Management of gastrointestinal tract defects
Overview
Despite vast improvements in preoperative optimization of patients, advancements in surgical technology and methodology, and the introduction of enhanced recovery protocols, complications following gastrointestinal (GI) surgery continue to be an unfortunate reality. More than 6 million GI tract surgeries were performed in the US in 2009 (1). This statistic also includes endoscopic interventions. World Health Organization data reveals a 38% increase globally in surgical procedures for the years 2004–2012 (1,2). With increasing rates of surgical procedures involving the GI tract globally, so too will the volume of complications, among those being anastomotic leak or perforation; perhaps the most feared complication.
Leaks can result from iatrogenic injury, intestinal resection, or anastomosis formation. The management this patient population in the acute setting is a complex process associated with high re-operative morbidity and mortality. Amongst our surgical endoscopists, “Leak is a ‘four-letter’ word.” The management of leak, traditionally with open surgical methods, has now largely evolved to include or have been replaced by laparoscopic and percutaneous methodologies. However, as endoscopic techniques and tools evolve and advance for both diagnostic and therapeutic applications, novel endoluminal and transluminal management options for leak continue to progress. In some centers, these methods are becoming a first list treatment option in select patient populations where appropriate.
This manuscript will review the endoscopic management of GI defects, including acute perforations, leaks, and chronic fistulae.
Full thickness luminal defects
Full thickness luminal defects can present in several ways. This ranges from asymptomatic microperforations which require no surgical intervention, to larger acute defects with associated peritonitis or sepsis, necessitating urgent or emergent intervention. Such defects present immediately (such as a colonoscopic perforation), acutely (such as an anastomotic leak or missed enterotomy within the first several days of a surgical procedure), subacutely (such as an asymptomatic leak that walls itself off) or as a chronic fistula (as in a well-established enterocutaneous tract). Interpreting the literature on full thickness luminal defects in a meaningful way is challenging because of the huge variation in clinical presentation. These defects vary in their location, occurring anywhere in the esophagus, stomach, duodenum, small intestine, or colon, or rectum to varying degrees. The size and number of defects is also variable, and multiple defects in a similar location can be a diagnostic challenge. The etiology of the defect as it pertains to location and chronicity, either acute, subacute, or chronic, is also a variable that bears consideration. Lastly, the patient’s clinical state, including their nutritional status and degree of immunocompromise, can play a role in the management of the defect itself. It can also be a factor in determining the goal of endoscopic therapy, whether it is being used as definitive management or as a temporizing measure to delay future surgical intervention.
Most defects managed in our current practice are related to a recent procedural intervention, endoscopy, or surgery (3). Diagnostic flexible esophagogastroduodenoscopy (EGD) has a reported rate of iatrogenic perforation of approximately 0.03%, most of which are reported to occur in the esophagus (4,5). The incidence of perforation during a screening or diagnostic colonoscopy is estimated at 1:1,400 patients. This rate is understandably significantly lower than that for a therapeutic colonoscopy, reported as an estimated 1:1,000 patients (6). An increase in the incidence of perforations associated with interventions has been seen as endoscopy has evolved over the years to include more therapeutic options. These procedures include stent placement, endoscopic myotomy, resection of dysplastic or neoplastic tissue, dilation, and others. Many of these resultant GI tract defects will require further management. An anastomotic leak following esophagectomy is reported in 8–10% of cases (7-9). Sleeve gastrectomy as performed in the bariatric population, is associated with a 1.5–7% rate of staple line leaks (10,11) while a leak rate of 1.7–2.5% after Roux-en-Y gastric bypass (RYGB) anastomoses is reported (12). While the rate of persistent fistula after PEG tube removal may be considered low at 1.1%, it remains a significant problem based on the sheer number of PEG tubes performed on an annual basis, which is likely underrepresented in the literature (13).
GI tract defects often necessitate complex and multimodal management strategies. They require careful communication and planning across the multispecialty care team managing the patient. This often includes the primary surgeon, endoscopist, interventional radiologist, and a critical care provider, all working together to manage a complex set of issues. Historically, a GI tract perforation was once classified as a contraindication to endoscopic procedures for fear of worsening the defect. With the evolution of new techniques and tools, endoscopists have garnered tremendous experience in the diagnosis and management of full thickness luminal defects. Endoscopic therapies applied with a multi-modal approach for leaks, perforations, and fistulae are now part of the treatment algorithm for these GI tract complications, and often times can be first-line therapies at select centers. Even in cases where definitive endoscopic repair is not possible, there may be temporizing procedures available endoscopically that can be used to mitigate the clinical situation and allow for pre-operative optimization, whether that involves nutritional rehabilitation or just symptom mitigation to allow for a delayed surgical repair in a more ideal setting.
During the evaluation and management of every patient with a GI tract defect, regardless of location or etiology, we follow the same series of steps. This is done in an attempt to standardize what is otherwise a highly diverse assemblage of clinical presentations, leak locations, and anatomical considerations. Step 1 is a diagnostic endoscopy with fluoroscopy. We use this to delineate the native anatomy as well as to elucidate the number, location, and size of the leak or leaks we are attempting to manage. Step 2 focuses on the management of the extraluminal cavity. This may include transluminal washout and debridement, as well as endoscopically assisted drain placement or repositioning of an existing drain. Step 3 is the management of distal obstruction, if one is present, as this is an impetus for persistent leak or fistula in many cases. Step 4 is the management of the GI tract defect itself. It is our practice to give preference to definitive endoscopic closure methods whenever feasible. Lastly, step 5 is the placement of distal feeding access if the circumstances deem it necessary.
Step 1: endoscopic leak diagnosis
While CT scans are very sensitive and specific for the finding of extra-luminal air and can be generally obtained quickly, they can be read as equivocal for leak with some frequency (14). This is particularly the case in patients following surgical procedures, in whom the finding of intraperitoneal or intra-thoracic air may not be unexpected, depending on the type of surgery (open vs. laparoscopic) and the time since the procedure. Leaks can also be detected using real time fluoroscopy with administration of water-soluble contrast or thin barium. However, similar to CT scans, they also have a recognized rate of false negative studies. A delay in diagnosis of an enteric leak can have dire consequences for the patient, so maximizing diagnostic accuracy is critical. The use of flexible endoscopy may provide additional diagnostic information and be of clinical relevance in a select group of patients for whom clinical presentation, physical exam and imaging findings are all equivocal for leak.
In order to perform a thorough evaluation of the patient with a leak, access to a variety of endoscopes is required. Our armamentarium includes standard diagnostic endoscopes, ultraslim endoscopes, single and dual channel therapeutic endoscopes, colonoscopes, and even digital ureteroscopes or choledochoscopes for select cases. These additional scopes provide the ability to traverse fistula or drain tracts. In some cases, access from both from the luminal side and in a percutaneous fashion can be useful. It also gives us the ability to traverse luminal defects without increasing their size, for instance when doing a percutaneous washout or drain repositioning. This broad range of scope lengths and diameters also helps to ensure that even leaks that occur beyond the reach of a traditional scope from the mouth or anus are potentially accessible by alternative routes.
In circumstances such as these, we perform an endoscopic evaluation exclusively in an operating room setting. This ensures that the tools, equipment, and personnel needed to manage both the potential complications of endoscopic insufflation in the setting of a GI tract defect, as well as to afford us the opportunity to manage any leak not amenable to endoscopic therapy, are readily available should the need arise. As described below, it is often necessary to perform endoscopic repositioning or exchange of percutaneous drains. This requires that the endoscopist have ready access to minor procedure trays, suture material, and replacement drains. These items are all easily available in the operating suite. It is our practice to intubate all patients with an upper GI tract leak for both comfort and airway protection during endoscopic intervention. Endotracheal intubation can afford the endoscopist the limited aspiration risk while using water-soluble contrast in potentially high volumes as required. Intubation, and the deep sedation associated with it, can also allow large closure devices, therapeutic endoscopes, and delivery systems to be inserted through the oropharynx with as little difficulty or discomfort to the patient as possible. Patients who are undergoing lower endoscopy do not specifically require intubation. However, when the procedural time has the potential to be prolonged, or if the patient is to remain in supine position with the potential for significant abdominal insufflation and distension, intubation may sometimes be appropriate both for patient comfort and safety.
In all cases, regardless of whether a leak is established or merely suspected, only CO2 insufflation is used during endoscopy. We minimize the flow rate of CO2 and only increase it as needed for visualization, with a caution to the anesthesia team that they will see a rise in their end tidal CO2 readings. It is absolutely imperative that during these the endoscopic air pump is turned off. The unregulated flow of room air can cause uncontrolled pneumoperitoneum, pneumomediastinum, or pneumothorax in the setting of a leak, and this insufflation can result in significant hemodynamic instability. This risk is not eliminated by the use of CO2 but it is significantly mitigated by the rapid absorption of CO2 by the tissues. Should a patient develop hemodynamic instability from inadvertent pneumoperitoneum during endoscopy, our preferred method of decompression is by the insertion of a Veress needle into the peritoneal cavity to relieve the intra-abdominal pressure. A contained leak or one that is either controlled by a drain, located exclusively within the retroperitoneum, or that is associated with a long and well-established fistula tract puts patients at lower than average risk for these types of complications. Regardless, we elect to utilized CO2 insufflation in all patients for whom endoscopy is being performed for management of a full thickness defect. In addition to the safety concerns, because the CO2 is rapidly reabsorbed, post procedure imaging is theoretically less complicated by any gas that has escaped across the luminal defect during the procedure. If large volumes of residual gas are seen on follow-up imaging, this is less likely to be the result of the CO2 used during endoscopy, and should heighten the suspicion for an ongoing or additional leak.
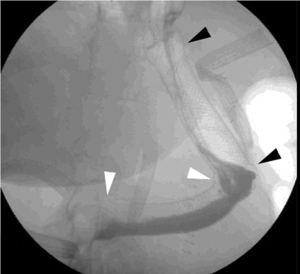
Effective endoscopic evaluation of GI tract defects requires the endoscopist to have the ability to use and interpret fluoroscopy images in real time. Drain injection (sinogram), fistula or wound injection, and through the scope (TTS) injection of contrast (either via the working channel itself or through an endoscopic injection catheter) are mandatory skills to identify and to document the successful closure of a GI defect (Figure 1). The endoscopist must be comfortable repositioning, and if necessary, replacing, percutaneous drains to ensure proper drainage of extraluminal cavities. Additionally, percutaneous drains can be inadvertently captured during closure of the luminal defect with both clips and suture devices, so the endoscopist should possess the necessary skill to move these drains away from the working space of the closure device. Fluoroscopy serves a vital function in this capacity, and again its importance cannot be overstated.
After the diagnostic endoscopy and fluoroscopy have been completed, we assemble a road map of the patient’s local-regional anatomy. We then pause to consider immediate goals. The first step is to delineate the team’s understanding of the patients locoregional anatomy as it pertains to the defect in question. This is done by creating an amalgamation of the endoscopic view, fluoroscopic images, surgical history, and preprocedural cross sectional imaging studies, in combination with the endoscopist’s understanding of the clinical presentation. This image is used for planning the next steps of defect management. Secondly, we discuss the objectives and goals of our pending endoscopic intervention. We discuss whether our goal will be definitive closure or a temporizing maneuver for defect control. If necessary, we also take this moment to review our findings and plans with the referring physician in real time. It is also at this point that some patients may undergo an immediate conversion to a surgical therapy as indicated, if they are determined to not be candidates for safe or successful endoscopic intervention (Figure 2). Lastly, we plot out the steps of the proposed intervention ensuring that all necessary equipment is in the room. This includes therapeutic endoscopes, surgical tools, drains, endoscopic tools and implants. We also mentally rehearse the steps of our proposed intervention to ensure that they flow in a logical fashion, and that no one step will complicate or preclude the following steps. We also take this time to discuss potential complications that may arise, as well as anticipated strategies for mediation of these complications to ensure that we have more than one option for troubleshooting the leak at our disposal.
Step 2: management of the extraluminal cavity
Patients with an immediate GI tract defect and those who have developed a stable fistula tract will generally have no extraluminal pocket requiring additional therapy. However, for patients presenting with an acute or sub-acute process, there may be an extraluminal cavity that must be managed in addition to the primary defect. An example of this would be a patient who is status post a bariatric procedure complicated by a leak that has developed several days later.
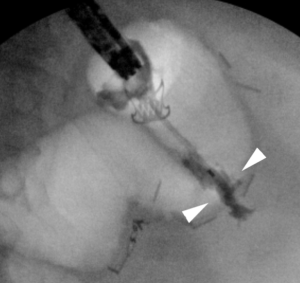
Small extraluminal collections may be irrigated out and the GI tract defect closed without placing a drain. The risk with this method is that some amount of contamination is being orphaned by the defect closure, and that subsequently an abscess requiring therapy could develop. Typically, these collections are amenable to percutaneous drainage and so this is a risk we accept, and maintain a high index of suspicion for in the time period following defect closure in these patients. We generally initiate (or continue) antibiotics following the procedure to help control the extraluminal contamination in patients who fall into this category. Large, undrained, extraluminal pockets benefit from the placement of a percutaneous drain. Depending on their skill set and comfort, a percutaneous drain can be placed by the endoscopist during the primary procedure under endoscopic or fluoroscopic guidance (Figure 3). Alternatively, drain placement can be performed before or after the endoscopic procedure by interventional radiology. There are also rare instances in which the endoscopist can serve as an assistant to our radiology colleague, where by the abscess cavity is insufflated or filled with irrigation fluid to reach a maximum size and thereby facilitate the creation of a target for percutaneous drainage.
For any defect above 5 mm in size with an associated extraluminal cavity, we generally traverse the defect with an ultraslim scope and enter the abscess cavity to attempt removal of gross debris. In our experience, this has included food, pills, or other foreign material in addition to purulent fluid. This also permits us to undertake debridement of necrotic tissue and irrigation of the cavity. This technique is conceptually similar to transgastric pancreatic necrosectomy. An ultraslim transnasal endoscope, which has a small (2 mm) working channel, is required to traverse a small defect. However, this scope does limit the ability to insufflate and aspirate, and has reduced optics compared to standard diagnostic endoscopes. Despite this, it is sufficient to permit all of the procedures necessary to manage the drain cavity without causing undue trauma or dilation of the defect itself. Larger debris can be removed with the assistance of a Roth net if necessary. Of note, the instrumentation available for these ultraslim scopes still easily facilitates endoscopic placement of percutaneous drains as well as endoscopically assisted relocation of poorly positioned drains (15,16).
Step 3: management of distal obstruction
A critical component of the diagnostic endoscopy portion of the procedure is evaluation for distal obstruction. In discussing the general principles of fistula management, the presence of a distal obstruction is a major contributing factor to failed fistula closure. Distal obstructions take a variety of forms. Most commonly, they are the consequence of a native or physiologic obstruction such as the lower esophageal sphincter, the pylorus, or the ileocecal valve. In patients with altered surgical anatomy, the distal obstruction may be an anastomotic stricture. An example of this would be a gastric bypass patient with a leak or fistula from the gastric pouch and a strictured gastro-jejunal anastomosis immediately distal to this, creating significant backpressure on the area of defect and perpetuating the leak. It can also be a more functional type obstruction, such as an angulation of the gastric conduit at the level of the incisura following a sleeve gastrectomy without true stricture, in patients with a sleeve leak at the angle of His. A similar effect is seen with a relative narrowing at the hiatus in the case of esophagectomy with gastric pull-through. Strictures may be managed by a variety of methods, including stent placement, and balloon dilation. Botulinum toxin injection and balloon dilation are both acceptable methods for management of physiologic obstructions at native sphincters. Failure to appropriately recognize and manage the downstream obstruction is one of the risk factors for ongoing issues related to the GI tract defect.
Step 4: management of GI tract defect
Defect size, chronicity, location, number and previous therapy are all factors which impact the decision of which endoscopic tool to use to address a specific type of defect. However, the decision should also take into account the endoscopist’s experience and comfort with the use of the tools available. An additional consideration is the type of endoscope able to access the defect. Many specialized endoscopes and the complex closure devices which they support are not readily available at every institution. We will discuss the benefits, drawbacks and limitations of each of these types of therapies below. Overall, we favor definitive defect closure if feasible and rely on non-definitive methodology only as a fallback or temporizing measure only in cases where definitive closure is not possible.
Non-definitive endoscopic therapy
Endoluminal stenting
The management of full thickness luminal defects with endoluminal stenting has become a widely accepted method. Theoretically, stent placement decreases flow across the fistula and promotes tract closure by allowing for diversion of enteric contents away from the leak or fistula tract, as flow across a tract is one of several forces which perpetuate a fistula. Typically, fully or partially covered self-expanding metal stents (SEMS) and self-expanding plastic stents (SEPS) have been used. We offer this method of management with several caveats. These devices should be used with caution because placing an endoluminal stent for management of a GI tract defect is an off-label use of these products in the US. If applied in this manor, this must be disclosed to the patient and their family as such. Additionally, leak of enteric contents around the stent is an almost universal finding when used for this indication. Therefore, while such stents may decrease flow across a defect, they cannot eliminate it completely. This allows for a mitigating effect, such as temporizing skin breakdown potentially, but may not allow enough decreased flow for the leak to close down completely. Lastly, endoluminal stents were primarily designed to provide radial force to open up an occlusion related to tumor. They cause tissue necrosis by their ability to generate radial force. Creating radial force on an area where tissue breakdown is already the primary disease process is somewhat counterintuitive, as this force and wall tension can lead to ischemia and theoretically impair the healing process. Stents can also tend to migrate when not anchored in position, or held in place by a stricture they are targeting. Despite this, the literature supports that stents can be a useful adjunct to the successful nonsurgical management of a full thickness luminal defect in select cases.
In a series of 34 patients with upper GI leaks or perforations managed with SEMS, 50% of patients had clinical success with closed leaks, but there was also a high rate of stent migration of 41% (17). In a small series of 9 patients who experienced staple line leaks following sleeve gastrectomy, 78% or 7 of the patients had successful healing with the use of SEMS. There was a correlation between higher success rate and earlier time to intervention with decreased duration of stent placement (18). This can also be interpreted potentially as defects that may have healed on their own regardless of stent placement. Stents have also been used in combination with additional endoscopic therapies including application of cyanoacrylate glue and over the scope clips (OTSC). These combinations have been shown to improve the successful rate of closure to up to 95% (19) with a rate of stent migration of only 19%. Again, the efficacy attributable to the stent in these combined cases cannot be elucidated. It is suggested by some authors that securing the stent proximally with either endoluminal suturing devices or endoclips may decrease the risk of stent migration. This can be a relatively simple adjunct to stent placement and can provide some benefit. Overall, the management of leaks and fistulae can require complex therapy, for which SEMS represent a useful adjunct and have been shown be more effective when used in combination with other techniques than when used in isolation (10). Again, the author’s preference is to use stents as a temporizing measure when other definitive closure methods are not feasible based on various factors.
When used for the management of a gastric sleeve leak, bend of the sleeve as well as the length of the gastric sleeve itself may require two stents to be placed. These stents are overlapped slightly so that the entire sleeve from the distal esophagus to the distal stomach can be included (Figure 4). These stents will also have a tendency to migrate unless held in place by a stricture. An endoclip or a TTS clip, an endoscopically placed suture, or a nasal bridal can be considered as methods to help maintain stent position (20). We typically use stents for large areas of GI breakdown that cannot be closed definitively. This includes defects such as an anastomotic disruption involving greater than 75% of the circumference of the bowel or a long, linear breakdown of a staple line with non-mobile tissue that renders endoscopic suturing impossible. However, as new data emerges regarding the safety and efficacy of endoluminal vacuum (E-VAC) therapy, stenting defects of this nature may become less prevalent as it is replaced by the endoluminal vacuum.
Endoluminal stents works best in areas of the GI tract that are either linear or follow a very mild curve. The overall construct of stents is a tubular, straight structure. When significantly bent, the devices have a tendency to migrate into a location in the GI tract where they can maintain their naturally straight design. Stent erosion can also be a significant issue, making them hard to remove, and also potentially contributing to further GI tract complications. In addition, the majority of delivery systems for fully covered stents are designed to be semi-rigid. It is therefore difficult to have them pass through bends in the GI tract and this presents an opportunity for injury. While there are commercially available, fully covered, TTS delivery systems for some stents, these devices are often too small to be used in the setting of leak because the delivery method limits their outer diameter to such a degree. We have previously described an over the scope method of stent deployment, but have found limited utility for this method in an era when definitive closure tools are now widely available (21). There are stents that are available outside the US designed specifically for management of bariatric upper GI defects such a sleeve leak. One such stent is the Niti-S Beta stent (TaeWoong Medical, South Korea) which has a double-bump shape designed to increase radial tension at specific locations and therefore prevent migration. A recent retrospective analysis of bariatric leaks managed with stents suggested that the success rate and the migration rate of the Niti-S Beta Stent was no different than other commercially available covered endoluminal stents (22).
E-VAC
E-VAC was first described for use in colorectal anastomotic leaks in 2008 (23). It has more recently been studied as a management strategy for complex fistulae and leaks in the upper GI tract. This method involves attaching a piece of vacuum sponge to the end of a nasogastric tube with suture. This device is then endoscopically placed at the area of interest (Figure 5). The sponge can be customized to the shape and size of the target defect, with limitations in size being determined by the method of endoscopic access. For instance, orally inserted endoluminal vacuum devices that must traverse cricopharyngeus and travel down the esophagus will potentially be more size limited than an endoluminal vacuum being used for a colorectal anastomotic defect. Once inserted, suction is applied. These devices, similarly to wound vacs applied to surgical wounds at skin level, promote wound contraction, enhanced granulation tissue formation, and also control of effluent in the case of enteric leaks. The caveat to this is that the vac sponge and the nasogastric tube can be easily overwhelmed by high volume or high viscosity of effluent. The frequency of dressing changes should be determined on a case by case basis, with the goal being to optimize control of the cavity without undue or excessive anesthetic exposure. While several countries have commercially available systems, there is presently no E-VAC system with FDA approval available in the US. As a result, the endoscopist is required to fashion the device prior to placement. This is done with the minimal equipment as described above, all of which is readily available in the operating room.
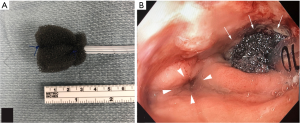
Recently, several series have investigated the safety and efficacy of this method for management of esophageal leaks and were published demonstrating significant clinical successes with E-VAC therapy. Successful defect closure was documented in between 78–100% of the cases (24-27). A subset of patients managed with a combination of E-VAC therapy and endoluminal stenting had complete healing reported in two series (26,27). To date, only one study has specifically studied the use of E-VAC therapy for management of staple line leaks after sleeve gastrectomy (28). The 9 patients in their series had complete resolution of their sleeve leaks confirmed by upper GI series, with 5 of the 9 having undergone combination therapy with concurrent stent placement (28). In general, the prevalence of E-VAC therapy is increasing as its applications have broadened beyond just colorectal defects. It has so far shown very promising results as a method of controlling and closing enteric leaks and fistulae. Unlike with stenting, E-VAC therapy allows for simultaneous drainage of the infection as well as defect management, with the additional benefit of endoscopic access for visual assessment. One review demonstrated a pooled success rate for E-VAC therapy of 90%, ranging between 70–100% of 200 cases of its application (29).
The drawbacks of E-VAC therapy are primarily the fact that it is quite labor-intensive and can also potentially necessitate a prolonged inpatient stay. The endoluminal vacuum itself can be connected to a portable wound vac suction device to potentially allow the patient to be discharged home, similar to a surgical wound vac dressing; however, this must be decided on a case by case basis in patients who are otherwise clinically appropriate for home. Similar to traditional wound vacuums, the sponge needs to be changed often, ranging from several days to a week, during E-VAC therapy. E-VAC therapy therefore requires that a capable endoscopist (or team of endoscopists) be available to remove and replace the sponge on a regular basis. This may not be feasible in a center with only limited access to therapeutic endoscopy capabilities. Additionally, larger defects may require a significant amount of time to close, potentially requiring extensive inpatient resources. In a clinically stable patient without systemic infection as a result of their GI tract defect, sponge changes may become a more routine procedure capable of being performed on an outpatient basis as the practice becomes more common. Anecdotally, our own institutional experience has been that E-VAC therapy can be used to manage large defects, including near complete anastomotic disruptions, in an otherwise clinically stable patient. As E-VAC therapy continues to evolve and a commercial product becomes available, it will likely become a staple tool in the armamentarium of the therapeutic endoscopist.
Sealants
Fibrin glues and tissue sealants can offer an additional adjunctive method for fistula management. These products form an acellular matrix and are also intended to promote a local inflammatory reaction. This reaction encourages tissue fusion, and thereby closure, in the case of leaks and fistulae. When used in isolation, Rabago et al. demonstrated a higher failure rate for tissue sealants used for the management of high output leaks and fistulae compared to low output (30), again implying that these products are better employed in an adjunctive fashion. Recently, a review showed a success rate ranging from 60–100% fistula closure managed with fibrin glue therapy (31). The variability in outcomes is attributable in part to the differences in the anatomy of the fistula tracts as well as the volume of output (31). Again, we view these products more as temporizing measures when definitive closure is not feasible or available.
Fistula plugs
Fistula plugs were initially developed for the management of fistula-in-ano. They have now been used to successfully close enteric fistulae in other portions of the GI tract as well. Porcine small intestinal mucosa is a bio-prosthetic collagen matrix which is designed to provide a scaffolding for tissue in-growth, without triggering a foreign body reaction (32). In one study, all 25 patients who were treated with fistula plugs for a persistent gastrocutaneous fistula after RYGB were found to have successful closure, however, there was some variation in the number of interventions required to achieve this end result (33). A 93% rate of successful closure was also seen in another series of patients with foregut fistulae arising from the stomach or proximal small intestine (34). While there is a subset of entero-atmospheric fistulae that will close spontaneously, overall the use of fistula plugs tends to decrease healing time. There may also be some clinical benefit to using both plugs and other modalities such as fibrin sealants as combination therapies.
Definitive endoscopic therapy
TTS clips
Initially designed for hemostasis and endoluminal marking, TTS clips are endoscopic clips that are passed through the endoscopic working channel and are deployed within the lumen of the GI tract. Reports began to emerge in the late 1990’s of their application in endoscopic closure of colonic and gastric perforations (35,36). Although they can be effective at closing smaller defects, their ability to close larger defects is very poor. These clips are relatively small so that they can fit down the working channel of the endoscope. They have a low grasping force, and are unable to grasp tissue deeper than the mucosal layer. These features make them more effective at closing surgically incised tissue with straight regular edges at the mucosal or submucosal level, and less effective at managing tissue defects with gaping, irregular edges such as result from blunt perforation (Figure 6). TTS clips are used routinely to close surgically incised mucosal edges that are the result of endoscopic submucosal dissection and per oral endoscopic myotomy, and their efficacy in this application has been well documented (37,38).
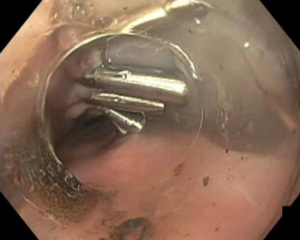
We primarily utilize TTS clips for closing defects in cases where the area cannot be safely or reliably accessed by an endoscope mounted with a dedicated closure device, based on size or distance from the mouth or anus. Alternatively, there may be situations in which reaching the defect endoscopically was so challenging in the first place that the endoscopist fears that having to repeat the maneuvers after removing the endoscope to affix a closure device will cause them to lose access to the defect altogether. Since TTS clips do not require the scope to be removed at all, they can provide a reasonable alternative in these situations. These endoclips can be easily removed if they are mal-deployed and, unlike larger OTSC, they are small enough that they can be placed near or around one another as indicated. TTS clips may have some advantage as both endoscopic suturing devices and OTSC when the defect is in a steep retroflex position, as these larger devices are difficult to use in full retroflex. However, the scope will need to be partially straightened temporarily to allow the clip to pass in most cases.
TTS clips have been shown to be successful in closing iatrogenic defects in the GI tract with clinical success rates ranging from 59–83% (39,40). It is felt that the limitation to their success is their small size, small closing force and mucosa-only tissue apposition, although in the right setting such as small defects that are not gaping, they can be quite effective. Since they are easily removed endoscopically, the endoscopist also does not burn any bridges in terms of subsequent therapies that may be required should the TTS clips prove to be ineffective.
OTSC
OTSC have more versatility compared to TTS clips in their application to defect closure. They can reliably achieve full thickness closure of defects measuring up to 2 cm in diameter when deployed under ideal circumstances (41). In the US there are presently two FDA approved types of OTSC: the Padlock Clip (US Endoscopy, Mentor, OH, USA), and the OTSC (Ovesco Endoscopy, Tubingen, Germany). These clips are comprised of an elastic biocompatible nitinol. They are packaged in an open position already mounted on a rigid distal cap attachment large diameter with a large diameter (Figure 7). This cap is carefully affixed to the endoscope with the clip in place, and the firing mechanism is strung through the working channel. The scope is then advanced into position such that the defect can be centered within the cap. Aggressive suction is applied to bring the tissue surrounding the defect into the cap. A grasping tool or tissue anchor can also be used as an accessory to bring tissue into the cap or help center the defect. The clip is then deployed utilizing a firing mechanism as described above. Caution must be exercised when using a grasping tool or tissue anchor, as it is possible to catch these devices in the clip if not drawn back sufficiently into the cap.
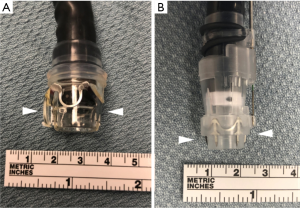
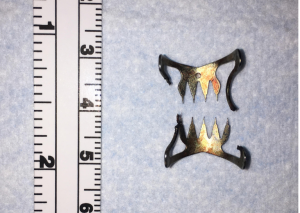
OTSCs achieve a robust closing force. They are capable of obtaining deep tissue closure, and in some cases full thickness bites. Despite this obvious advantage to the clip itself over other closure methods, the application of these clips requires the defect to be accessible by a therapeutic endoscope with a large rigid cap and clip affixed to the end of it. The therapeutic scope itself already provides some additional challenges due to its increased diameter and intrinsic rigidity, so reaching the defect is not always possible depending on the patient’s physical size and the exact location of the defect within the GI tract. Additionally, the while a benefit of these clips is the robust closure they provide, this same characteristic also makes their removal extremely challenging, with very few easily reproducible options available for many years (42). There is now a specific tool available for Ovesco OTSC clip removal (remOVE tool, OVESCO Endoscopy) which in essence fractures the clip at its hinge mechanism by applying direct current (Figure 8) (43). The ability to remove these clips is essential. In the event that a clip is misplaced, it cannot easily be worked around due to its relatively large size and extremely limited mobility once affixed to tissue. It may be necessary to removal a clip for the management of a recurrent or persistent leak. It may also be prudent to remove the clip prior to performing a formal surgical intervention where the clip has the potential to interact with a surgical stapler or energy device.
In an unselected cohort of 34 patients with a full thickness luminal defect managed by OTSC placement, 76.5% were found to achieve definitive closure (44). Defects located in the rectum, colon, or stomach, were more likely to be associated with favorable outcomes, as compared to other areas of the GI tract (44). In 2016, a similar success rate was demonstrated by Winder et al in their series of 22 patients. In their study, OTSC application was successfully used in the management of acute or sub-acute enteric leaks in 100% of cases, and in 76% of cases for chronic fistulae management with a median follow-up of 4.7 months (45). In general, immediate outcomes with the OTSC system are generally favorable, again highlighting the importance of concomitant fluoroscopy when undertaking complex therapeutic endoscopy for leaks and fistulae. However, long-term outcome data is limited. In one study that followed patients for a mean duration of 178 days, 53% of patients had an overall long-term successful closure, despite 89% successful closure on initial radiographic studies performed immediately after application of the OTSC (46,47). Another series of 92 patients with a total of 117 full thickness defects of the GI tract who underwent management with Ovesco clips were followed for 5.5 months. In this series there was an overall defect closure rate of 66.1% with 55.0% success for fistula closure and 79.6% success for leaks (48).
Endoluminal suturing devices
There are several reports in the literature on the use of prototype devices to manage full thickness defects, or the off label use of tissue plication devices. The Overstitch (Apollo Endosurgery, Austin, TX, USA) is a disposable device that attaches to the end of a double channel endoscope and is designed to allow safe and effective endoscopic suture placement (Figure 9). The new Overstitch Sx (Apollo Endosurgery) offers the same suturing capabilities with the added benefit of compatibility with a standard single lumen endoscope, as the working mechanism of the device travels parallel to the exterior of the scope rather than through the working channel. Endoluminal suturing devices offer greater versatility than can be achieved with currently available clipping devices. They provide the opportunity to have access to a choice of closure materials, including permanent or absorbable sutures. There is also no predetermined suturing pattern, so closure technique can be tailored to the defect in situ, including the placement of simple, running, figure of 8, and purse string suture patterns. There is no absolute limit on defect size. The mobility and tension of local tissue are the major factors determining whether or not a large defect can be closed with endoscopic suturing, in contrast to the fixed diameter limitations that apply when using OTSC. Lastly, these devices offer some amount of control over the depth of the tissue bites being taken.
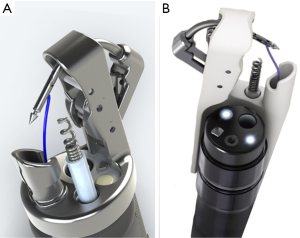
Studies have demonstrated that with these suturing devices, the endoscopist can take consistent subserosal suture bites in the colon without full thickness injury, but can also successfully manage full thickness gastric defects (49). Suture removal is as simple as cutting the stitch, and it is easy to place additional sutures around a previously placed stitch when necessary. This is in stark contrast to placing an OTSC in proximity to another OTSC where there can be significant clip/clip interaction requiring clip removal as described above.
There are some disadvantages of the original Overstitch device. These include the need for a specialized endoscope, as the device is only compatible with an Olympus 2T endoscope, thereby limiting its application to centers where this equipment is readily available. Additionally, the Overstich device cannot be used to reliable reach locations beyond the duodenum and descending colon, due to the fact that the 2T scope is shorter in length than standard diagnostic scopes. The outer diameter of the device may also limit its ability to be passed through the upper GI tract in pediatric populations or in individuals of smaller stature or with other esophageal pathology such as stricture. As mentioned above, the newer iteration of the Overstitch Sx is compatible with a single channel scope, however, it has a similar outer diameter when fully assembled and therefore has the same limitations with pediatric patients and smaller adults. In contrast to the ‘fire it and forget it’ mechanism of OTSCs, the Overstitch device requires more practice to use it safely and efficiently. This is especially true in situations where there is limited working space and difficulty with insufflation, as is commonly the case when dealing with large anastomotic defects or staple line leaks.
It is our practice to utilize the Overstitch for defects larger than 2 cm in size and for those defects in which an OTSC would clearly be insufficient. Long, linear breakdown of a gastric sleeve staple line, as well as a hemi circumferential breakdown of an anastomosis would be realistic examples of such cases. Notably, the Overstitch device is compatible for use with OTSCs. For the management of particularly large, complex, or recurrent defects we sometimes employ an ‘Ovesco over Overstitch’ method. In these cases, the Overstitch is first used to significantly reduce the size of a defect, and an Ovesco OTSC is then used to reinforce the primary suture closure (50). While there are several case reports outlining the broad range of application of the Overstich device, to date there have been no large case series published.
Conclusions
Overall, the endoscopic management of GI perforations, leaks and fistula are complex processes that often requires a multimodal approach. While they once required prolonged conservative management or morbid open surgical repair, these complications can now be managed with endoscopic techniques in various combinations in many cases. Novel and evolving endoscopic tools and therapies allow providers to achieve the highest possible success rate with the least associated patient morbidity and mortality. As these technologies continue to develop, new techniques and applications will continue to become available. With time, endoscopic interventions will hopefully become a widely accessible first-line therapy for management of this patient population.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Jeffrey M. Marks and Ryan M. Juza) for the series “Surgical Endoscopy: Essential Skills in Gastrointestinal Surgery” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: The series “Surgical Endoscopy: Essential Skills in Gastrointestinal Surgery” was commissioned by the editorial office without any funding or sponsorship. EM Pauli is a consultant for Boston Scientific Corp and Actuated Medical, Inc. He received research support, speaking and teaching honoraria from Cook Biotech, Inc. and C.R. Bard, Inc. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Center for Disease Control and Prevention. National Hospital Discharge Survey: 2010 Table, Procedures by selected patient characteristics. Available online: https://www.cdc.gov/nchs/nhds/nhds_tables.htm
- Rinaldi S, Lise M, Clavel-Chapelon F, et al. Body size and risk of differentiated thyroid carcinomas: findings from the EPIC study. Int J Cancer 2012;131:E1004-14. [Crossref] [PubMed]
- Winder JS, Pauli EM. Comprehensive management of full-thickness luminal defects: The next frontier of gastrointestinal endoscopy. World J Gastrointest Endosc 2015;7:758-68. [Crossref] [PubMed]
- Geraci G, Pisello F, Modica G, et al. G Chir 2009;30:502-6. [Complications of elective esophago-gastro-duodenoscopy (EGDS) Personal experience and literature review]. [PubMed]
- Merchea A, Cullinane DC, Sawyer MD, et al. Esophagogastroduodenoscopy-associated gastrointestinal perforations: a single-center experience. Surgery 2010;148:876-80; discussion 881-2. [Crossref] [PubMed]
- Panteris V, Haringsma J, Kuipers EJ. Colonoscopy perforation rate, mechanisms and outcome: from diagnostic to therapeutic colonoscopy. Endoscopy 2009;41:941-51. [Crossref] [PubMed]
- Rutegård M, Lagergren P, Rouvelas I, et al. Intrathoracic anastomotic leakage and mortality after esophageal cancer resection: a population-based study. Ann Surg Oncol 2012;19:99-103. [Crossref] [PubMed]
- Alanezi K, Urschel JD. Mortality secondary to esophageal anastomotic leak. Ann Thorac Cardiovasc Surg 2004;10:71-5. [PubMed]
- Sauvanet A, Mariette C, Thomas P, et al. Mortality and morbidity after resection for adenocarcinoma of the gastroesophageal junction: predictive factors. J Am Coll Surg 2005;201:253-62. [Crossref] [PubMed]
- Juza RM, Haluck RS, Pauli EM, et al. Gastric sleeve leak: a single institution's experience with early combined laparoendoscopic management. Surg Obes Relat Dis 2015;11:60-4. [Crossref] [PubMed]
- Sakran N, Goitein D, Raziel A, et al. Gastric leaks after sleeve gastrectomy: a multicenter experience with 2,834 patients. Surg Endosc 2013;27:240-5. [Crossref] [PubMed]
- Morales MP, Miedema BW, Scott JS, et al. Management of postsurgical leaks in the bariatric patient. Gastrointest Endosc Clin N Am 2011;21:295-304. [Crossref] [PubMed]
- McElrath L, Pauli EM, Marks JM. Hernia formation and persistent fistula after percutaneous endoscopy gastrostomy: unusual complications of a common procedure. Am Surg 2012;78:E200-1. [PubMed]
- Del Gaizo AJ, Lall C, Allen BC, et al. From esophagus to rectum: a comprehensive review of alimentary tract perforations at computed tomography. Abdom Imaging 2014;39:802-23. [Crossref] [PubMed]
- Juza RM, Fitzgerald K, Gusani N, et al. Transluminal Endoscopic Drain Repositioning for Post-operative Gastrointestinal Leaks: A Case Series. In: Scientific Session of the 16th World Congress of Endoscopic Surgery, Jointly Hosted by Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) & Canadian Association of General Surgeons (CAGS), Seattle, Washington, USA, 11–14 April 2018: Poster Abstracts. Surg Endosc 2018;32:S130-359.
- Juza RM, Fitzgerald K, Pauli EM. Transluminal Endoscopic Drain Repositioning for Post-Operative Gastrointestinal Leaks. In: Scientific Session of the 16th World Congress of Endoscopic Surgery, Jointly Hosted by Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) & Canadian Association of General Surgeons (CAGS), Seattle, Washington, USA, 11–14 April 2018: Poster Abstracts. Surg Endosc 2018;32:S130-359.
- van den Berg MW, Kerbert AC, van Soest EJ, et al. Safety and efficacy of a fully covered large-diameter self-expanding metal stent for the treatment of upper gastrointestinal perforations, anastomotic leaks, and fistula. Dis Esophagus 2016;29:572-9. [Crossref] [PubMed]
- Simon F, Siciliano I, Gillet A, et al. Gastric leak after laparoscopic sleeve gastrectomy: early covered self-expandable stent reduces healing time. Obes Surg 2013;23:687-92. [Crossref] [PubMed]
- Southwell T, Lim TH, Ogra R. Endoscopic Therapy for Treatment of Staple Line Leaks Post-Laparoscopic Sleeve Gastrectomy (LSG): Experience from a Large Bariatric Surgery Centre in New Zealand. Obes Surg 2016;26:1155-62. [Crossref] [PubMed]
- Pauli EM, Marks JM. Endoscopic Tools and Techniques for Strictures and Stenoses. In: Marks JM, Dunkin BJ. editors. Principles of Flexible Endoscopy for Surgeons. New York: Springer, 2013: P105-18.
- Pauli EM, Schomisch SJ, Blatnik JA, et al. A novel over-the-scope deployment method for enteral stent placement. Surg Endosc 2013;27:1410-1. [Crossref] [PubMed]
- Boerlage TCC, Houben GPM, Groenen MJM, et al. A novel fully covered double-bump stent for staple line leaks after bariatric surgery: a retrospective analysis. Surg Endosc 2018;32:3174-80. [Crossref] [PubMed]
- Weidenhagen R, Gruetzner KU, Wiecken T, et al. Endoscopic vacuum-assisted closure of anastomotic leakage following anterior resection of the rectum: a new method. Surg Endosc 2008;22:1818-25. [Crossref] [PubMed]
- Pournaras DJ, Hardwick RH, Safranek PM, et al. Endoluminal Vacuum Therapy (E-Vac): A Treatment Option in Oesophagogastric Surgery. World J Surg 2018;42:2507-11. [Crossref] [PubMed]
- Smallwood NR, Fleshman JW, Leeds SG, et al. The use of endoluminal vacuum (E-Vac) therapy in the management of upper gastrointestinal leaks and perforations. Surg Endosc 2016;30:2473-80. [Crossref] [PubMed]
- Bludau M, Fuchs HF, Herbold T, et al. Results of endoscopic vacuum-assisted closure device for treatment of upper GI leaks. Surg Endosc 2018;32:1906-14. [Crossref] [PubMed]
- Bludau M, Holscher AH, Herbold T, et al. Management of upper intestinal leaks using an endoscopic vacuum-assisted closure system (E-VAC). Surg Endosc 2014;28:896-901. [Crossref] [PubMed]
- Leeds SG, Burdick JS. Management of gastric leaks after sleeve gastrectomy with endoluminal vacuum (E-Vac) therapy. Surg Obes Relat Dis 2016;12:1278-85. [Crossref] [PubMed]
- Kuehn F, Loske G, Schiffmann L, et al. Endoscopic vacuum therapy for various defects of the upper gastrointestinal tract. Surg Endosc 2017;31:3449-58. [Crossref] [PubMed]
- Rábago LR, Ventosa N, Castro JL, et al. Endoscopic treatment of postoperative fistulas resistant to conservative management using biological fibrin glue. Endoscopy 2002;34:632-8. [Crossref] [PubMed]
- Assenza M, Rossi D, De Gruttola I, et al. Enterocutaneous fistula treatment: case report and review of the literature. G Chir 2018;39:143-51. [PubMed]
- Ansaloni L, Cambrini P, Catena F, et al. Immune response to small intestinal submucosa (surgisis) implant in humans: preliminary observations. J Invest Surg 2007;20:237-41. [Crossref] [PubMed]
- Maluf-Filho F, Hondo F, Halwan B, et al. Endoscopic treatment of Roux-en-Y gastric bypass-related gastrocutaneous fistulas using a novel biomaterial. Surg Endosc 2009;23:1541-5. [Crossref] [PubMed]
- Filgate R, Thomas A, Ballal M. Treatment of foregut fistula with biologic plugs. Surg Endosc 2015;29:2006-12. [Crossref] [PubMed]
- Binmoeller KF, Grimm H, Soehendra N. Endoscopic closure of a perforation using metallic clips after snare excision of a gastric leiomyoma. Gastrointest Endosc 1993;39:172-4. [Crossref] [PubMed]
- Yoshikane H, Hidano H, Sakakibara A, et al. Endoscopic repair by clipping of iatrogenic colonic perforation. Gastrointest Endosc 1997;46:464-6. [Crossref] [PubMed]
- Orenstein SB, Raigani S, Wu YV, et al. Peroral endoscopic myotomy (POEM) leads to similar results in patients with and without prior endoscopic or surgical therapy. Surg Endosc 2015;29:1064-70. [Crossref] [PubMed]
- Ponsky JL, Marks JM, Pauli EM. How i do it: per-oral endoscopic myotomy (POEM). J Gastrointest Surg 2012;16:1251-5. [Crossref] [PubMed]
- Magdeburg R, Collet P, Post S, et al. Endoclipping of iatrogenic colonic perforation to avoid surgery. Surg Endosc 2008;22:1500-4. [Crossref] [PubMed]
- Cho SB, Lee WS, Joo YE, et al. Therapeutic options for iatrogenic colon perforation: feasibility of endoscopic clip closure and predictors of the need for early surgery. Surg Endosc 2012;26:473-9. [Crossref] [PubMed]
- Al Ghossaini N, Lucidarme D, Bulois P. Endoscopic treatment of iatrogenic gastrointestinal perforations: an overview. Dig Liver Dis 2014;46:195-203. [Crossref] [PubMed]
- Kapadia S, Nagula S, Kumta NA. Argon plasma coagulation for successful fragmentation and removal of an over-the-scope clip. Dig Endosc 2017;29:820-1. [Crossref] [PubMed]
- Schmidt A, Riecken B, Damm M, et al. Endoscopic removal of over-the-scope clips using a novel cutting device: a retrospective case series. Endoscopy 2014;46:762-6. [Crossref] [PubMed]
- Raithel M, Albrecht H, Scheppach W, et al. Outcome, comorbidity, hospitalization and 30-day mortality after closure of acute perforations and postoperative anastomotic leaks by the over-the-scope clip (OTSC) in an unselected cohort of patients. Surg Endosc 2017;31:2411-25. [Crossref] [PubMed]
- Winder JS, Kulaylat AN, Schubart JR, et al. Management of non-acute gastrointestinal defects using the over-the-scope clips (OTSCs): a retrospective single-institution experience. Surg Endosc 2016;30:2251-8. [Crossref] [PubMed]
- Rogalski P, Daniluk J, Baniukiewicz A, et al. Endoscopic management of gastrointestinal perforations, leaks and fistulas. World J Gastroenterol 2015;21:10542-52. [Crossref] [PubMed]
- Law R, Wong Kee Song LM, Irani S, et al. Immediate technical and delayed clinical outcome of fistula closure using an over-the-scope clip device. Surg Endosc 2015;29:1781-6. [Crossref] [PubMed]
- Morrell DJ, Winder JS, Johri A, et al. Over-the-scope clip management of non-acute, full-thickness gastrointestinal defects. Surg Endosc 2019; [Epub ahead of print]. [Crossref] [PubMed]
- Pauli EM, Delaney CP, Champagne B, et al. Safety and effectiveness of an endoscopic suturing device in a human colonic treat-and-resect model. Surg Innov 2013;20:594-9. [Crossref] [PubMed]
- Alli VV, Strong AT, Allemang MT, et al. Results of the Ovesco-Over-Overstitch Technique for Managing Bariatric Surgical Complications. In: Scientific Session of the 16th World Congress of Endoscopic Surgery, Jointly Hosted by Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) & Canadian Association of General Surgeons (CAGS), Seattle, Washington, USA, 11–14 April 2018: Poster Abstracts. Surg Endosc 2018;32:S236.
Cite this article as: Witte SR, Pauli EM. Management of gastrointestinal tract defects. Ann Laparosc Endosc Surg 2019;4:67.