC =94.7; |
Overall complic.: |
TC =3.1; |
TC =3.1; |
5-year OS: |
| Major complic.: |
5-year DFS: |
|||||||||||
| Matsuda et al. 2018, 2007–2017, n=72 | Japan | Mid-TCC | Case-control | TC =34 vs. ERC =38 | Laparoscopy | I =33; |
NA | 100 | TC =29.4; |
TC =5.9; |
NA | 5-year OS: |
| 5-year DFS: |
||||||||||||
| Chong et al. 2016, 1995–2013, n=1,066 | Korea | TCC | PSM case-control | TC =127 vs.
|
Open (n=649); laparoscopy (n=417) | I =180; |
NA | NA | TC =19.6; EC =26.7; | TC =0.8; |
TC =0; |
5-year OS: |
| 5-year DFS: |
||||||||||||
| Guan et al. 2017, 2004–2013, n= 10,344 | China | TCC | PSM case-control | TC =4,431 vs.
|
Open | I=2210; II=4575; III=3559 | EC =80.3; |
NA | NA | NA | NA | 5-year CSS: |
| Studies on different procedures for splenic flexure colon cancer resection | ||||||||||||
| de’Angelis et al. 2016, 2000–2013, n=68 | France | SFC | Matched case-control | ERC =27 vs.
|
Laparoscopy | 0=2; I=6; |
ERC =91.6; |
100 | ERC =22.2; |
ERC =3.7; |
ERC =0; |
5-year OS: |
| 5-year DFS: |
||||||||||||
| Gravante et al. 2016, 2003–2012, n=98 | UK | SFC | Case-control | ERC =64 vs.
|
Open =85; |
NA | ERC =78.1; |
ERC =98.4; |
ERC =21.9; |
ERC =6.3; |
ERC =1.6; |
Mean OS: |
| Odermatt et al. 2014, 1996–2011, n=68 | UK | SFC | Case-control study | ERC =38 vs.
|
Open =58; |
I =9; |
ERC =44.7; |
ERC =100; |
NA | ERC =10.5; |
ERC =7.9; |
5-year OS: |
| 5-year DFS: |
||||||||||||
| Nakashima et al. 2011, 2003–2010, n=55 | Japan | SFC | Case-control study | ERC =1; |
Open =33 vs. laparoscopy =22 | NA | NA | Open =91; |
Open =36; |
Open =0; |
Open =0; |
NA |
| Manceau et al. 2018, 2000–2016, n=65 | France | SFC | Case Series | STC =65 | Open | I =7; |
94 | 100 | NA | NA | NA | NA |
| Studies on different approaches for TCC resection | ||||||||||||
| Ozben et al. 2018, 2014–2017, n=29 | Turkey | TCC | Case series | ERC =12; |
Robotic | NA | NA | 100 | 24.1 | 0.0 | 0.0 | NA |
| de’Angelis et al. 2015, 2013–2014, n=44 | France | TCC | Matched case-control | LAP-TC =22 vs. Robotic-TC =22 | LAP robotic | I =2; |
LTC =95.5; |
100 | LAP-TC =9.1; |
LAP-TC =4.5; |
0.0 | NA |
a, tumors classifications according to the AJCC TNM system. TCC, transverse colon cancer; LN, lymph node; PSM, propensity score matched; TC, transverse colectomy; EC, extended colectomy; ERC, extended right colectomy; ELC, extended left colectomy; OS, overall survival; DFS, disease-free survival; NA, not available or nor applicable; SFC, splenic flexure cancer; LC, left colectomy; LAP, laparoscopic; STC, subtotal colectomy; AJCC, American Joint Committee on Cancer.
Definitions and technical notes
Different surgical procedures are described for the resection of TCC, ranging from segmental colectomy to STC. No consensus is reached on the specific indication of each procedure; rather, the surgical choice stands upon surgeon’s experience and clinical considerations.
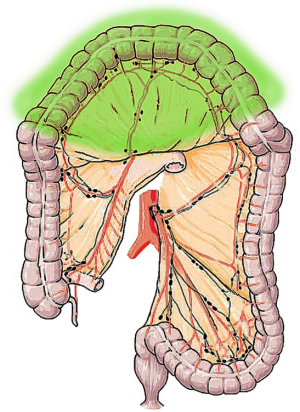
The more bowel-sparing approach is the TC, which is the resection of any length of bowel from the hepatic to the splenic flexure with the accompanying lympho-vascular supply along the middle colic pedicle (8,13,14). A transverse colon resection leaves short transverse stumps that require complex technical reconstruction, such as right colonic transposition or intestinal derotation, which may increase the risk of leak (15,16). In general, TC is associated with shorter margin lengths and fewer lymph nodes resection than more extended procedures (Figure 1).
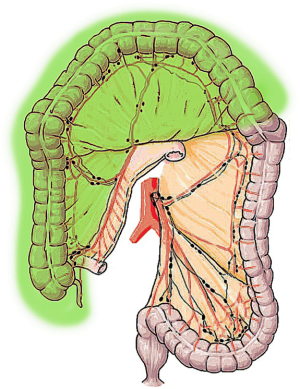
ERC consists in the ligation of the ileocolic, right colic and middle colic vessel to achieve the resection of distal ileum, cecum, ascending colon and the transverse colon up to the splenic flexure (17-20). In this procedure, the ascending branch of the left colic artery is preserved providing the blood supply to the distal anastomosis. In its first description, ERC was originally indicated for the treatment of the right-sided colon cancer but it was subsequently performed for transverse colon resections as well (9,17). ERC shows technical advantages when compared to TC, mainly related to the ileo-colonic anastomosis, which can be performed without tension being the ileum a highly mobile segment of the bowel (Figure 2) (17).
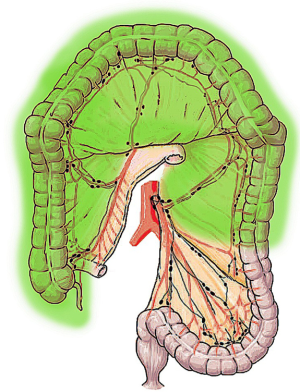
STC refers to the resection of the colon up to the junction between the descending colon and the sigmoid colon (21). STC includes the ligation of the ileocolic, right colic, middle colic and left colic pedicles (22) (Figure 3). Consequently, STC is an extensive resection, also indicated when there is cancer on both the right and left sides of the colon and in case of familial adenomatous polyposis (FAP) or hereditary non-polyposis colon cancer (HNPCC), as a way to prevent CRC (called prophylactic colectomy).
In the present article, we will discuss the outcomes of these three surgical procedures performed for the resection of TCC. ERC and STC, together with LC, as left hemicolectomy or left segmental colectomy, have been described for SFC resection (12). It must be noted that in the current literature is still frequent to read definitions of ERC and STC that overlap. However, these are two different procedures, and the two terms should not be used interchangeably. Sometimes, both procedures are assimilated (considered as the same group of patients); in these cases, they will refer to extended colectomy (EC).
Resection of hepatic flexure and mid-TCC
Literature concerning colon cancers located at the hepatic flexure and mid- transverse colon is limited: randomized clinical trials and large studies reporting long-term outcomes are lacking (9). It is thus difficult to identify an evidence-based gold standard treatment for TCCs. Consequently, the choice of performing TC, ERC or STC is often left to the surgeon's preference and experience.
ERC is generally performed when malignant lesions are located at the hepatic flexure or proximal to the mid- transverse colon (11,23). Leijssen et al. (24) conducted a study on a sample of 103 stage I–III colon cancer patients matched on propensity scores to compare TC vs. EC (both right and left EC). EC patients tended to have worse short-term outcomes than TC patients, but no procedure-related differences were found for 5-year overall and DFS rates (24). Matsuda et al. (23) reported a series of 72 patients, of whom 38 underwent ERC and 34 TC; comparable were observed in terms of oncological outcomes, but fewer overall postoperative complications occurred in patients operated on by ERC. In a Korean study, Chong et al. (11) evaluated a sample of 1,066 patients with TCC. The authors reported no differences in terms of oncological outcomes and survival between EC and TC after propensity score matching, resulting in a balanced sample of 254 patients. However, they found a significantly longer specimen and a higher number of harvested lymph nodes in the EC group (11). A population-based study by Guan et al. (25) compared TC (partial colectomy) to EC in 10,344 patients with TCC and showed similar 5-year cancer-specific survival between the two procedures (67.5% for TC and 66.5% for EC), which was also confirmed after propensity score matching, despite of less nodes examined by TC than EC. Overall data supported that node retrieval is adequate to the tumor stage even when performing TC, that the surgical approach is not an independent prognostic factor for patients with TCC, but subgroup analyses showed that in patients with tumor sized ≥5 cm, no survival benefit can be obtained by TC compared to EC (25).
The most recent meta-analysis on the topic selected 5 articles comparing TC and EC (comprising both ERC and LC), including overall 11,687 patients, of whom 4,664 underwent TC and 7,023 EC (9). Pooled data analyses showed no difference in terms of short- and long-term outcomes, comprising postoperative complication rates, although a statistically significant difference was found in favor of EC for the number of harvested lymph nodes.
Only one study compared ERC vs. TC (23), and none directly ERC vs. STC outcomes, thus questions remains open about the potential oncologic benefits associated with a more extended colonic resection and regarding the influence of the different intestinal reconstructions on leak rates. Therefore, it is still unknown which intervention should be preferred for the treatment of hepatic flexure and mid-TCCs.
Resection of splenic flexure colon cancer
For the great majority of studies concerning colon cancer located at the splenic flexure, the exact location, meaning the distal part of the transverse or the proximal part of the left colon, is rarely described. Thus, some of these tumors could eventually be located in the descending colon up to 10–15 cm from the splenic flexure (26,27). However, the splenic flexure is often considered as a possible location of TCC, for which we find the same type of debate about the required extension of resection and the type of surgical procedure to be preferred. Indeed, for SFCs, segmental colectomy, LC, ERC, and STC are described (12). As for mid- transverse colon resection, resection of the whole tumor mass and lymph nodes at the splenic flexure is challenging, due to the anatomical variability between patients (18,28,29). In most cases the bloody supply is carried out by the inferior mesenteric artery (IMA) via the left colic artery, but in other cases it is carried by the SMA via the left branches of the MCA. Consequently, to achieve an adequate lymph-nodal dissection, the surgeon must take into account these anatomical variants (22). The only systematic review and meta-analysis conducted so far considered 12 retrospective studies, including 569 SFC patients, and compared LC vs. ERC (12); pooled data analysis showed no significant procedure-related differences for the oncologic quality of the resection and postoperative outcomes. However, due to the limited literature and the overall moderate to poor quality of evidence, the authors concluded that further studied are needed to elucidate which is the optimal extent of SFC surgical resection (12). Indeed, some authors argue for a more aggressive treatment in case of SFC, such as STC, to achieve a more accurate lymph node resection (22). Manceau et al. reported data on elective STC with ileo-sigmoid anastomosis in the treatment of transverse colon and SFCs, describing the procedure as a safe and associated with a good quality of life (21).
Resection in emergency settings
Despite the implementation of screening programs, 15–30% of CRCs required emergency surgical interventions. Large bowel obstructions represent 80% of emergencies related to colon cancer, whereas perforations the remaining 20% (30,31). Emergency surgery is a risk factor for poorer prognosis and it is associated with morbidity rates that are twice higher than those observed in elective settings. Similarly, postoperative mortality ranges between 15% and 30% vs. 1% and 5% for elective surgery (32). A prospective multicenter study coordinated by the Association Française de Chirurgie (AFC) identified emergency surgery as an independent risk factor of mortality in the treatment of CRC (33).
The management of the TCC in emergency depends on its exact localization, on the patient’s general conditions, and on the surgeon’s experience (30,32). However, in general, emergencies for TCC require an extensive resection that is more likely performed by open surgery (17). In case of colonic occlusion associated to TCC, with or without perforation, a right colectomy or ERC the most performed procedures (34). The choice of a primary anastomosis largely depends on the patient’s clinical status; it should be avoided whenever the patient is unfit and the risk of anastomotic leakage is high in favor to a double-barreled ileo-colostomy (35). In patients with colonic occlusion without perforation and hemodynamically stable, a loop colostomy may be performed as first step surgery, to allow colonic decompression with a minimal surgical trauma and the implementation of intensive resuscitation, prior to the second-stage colectomy (30). However, several studies found no differences in terms of mortality and morbidity rates when comparing to primary resection (36,37).
STC or total colectomy with ileo-rectal anastomosis has been proposed as an alternative to avoid a stoma and overcome the problem of an unprepared distended colon. In general, this procedure should be taken in to account when the occlusion causes right colon ischemia, when the cancer is distant from the perforation site (diastatic cecal perforation), when simultaneous tumor resections should be associated, or in the presence of a distal TCC near the splenic flexure.
Postoperative complications and long-term outcomes
TCCs are often burdened by poorer outcomes compared to other colonic locations. Mortality rates range between 0% to 8%, whereas complication rates range between 6% to 44% (Table 1). The majority of the studies do not find significant procedure-related differences in term of postoperative outcomes and R0 resection. Although a significantly higher number of lymph nodes is harvested during more extended colonic resection (e.g., ERC, EC), at least 12 nodes are retrieved in the great majority of cases. Concerning overall and DFS, the reported 5-year OS range between 73.5% and 90.3% (11,23-25) (Table 1). Differences are noted depending on the American Joint Committee on Cancer (AJCC) stage of disease, with ranges comprised between 87.7% and 93.7% for stage-II disease and between 64.2% and 89.7% in stage-III cancer (24). DFS rates also varied between 85.5% and 94.4% in stage-II disease and between 53.3% and 79.0% in stage-III disease (24). However, no procedure-related difference has been reported in term of survival; all studies comparing TC vs. ERC or EC observed similar long-term (up to 5 years) outcomes.
Concerning articles focusing on cancers located at the splenic flexure, most of them compared ERC vs. LC in small sized retrospective comparative studies (17,18,38). Postoperative mortality ranged between 0% and 7.9%, whereas morbidity varied from 14.7% to 29.6% (17,18,38,39). In general, no procedure-related difference was noted for both short- and long-term outcomes.
One of the most severe complications of colonic resections is anastomotic leakage, with a reported incidence in the literature ranging between 2% and 12% (40). Anastomotic leakage may be difficult to detect and could lead to important sequelae such as infections, abscess and peritonitis, with a higher risk of cancer recurrence and patient death (41,42).
Ileo-sigmoid anastomosis and ileo-rectal anastomosis after STC are characterized by a high incidence of anastomotic leak, especially if they are performed in a one-step procedure with the colectomy (21,43). Also, TC was associated with a non-negligible rate of anastomotic leak (42). By performing a colo-colic anastomosis, the short transverse stumps left and the possibility of right colonic transposition or intestinal derotations, can increase the odds of leak (15,16). STC have been also related with prolonged operative times and higher incidence of postoperative diarrhea and electrolyte disorders (44).
Open surgery, LAP surgery, and robotic surgery for TCC
Due to the expected technical difficulties, the resection of TCC has been more likely performed by open surgery (45). Authors advocated that it is easier to achieve an adequate lymph node harvest around the MCA, often characterized by vascular variability, and a safer dissection of the transverse colon without damaging important structures as the pancreas, the duodenum, the spleen, and the superior mesenteric vein (46,47). As commented, appropriate mobilization of the transverse colon and high division of the middle colic vessels are considered technically challenging (14,48). However, randomized controlled studies and meta-analyses showed that LAP surgery for CRC (although located in different sites than transverse colon) is safe and feasible. LAP procedures are associated with similar oncological outcomes than those obtained by open surgery while offering several advantages related to the minimally invasive approach, namely early recovery of bowel movements, shorter hospital stay, and reduced use of analgesics (49-51). Due to the accumulating experience in performing laparoscopy, studies emerged also for cancers specifically located in the transverse colon (14,45). In 2010, a retrospective study by Zmora et al. (14) showed that the LAP approach is feasible for TCC and it is associated with a low rate of conversions and intraoperative complications. Improved short- and long-term outcomes, particularly improved recovery and lymph-node harvesting, have been reported by LAP approach (52). In 2017, Wu et al. conducted a meta-analysis considering 13 studies and 1 conference abstract for a total of 1,728 patients (45). demonstrated that LAP surgery for TCC is associated with higher operative time but lower blood loss, fewer postoperative complications, shorter bowel recovery and length of hospital stay, with equivalent oncologic outcomes than open surgery (45). In 2018, another meta-analysis by Gavriilidis et al. based upon 8 studies reported that there were no survival benefits of LAP TC over open TC up to 5 years (48). Open surgery was associated with 38 min shorter operative time than laparoscopy. However, LAP colectomy was associated with earlier postoperative recovery and consequently a hospital stay shorter by 4.5 days compared to open surgery (48).
Colon mobilization and lymphadenectomy require LAP expertise, severe complications may occur if the dissection is conducted in a wrong plane (53). LAP surgery might be even more technically demanding for the visualization of the mesenteric root, in patients obese or with previous upper abdominal surgery (14). Recently, the study by Hamabe et al. proposed a classification into three subtypes of the intravascular relationships between the first jejunal vein (FJV), the SMA, and the MCA. This classification could be for TC LAP oncologic resections (46). Therefore, in the presence of good surgical ability and in selected patients, both ERC and STC appear to be feasible by laparoscopy (54,55), although a high rate of conversion (up to 15%) and longer operative time has been reported (49,54,55). On the other hand, higher rates of incisional hernia have been reported (6–8.3%) after open STC (56,57), compared to no incisional hernia formation with a LAP approach. Small bowel obstruction is another possible long-term complication of STC, but the meta-analysis by Tilney et al. showed no differences between open and LAP surgery (55). Therefore, a LAP approach seems to be preferable at elective setting, depending on surgeon’s experience in minimally invasive surgery.
Finally, the use of robotic surgery for TCCs is limited to few retrospective studies (8,58-60). In general, robotic surgery may represent a possible alternative for colon cancer resection offering some technical advantages compared to laparoscopy, such as less rigid instrumentations, more ergonomics, and a greater maneuverability (61). For these reasons, it might be useful to overcome some of the technical difficulties related to the transverse colon location. One of the first description of robotic colectomy for TCC (AJCC stages I to III) was published by de’Angelis et al. in 2015 (8). The authors compared 22 robotic TC vs. 22 matched LAP TC and showed longer operating times when operating on via the robotic approach. However, no approach-related differences were noted for any other short-term outcome evaluated. Other studies confirmed that robotic surgery allows performing oncologically adequate dissections of the transverse colon with radical lymphadenectomy and acceptable and short-term outcomes (58,59,61). A case-report described robotic transverse colon resections by STC (62). Robotic surgery might be associated with some advantages in the construction of intracorporal anastomosis thanks to the endo-wrist function, the stability of the robotic arms, and the 3D enhanced view, but further studies are needed to elucidate the true role of robotic surgery for TCC.
Conclusions
Current literature on TCC resection is still limited. Less aggressive surgical procedures, such as TC, may to be preferred for early-stage cancers, whereas ERC and STC should be considered for advanced stages or at emergency presentations. Whether the oncologic principle of surgery is met, no procedure-related differences are reported in the available retrospective, mainly small-sized, studies. LAP surgery appeared to be safe and feasible for transverse colon resections, especially in elective settings and if the surgeon is experienced with this technique. Robotic surgery might be considered as a valid alterative allowing simplifying some technical LAP difficulties, but the literature is too limited to draw definitive conclusions. Finally, the scientific community should find a consensus on standardized definitions for TC, ERC and STC, in order to elucidate clinicians and surgeons on the indications and contraindications of each surgical intervention for the treatment of cancer located in the transverse colon.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Roberto Bergamaschi and Mahir Gachabayov) for the series “Right Colon Cancer Surgery: Current State” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2019.07.01). The series “Right Colon Cancer Surgery: Current State” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Ferlay J, Colombet M, Soerjomataram I, et al. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer 2018;103:356-87. [Crossref] [PubMed]
- Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018;391:1023-75. [Crossref] [PubMed]
- De Angelis R, Sant M, Coleman MP, et al. Cancer survival in Europe 1999-2007 by country and age: of EUROCARE--5-a population-based study. Lancet Oncol 2014;15:23-34. [Crossref] [PubMed]
- Bertelsen CA, Neuenschwander AU, Jansen JE, et al. Disease-free survival after complete mesocolic excision compared with conventional colon cancer surgery: a retrospective, population-based study. Lancet Oncol 2015;16:161-8. [Crossref] [PubMed]
- Morikawa E, Yasutomi M, Shindou K, et al. Distribution of metastatic lymph nodes in colorectal cancer by the modified clearing method. Dis Colon Rectum 1994;37:219-23. [Crossref] [PubMed]
- Stelzner S, Hohenberger W, Weber K, et al. Anatomy of the transverse colon revisited with respect to complete mesocolic excision and possible pathways of aberrant lymphatic tumor spread. Int J Colorectal Dis 2016;31:377-84. [Crossref] [PubMed]
- de'Angelis N, Alghamdi S, Renda A, et al. Initial experience of robotic versus laparoscopic colectomy for transverse colon cancer: a matched case-control study. World J Surg Oncol 2015;13:295. [Crossref] [PubMed]
- Milone M, Manigrasso M, Elmore U, et al. Short- and long-term outcomes after transverse versus extended colectomy for transverse colon cancer. A systematic review and meta-analysis. Int J Colorectal Dis 2019;34:201-7. [Crossref] [PubMed]
- Kawada K, Nishikawa G, Shinohara H, et al. Laparoscopic Transverse Colectomy. In: (eds) SY, editor. Laparoscopic Surgery for Colorectal Cancer. Tokyo: Springer; 2016.
- Chong CS, Huh JW, Oh BY, et al. Operative Method for Transverse Colon Carcinoma: Transverse Colectomy Versus Extended Colectomy. Dis Colon Rectum 2016;59:630-9. [Crossref] [PubMed]
- Martínez-Pérez A, Brunetti F, Vitali GC, et al. Surgical Treatment of Colon Cancer of the Splenic Flexure: A Systematic Review and Meta-analysis. Surg Laparosc Endosc Percutan Tech 2017;27:318-27. [Crossref] [PubMed]
- Akiyoshi T, Kuroyanagi H, Fujimoto Y, et al. Short-term outcomes of laparoscopic colectomy for transverse colon cancer. J Gastrointest Surg 2010;14:818-23. [Crossref] [PubMed]
- Zmora O, Bar-Dayan A, Khaikin M, et al. Laparoscopic colectomy for transverse colon carcinoma. Tech Coloproctol 2010;14:25-30. [Crossref] [PubMed]
- Bakker IS, Grossmann I, Henneman D, et al. Risk factors for anastomotic leakage and leak-related mortality after colonic cancer surgery in a nationwide audit. Br J Surg 2014;101:424-32; discussion 32. [Crossref] [PubMed]
- Dumont F, Da Re C, Goere D, et al. Options and outcome for reconstruction after extended left hemicolectomy. Colorectal Dis 2013;15:747-54. [Crossref] [PubMed]
- Gravante G, Elshaer M, Parker R, et al. Extended right hemicolectomy and left hemicolectomy for colorectal cancers between the distal transverse and proximal descending colon. Ann R Coll Surg Engl 2016;98:303-7. [Crossref] [PubMed]
- de'Angelis N, Hain E, Disabato M, et al. Laparoscopic extended right colectomy versus laparoscopic left colectomy for carcinoma of the splenic flexure: a matched case-control study. Int J Colorectal Dis 2016;31:623-30. [Crossref] [PubMed]
- Zhao LY, Chi P, Ding WX, et al. Laparoscopic vs open extended right hemicolectomy for colon cancer. World J Gastroenterol 2014;20:7926-32. [Crossref] [PubMed]
- Zhao LY, Liu H, Wang YN, et al. Techniques and feasibility of laparoscopic extended right hemicolectomy with D3 lymphadenectomy. World J Gastroenterol 2014;20:10531-6. [Crossref] [PubMed]
- Manceau G, d'Annunzio E, Karoui M, et al. Elective subtotal colectomy with ileosigmoid anastomosis for colon cancer preserves bowel function and quality of life. Colorectal Dis 2013;15:1078-85. [PubMed]
- Manceau G, Mori A, Bardier A, et al. Lymph node metastases in splenic flexure colon cancer: Is subtotal colectomy warranted? J Surg Oncol 2018;118:1027-33. [Crossref] [PubMed]
- Matsuda T, Sumi Y, Yamashita K, et al. Optimal Surgery for Mid-Transverse Colon Cancer: Laparoscopic Extended Right Hemicolectomy Versus Laparoscopic Transverse Colectomy. World J Surg 2018;42:3398-404. [Crossref] [PubMed]
- Leijssen LGJ, Dinaux AM, Amri R, et al. A Transverse Colectomy is as Safe as an Extended Right or Left Colectomy for Mid-Transverse Colon Cancer. World J Surg 2018;42:3381-9. [Crossref] [PubMed]
- Guan X, Zhao Z, Yang M, et al. Whether partial colectomy is oncologically safe for patients with transverse colon cancer: a large population-based study. Oncotarget 2017;8:93236-44. [Crossref] [PubMed]
- Kim MK, Lee IK, Kang WK, et al. Long-term oncologic outcomes of laparoscopic surgery for splenic flexure colon cancer are comparable to conventional open surgery. Ann Surg Treat Res 2017;93:35-42. [Crossref] [PubMed]
- Beisani M, Vallribera F, García A, et al. Subtotal colectomy versus left hemicolectomy for the elective treatment of splenic flexure colonic neoplasia. Am J Surg 2018;216:251-4. [Crossref] [PubMed]
- Bourgouin S, Bege T, Lalonde N, et al. Three-dimensional determination of variability in colon anatomy: applications for numerical modeling of the intestine. J Surg Res 2012;178:172-80. [Crossref] [PubMed]
- Shaikh IA, Suttie SA, Urquhart M, et al. Does the outcome of colonic flexure cancers differ from the other colonic sites? Int J Colorectal Dis 2012;27:89-93. [Crossref] [PubMed]
- Pisano M, Zorcolo L, Merli C, et al. 2017 WSES guidelines on colon and rectal cancer emergencies: obstruction and perforation. World J Emerg Surg 2018;13:36. [Crossref] [PubMed]
- de' Angelis N. Colorectal cancer research: A state of the art. In: de'Angelis N, Di Saverio S, Brunetti F, editors. Emergency Surgical Management of Colorectal Cancer Switzerland Springer Nature 2019. p. 1-13.
- Picarello E, Zanghi C, Fugazzola P, et al. Emergencies related to the primary colon cancer In: de'Angelis N, Di Saverio S, Brunetti F, editors. Emergency Surgical Management of Colorectal Cancer Switzerland: Springer Nature 2019. p. 91-9.
- Alves A, Panis Y, Mathieu P, et al. Postoperative mortality and morbidity in French patients undergoing colorectal surgery: of a prospective multicenter study. Arch Surg 2005;140:278-83, discussion 84. [Crossref] [PubMed]
- Shimura T, Joh T. Evidence-based Clinical Management of Acute Malignant Colorectal Obstruction. J Clin Gastroenterol 2016;50:273-85. [Crossref] [PubMed]
- Felli E, Brunetti F, Disabato M, Salloum C, Azoulay D, De'angelis N. Robotic right colectomy for hemorrhagic right colon cancer: a case report and review of the literature of minimally invasive urgent colectomy. World J Emerg Surg 2014;9:32. [Crossref] [PubMed]
- Yang HY, Wu CC, Jao SW, et al. Two-stage resection for malignant colonic obstructions: the timing of early resection and possible predictive factors. World J Gastroenterol 2012;18:3267-71. [PubMed]
- Gainant A. Emergency management of acute colonic cancer obstruction. J Visc Surg 2012;149:e3-e10. [Crossref] [PubMed]
- Odermatt M, Siddiqi N, Johns R, et al. Short- and long-term outcomes for patients with splenic flexure tumours treated by left versus extended right colectomy are comparable: a retrospective analysis. Surg Today 2014;44:2045-51. [Crossref] [PubMed]
- Nakashima M, Akiyoshi T, Ueno M, et al. Colon cancer in the splenic flexure: comparison of short-term outcomes of laparoscopic and open colectomy. Surg Laparosc Endosc Percutan Tech 2011;21:415-8. [Crossref] [PubMed]
- Daams F, Luyer M, Lange JF. Colorectal anastomotic leakage: aspects of prevention, detection and treatment. World J Gastroenterol 2013;19:2293-7. [Crossref] [PubMed]
- Nikolian VC, Kamdar NS, Regenbogen SE, et al. Anastomotic leak after colorectal resection: A population-based study of risk factors and hospital variation. Surgery 2017;161:1619-27. [Crossref] [PubMed]
- Schiff A, Roy S, Pignot M, et al. Diagnosis and Management of Intraoperative Colorectal Anastomotic Leaks: A Global Retrospective Patient Chart Review Study. Surg Res Pract 2017;2017:3852731 [Crossref] [PubMed]
- Segelman J, Mattsson I, Jung B, et al. Risk factors for anastomotic leakage following ileosigmoid or ileorectal anastomosis. Colorectal Dis 2018;20:304-11. [Crossref] [PubMed]
- Cuffy M, Abir F, Audisio RA, et al. Colorectal cancer presenting as surgical emergencies. Surg Oncol 2004;13:149-57. [Crossref] [PubMed]
- Wu Q, Wei M, Ye Z, et al. Laparoscopic Colectomy Versus Open Colectomy for Treatment of Transverse Colon Cancer: A Systematic Review and Meta-Analysis. J Laparoendosc Adv Surg Tech A 2017;27:1038-50. [Crossref] [PubMed]
- Hamabe A, Park S, Morita S, et al. Analysis of the Vascular Interrelationships Among the First Jejunal Vein, the Superior Mesenteric Artery, and the Middle Colic Artery. Ann Surg Oncol 2018;25:1661-7. [Crossref] [PubMed]
- Jin G, Tuo H, Sugiyama M, et al. Anatomic study of the superior right colic vein: its relevance to pancreatic and colonic surgery. Am J Surg 2006;191:100-3. [Crossref] [PubMed]
- Gavriilidis P, Katsanos K. Laparoscopic Versus Open Transverse Colectomy: A Systematic Review and Meta-Analysis. World J Surg 2018;42:3008-14. [Crossref] [PubMed]
- Abraham NS, Byrne CM, Young JM, et al. Meta-analysis of non-randomized comparative studies of the short-term outcomes of laparoscopic resection for colorectal cancer. ANZ J Surg 2007;77:508-16. [Crossref] [PubMed]
- Ohtani H, Tamamori Y, Arimoto Y, et al. A meta-analysis of the short- and long-term of randomized controlled trials that compared laparoscopy-assisted and open colectomy for colon cancer. J Cancer 2012;3:49-57. [Crossref] [PubMed]
- Stage JG, Schulze S, Moller P, et al. Prospective randomized study of laparoscopic versus open colonic resection for adenocarcinoma. Br J Surg 1997;84:391-6. [Crossref] [PubMed]
- Agarwal S, Gincherman M, Birnbaum E, et al. Comparison of long-term follow up of laparoscopic versus open colectomy for transverse colon cancer. Proc (Bayl Univ Med Cent) 2015;28:296-9. [Crossref] [PubMed]
- Yamamoto M, Okuda J, Tanaka K, et al. Clinical outcomes of laparoscopic surgery for advanced transverse and descending colon cancer: a single-center experience. Surg Endosc 2012;26:1566-72. [Crossref] [PubMed]
- Abraham NS, Young JM, Solomon MJ. Meta-analysis of short-term outcomes after laparoscopic resection for colorectal cancer. Br J Surg 2004;91:1111-24. [Crossref] [PubMed]
- Tilney HS, Lovegrove RE, Purkayastha S, et al. Laparoscopic vs open subtotal colectomy for benign and malignant disease. Colorectal Dis 2006;8:441-50. [Crossref] [PubMed]
- Pokala N, Delaney CP, Senagore AJ, et al. Laparoscopic vs open total colectomy: a case-matched comparative study. Surg Endosc 2005;19:531-5. [Crossref] [PubMed]
- Seshadri PA, Poulin EC, Schlachta CM, et al. Does a laparoscopic approach to total abdominal colectomy and proctocolectomy offer advantages? Surg Endosc 2001;15:837-42. [Crossref] [PubMed]
- Ozben V, de Muijnck C, Esen E, et al. Is Robotic Complete Mesocolic Excision Feasible for Transverse Colon Cancer? J Laparoendosc Adv Surg Tech A 2018;28:1443-50. [Crossref] [PubMed]
- Jung KU, Park Y, Lee KY, et al. Robotic transverse colectomy for mid-transverse colon cancer: surgical techniques and oncologic outcomes. J Robot Surg 2015;9:131-6. [Crossref] [PubMed]
- D'Annibale A, Pernazza G, Morpurgo E, et al. Robotic right colon resection: evaluation of first 50 consecutive cases for malignant disease. Ann Surg Oncol 2010;17:2856-62. [Crossref] [PubMed]
- Isik O, Gorgun E. How Has the Robot Contributed to Colon Cancer Surgery? Clin Colon Rectal Surg 2015;28:220-7. [Crossref] [PubMed]
- Rosen SA, Olson TJP, Peter SD, et al. Robotic-assisted subtotal colectomy for synchronous colon cancers in a patient with indeterminate colitis - a video vignette. Colorectal Dis 2018;20:1153-4. [Crossref] [PubMed]
Cite this article as: Martínez-Pérez A, Reitano E, Gavriilidis P, Genova P, Moroni P, Memeo R, Brunetti F, de’Angelis N. What is the best surgical option for the resection of transverse colon cancer? Ann Laparosc Endosc Surg 2019;4:69.