
Laparoscopy in the diagnosis and treatment of peritoneal metastases
Introduction
Interest in the management of peritoneal metastases over the last two decades has markedly increased. Numerous manuscripts and several textbooks recently published attest to this greatly expanded effort to manage peritoneal metastases. This change emanates from new and increasingly successful treatments that prevent peritoneal metastases, sometimes prolong the survival of these cancer patients, and in some special cases result in cure. These benefits to patients with peritoneal metastases are regularly expanded by clinical and laboratory research efforts designed to refine management strategies. Laparoscopy is currently being explored for improved diagnosis and more beneficial treatments of this condition. This manuscript attempts to critically evaluate the role for laparoscopy in patients with peritoneal metastases. Clear definition of the role for laparoscopy in gastrointestinal and gynecologic malignancies that frequently show peritoneal metastases as part of their natural history is sought.
Prevalence of peritoneal metastases in primary and recurrent abdominal and pelvic malignancies
It is well established that gastrointestinal and gynecologic malignancy may disseminate from the primary site through lymphatic channels to regional lymph nodes, through the blood stream to liver, lung or other systemic sites and through the peritoneal space to nearby or distant peritoneal surfaces. For lymphatic, blood borne, or transcoelomic metastases to occur, a complex of cell surface events must happen so that the cancer cells released from the primary malignancy can implant, become vascularized, and then progress as a cancer nodule. Table 1 collects data to establish the incidence of peritoneal metastases with the common gastrointestinal and gynecologic malignancies at the time of primary diagnosis. By far, the largest incidence of peritoneal metastases present at the time of initial diagnosis is ovarian cancer. Nearly 90% of ovarian cancers have peritoneal dissemination at the time of diagnosis. For gastrointestinal cancer, stomach cancer has the highest incidence of peritoneal metastases at the time of diagnosis of the disease. As high as 20% of patients will have peritoneal metastases at presentation. Another 20% will have no visible peritoneal implants but will have free cancer cells within the peritoneal space that can be detected by cytology. Other gastrointestinal cancers at the time of initial presentation with the exception of appendiceal neoplasms, have a lower incidence of peritoneal metastases.
Table 1
| Primary site | Incidence of peritoneal spread (%) |
|---|---|
| Gastric cancer | 20 |
| Colon cancer | 10 |
| Rectal cancer | 20 |
| Pancreas cancer | 5 |
| Appendiceal neoplasms | 80 |
| Small bowel adenocarcinoma | 30 |
| Ovarian cancer | 90 |
| Endometrial | 10 |
| Cervical | 5 |
| Retroperitoneal or visceral sarcoma | 5 |
Incidence of peritoneal metastases diagnosed in follow-up
With the recurrence of a gastrointestinal malignancy, the incidence of peritoneal metastases is greatly increased. Although the mechanism whereby this large change in the incidence of peritoneal metastases occurs is far from established, the surgical intervention used to resect the primary cancer is at least, in part, responsible for peritoneal dissemination. Table 2 presents the incidence of peritoneal dissemination that has been documented with recurrent disease. With ovarian cancer, by far the most common anatomic site for disease recurrence is peritoneal surfaces. For endometrial cancer, peritoneal metastases are estimated at 30%. Even cervical cancer can disseminate to peritoneal surfaces and this is estimated in 10% of patients. The incidence of peritoneal metastases with recurrent disease in gastric and pancreatic cancer escalates to 60%. For colon cancer, the incidence with recurrence is 20% and 30% for rectal cancer.
Table 2
| Primary site | Incidence of peritoneal spread (%) |
|---|---|
| Gastric cancer | 60 |
| Colon cancer | 20 |
| Rectal cancer | 30 |
| Pancreas cancer | 65 |
| Appendiceal neoplasms | 100 |
| Small bowel adenocarcinoma | 60 |
| Ovarian cancer | 100 |
| Endometrial | 30 |
| Cervical | 10 |
| Retroperitoneal or visceral sarcoma | 70 |
Control of peritoneal dissemination of rectal cancer
In the past local-regional recurrence and peritoneal dissemination of rectal cancer was far more common than seen with colon cancer. This high incidence of local treatment failure was related to an unnecessarily traumatic rectal cancer excision. Currently, there is a low incidence of resection site disease or peritoneal dissemination from rectal cancer. This is thought to be caused by two standard of care treatments that work to contain the malignant process as it is being resected. First, all advanced rectal cancers receive neoadjuvant radiation and chemotherapy to shrink the cancer and at least, in part, devitalize it. Second, a “no touch isolation technique” called total mesorectal excision (TME) is used. This resection technique maintains intact, as much as possible, the fatty tissues and fascial envelope that surrounds the rectal cancer. This results not only in complete rectal cancer clearance but also absolute containment of all the malignant cells of the primary tumor. Of course, liver metastases or distant lymph nodal metastases may occur.
Adverse events associated with laparoscopy that must be considered prior to its application in patients with peritoneal metastases
From this description of the frequent occurrence of peritoneal metastases from gastrointestinal and gynecologic malignancy, the need for diagnostic and therapeutic tools to manage this process becomes obvious. Direct visualization and biopsy confirmation of cancer present on peritoneal surfaces is possible by laparoscopy. Also, there are therapeutic modalities that can be implemented with the laparoscope. However, laparoscopy is an invasive modality and its associated adverse events should be characterized. It requires full thickness penetration of the abdominal wall at 1–5 places to accomplish the desired goals. Diagnostic laparoscopy will usually only require a single periumbilical port site but therapeutic laparoscopy will usually require additional port sites. An additional 4 port sites are not uncommon.
The incidence of adverse events associated with diagnostic laparoscopy is dependent, in large part, on the extent of prior surgery and the intestinal adhesions that have resulted. Although these complications are seldom life threatening, they may require major surgical intervention to resolve the trauma induced by the laparoscopic procedure. Bowel perforation, bladder perforation, and uncontrolled bleeding will almost always require a surgical intervention but are almost always resolvable. There is a second complication that may result from laparoscopy, this should be characterized as an oncologic complication. The port sites may become seeded by malignant metastases (Figure 1). These port site metastases have a pyramidal shape with the base of the pyramid at the peritoneal surface. Cancer cells at high density enter the subperitoneal space as a result of the increased pressure required to distend the abdominal wall and maintain visualization with the laparoscope. The dense subperitoneal lymphatic plexus may allow cancer cells to migrate several centimeters from the actual trochar site. Also, cancer cells are able to move along the layers of the abdominal wall penetrated by the trochar. This movement may be facilitated by increase intraabdominal pressure and air and small amounts of fluid moving along the trochar tunnel. Further seeing of a trochar site may occur as cancer cells adherent to the end of a trochar are wiped from the trochar surface as it is removed from the abdominal wall. After the trochar is removed cancer cells in peritoneal fluid may enter the wounded site, adhere to traumatized surfaces and then progress.
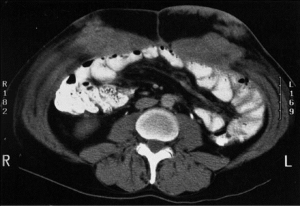
Several manuscripts that describe trochar site metastases emphasize the following important aspects concerning laparoscopy in patients who may have peritoneal metastases. First, if trochar site metastases are diagnosed, in virtually all patients peritoneal metastases, often of a large extent, are also present (1). Second, if laparoscopy is performed in a patient who may have peritoneal metastases or peritoneal mesothelioma, all port sites should be located in the midline. If port site metastases occur in the midline they can be resected as part of a midline abdominal incision. Port site metastases in or along the linea alba remain confined to this space. In contrast, port site metastases within the rectus muscle spread along the muscle fibers diffusely. Port site metastases within the lateral aspects of the abdominal wall are much more difficult to resect realizing that the trochar site may not go straight through the abdominal wall but may pass obliquely and somewhat irregularly through the multiple muscle and fascial layers of the abdominal wall.
If biopsies or confirmation of malignancy is necessary, the tissues that are obtained may be inadequate to make a definitive diagnosis. If tissues are examined on site and are inadequate for diagnosis more tissue can be obtained at the time of laparoscopy without the need for a second intervention.
A final caveat involves biopsy from the undersurface of the hemidiaphragm. With surprising frequency a biopsy at this anatomic site may result in a perforation of the diaphragm and consequent pneumothorax requiring a thoracostomy tube. Air from the laparoscopy will rapidly enter the thoracic space. Of course, cancer contamination of the pleural space from a full thickness penetration of the hemidiaphragm may occur.
Laparoscopy to determine the presence of peritoneal metastases
An important determination in many cancer patients prior to the initiation of treatment is the accurate assessment of the peritoneal space for the presence versus absence of metastatic disease. Can laparoscopy definitively determine that no progression of cancer in the peritoneal space has occurred? Or is an open procedure with complete division of all adhesions required to rule out peritoneal metastases? Passot and colleagues from the French Peritoneal Metastases Working Group prospectively evaluated 50 patients undergoing laparoscopy to determine the extent of peritoneal metastases (2). At the same intervention as laparoscopic exploration, open exploration with lysis of all adhesions and complete visualization of all parietal and visceral peritoneal surfaces occurred. Laparoscopy was possible in 44 of these 50 patients and deemed satisfactory by the surgeon in 52% (26 of 50 patients). Colorectal peritoneal recurrence was found in 58% by laparoscopic examination and by 68% by open surgery. If patients had a “satisfactory laparoscopy” there is a high concordance (24 of 25 patients). However, with a difficult laparoscopy only 38% of the peritoneal metastases were visualized.
Preoperative laparoscopy in gastric cancer patients
An important application of laparoscopy in gastric cancer clinical trials and selection of patients for gastrectomy has been described by Bagwell and coworkers (3). For gastric cancer, chemotherapy is used prior to gastrectomy to downstage the primary tumor mass and facilitate complete clearance and absolute containment of the disease. This strategy cannot be fulfilled if peritoneal metastases are present at the time of gastrectomy. The gastric cancer implants respond less well than the primary cancer to systemic chemotherapy. In patients with advanced gastric cancer pre-treatment laparoscopy is used to identify patients with peritoneal metastases or positive peritoneal cytology and arrange for special treatments of these patients prior to any attempt at a potentially curative gastrectomy.
Repeated laparoscopy as a monitor of intraperitoneal chemotherapy response
In the past peritoneal metastases from gastric cancer was considered a terminal condition with no successful treatment options and a rapid demise of the patient was predicted. Two clinical investigators in Japan have reported some success in prolonging the survival of these patients, and even performing potentially curative gastrectomy in the responder patients (4,5). In these gastric cancer patients with peritoneal metastases the chemotherapy is given by intraperitoneal administration using a permanent intraperitoneal port. In the treatments used by Yonemura and colleagues, the chemotherapy is a combined systemic cisplatin and intraperitoneal docetaxel plus oral S1 administration (bidirectional chemotherapy). To assess the responses to intraperitoneal chemotherapy repeated laparoscopy is performed approximately every two months. If peritoneal metastases are seen by serial laparoscopy the intraperitoneal chemotherapy through a port combined with systemic chemotherapy is continued. In those patients in whom peritoneal metastases completely respond on both parietal and visceral surfaces a gastrectomy to remove all visible evidence of gastric cancer is considered. Approximately 75% of the patients treated undergo gastrectomy with 10% 5-year survivors if response to neoadjuvant treatments were complete. In the studies by Yonemura, serial laparoscopy up to 7 interventions is an essential part of the monitoring of gastric cancer treatment strategies.
Determination of the presence versus absence of peritoneal metastases in patients with primary gastrointestinal malignancy
In nearly all patients with an abdominal or pelvic primary cancer, the presence of peritoneal metastases will change the initial treatment plan. If radiologic workup shows trace ascites or unexpected nodules are present, laparoscopy with biopsy should precede definitive treatment. In patients with pancreas cancer, the presence of peritoneal metastases will almost always call for cancellation of the pancreaticoduodenectomy or distal pancreatectomy. Similarly, peritoneal metastases with gastric cancer will change the surgical treatment plan (3). All patients who are eligible for gastrectomy with advanced gastric cancer should have a laparoscopy prior to the intervention. In primary colon or rectal cancer, patients should have laparoscopy if peritoneal metastases are suspected. If small volume peritoneal disease is documented by laparoscopic biopsy, open colorectal cancer resection, peritonectomy of the peritoneal metastases, and a perioperative intraperitoneal chemotherapy regimen is indicated (6). It is in the patient’s interest to devise a strategy that incorporates all necessary treatments into the initial surgical intervention that is used to resect the primary cancer (7). Other gastrointestinal cancers such as small bowel adenocarcinoma and appendiceal adenocarcinoma should have the presence versus absence of peritoneal metastases determined prior to a routine cancer resection. If peritoneal metastases are present, the management becomes much different from that routinely employed.
In summary, before resection of a gastrointestinal cancer, the presence of peritoneal surface disease must be determined prior to the routine visceral resection. If unexpected peritoneal metastases are documented as a laparoscopic colorectal cancer resection is being initiated, the best course of action for these patients is a discontinuation of the laparoscopic resection. These patients should then be referred to a peritoneal surface oncology treatment center for definitive management (8).
Therapeutic laparoscopy
The extent of peritoneal dissemination within the abdomen and pelvis is quantitated by the peritoneal cancer index (PCI), a radiologic PCI (RPCI) and a laparoscopic PCI (LPCI) (9). Often, if peritoneal metastases are suspected by radiologic examination, then laparoscopy is indicated. If the laparoscopic PCI is low and all the disease is resectable by laparoscopic peritonectomy, a laparoscopic cytoreductive surgery with laparoscopic HIPEC may be considered. Also, the extent of adhesions from prior surgery must be considered. Laparoscopic resection of all disease should be performed and a greater omentectomy and resection of the falciform ligament added to the laparoscopic disease resection.
The most common indication for laparoscopic cytoreductive surgery plus laparoscopic HIPEC is appendiceal mucinous neoplasm (10-12). Tumor deposits can be isolated to the peri-appendiceal area and they are present to a limited extent. Also, the appendiceal peritoneal metastases are minimally invasive so that peritonectomy provides for complete resection. The therapeutic laparoscopy is followed by laparoscopic HIPEC.
In some diseases a single laparoscopic cytoreduction with HIPEC may not be sufficient to control the disease. Badgwell and coworkers have reported on multiple treatments of gastric cancer peritoneal metastases with repeated HIPEC (13). They repeatedly performed laparoscopic HIPEC with mitomycin C (30 mg) and cisplatin (200 mg). These 19 patients underwent 38 laparoscopic HIPEC procedures. There was no 30-day mortality. Five patients went on to receive a potentially curative gastrectomy. The median overall survival from date of diagnosis of metastatic disease was 30.2 months and the median overall survival from the first laparoscopic HIPEC was 20.3 months.
Laparoscopic HIPEC for the management of debilitating malignant ascites
Another therapeutic application of laparoscopic HIPEC is the alleviation of malignant ascites. Often debilitating ascites from a malignancy can be palliated with systemic chemotherapy. However, in those patients who fail this systemic approach, laparoscopic HIPEC can be recommended. Numerous favorable reports from the literature have been presented (14-16). Although no randomized comparisons of laparoscopic HIPEC versus best palliative care are available it should be a treatment option available to patients not responsive to less invasive management strategies.
Laparoscopic administration of pressurized intraperitoneal aerosolized chemotherapy (PIPAC)
A new treatment modality utilizing the laparoscope to serially assess responses and simultaneously serially administer intraperitoneal chemotherapy is PIPAC. The aerosolized chemotherapy improves drug distribution as compared to a solubilized chemotherapy. Several reports of disease regression or stabilization with favorable palliative effects are reviewed (17). Use of PIPAC to date has not occurred along with cytoreductive surgery and to this point in time its treatment with curative intent has not been reported. However, this new modality for intraperitoneal chemotherapy delivery holds promise for peritoneal metastases management.
Minimally invasive surgery by laparoscopy using laparoscopic HIPEC to prevent peritoneal metastases in high risk groups
Increasingly, minimally invasive techniques are being reported for the resection of primary gastrointestinal cancer. The laparoscopic resection of colon and upper rectal cancer has been accepted as an alternative to laparotomy (18). Also, reports of gastric cancer and pancreas cancer resections by the laparoscope or robotic techniques have appeared (19,20). In all these resections there are selected patients who are at high risk for peritoneal metastases as a result of traumatic dissemination of cancer cells intraoperatively. The challenge for optimal care of these high risk patients is to identify them either preoperatively or intraoperatively and then employ prophylactic perioperative intraperitoneal chemotherapy to reduce the possibility for subsequent peritoneal metastases. The high risk clinical features for colorectal cancer are T4 disease, N2 lymph node involvement, positive margins of resection, positive peritoneal cytology, ovarian metastases and small numbers of peritoneal metastases. Laparoscopic resection along with laparoscopic HIPEC can be used as a new treatment strategy for these selected patients (21).
Radiologic versus laparoscopic identification and quantification of peritoneal metastases
In some clinical situations radiology and laparoscopy are in competition to provide information concerning the presence versus absence of peritoneal dissemination of cancer or the estimate of extent disease by PCI (9). Almost invariably, the radiologic studies are performed first. If the peritoneal metastases are from a mucinous primary cancer, the disease is usually well depicted by CT using an optimal peritoneal metastases protocol that involves maximal oral and maximal intravenous contrast. If there is very small volume mucinous malignancy this can be accurately depicted on MRI. However, with intestinal type (non-mucinous) cancer, the cancer nodules less than 2 cm in diameter are often not depicted (22,23). If the patient is symptomatic and a surgery is necessary, laparoscopy is not needed. However, if the patient has small volume peritoneal metastases and no concerning radiologic features, laparoscopic assessment may be crucial towards the decision to perform cytoreductive surgery plus perioperative intraperitoneal chemotherapy as compared to the treatment option of best supportive care. In performing the laparoscopy midline ports are preferable in order to avoid subsequent port site recurrence. The laparoscopic assessment is directed at the small bowel and its mesentery, the porta hepatis, and the gastrohepatic ligament. Unresectable disease at one or more of the sites will indicate a laparoscopic contraindication to cytoreductive surgery plus HIPEC (24). When radiologic studies are not definitive, the diseases that may profit most from laparoscopic assessment are those cancers that show an early invasion into the peritoneal surface. Gastric cancer should have a laparoscopy prior to intervention of advanced disease because of the poor detection of the peritoneal metastases invading peritoneal surfaces. The poorly differentiated and signet ring colon cancers also may have extensive mesenteric disease with a totally normal radiologic workup.
Conclusions
In summary, laparoscopy has multiple applications in patients with peritoneal metastases. Although there is some morbidity and even possible mortality, especially in patients who have had extensive prior surgery, proper patient selection goes far to avoid complications. Diagnostic information may be required to assess the response to chemotherapy. Determination of the presence versus absence of peritoneal metastases is always necessary to plan an optimal surgical intervention for removal of a primary gastrointestinal cancer. If peritoneal metastases are present they are most effectively treated at the time of primary cancer resection trying to avoid the second-look laparotomy. Therapeutic laparoscopy when combined with laparoscopic HIPEC may allow definitive treatment of peritoneal metastases of minimal extent. Careful workup of the primary malignancy may reveal that it is advanced. This then would indicate open resection with wide excision and peritonectomy around the primary malignancy. Also, perioperative chemotherapy may be necessary for the control of peritoneal metastases or their prevention. Laparoscopic HIPEC may be used repeatedly for gastric cancer, for palliation of malignant ascites unresponsive to systemic chemotherapy and for the delivery of PIPAC. A unique opportunity to prevent peritoneal metastases is the use of laparoscopic HIPEC in patients at high risk after laparoscopic gastrointestinal cancer resection. Radiologic and laparoscopic assessment of peritoneal metastases can be used to complement each other.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2019.04.04). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kwong ML, Sampah ME, Bello BL, et al. Port site metastases after minimally invasive resection for colorectal cancer: A retrospective study of 13 patients. Surg Oncol 2019;29:20-4. [Crossref]
- Passot G, Dumont F, Goéré D, et al. Multicentre study of laparoscopic or open assessment of the peritoneal cancer index (BIG-RENAPE). Br J Surg 2018;105:663-7. [Crossref] [PubMed]
- Badgwell B, Cormier JN, Krishnan S, et al. Does neoadjuvant treatment for gastric cancer patients with positive peritoneal cytology at staging laparoscopy improve survival? Ann Surg Oncol 2008;15:2684-91. [Crossref] [PubMed]
- Canbay E, Mizumoto A, Ichinose M, et al. Outcome data of patients with peritoneal carcinomatosis from gastric origin treated by a strategy of bidirectional chemotherapy prior to cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in a single specialized center in Japan. Ann Surg Oncol 2014;21:1147-52. [Crossref] [PubMed]
- Ishigami H, Fujiwara Y, Fukushima R, et al. Phase III Trial Comparing Intraperitoneal and Intravenous Paclitaxel Plus S-1 Versus Cisplatin Plus S-1 in Patients With Gastric Cancer With Peritoneal Metastasis: PHOENIX-GC Trial. J Clin Oncol 2018;36:1922-9. [Crossref] [PubMed]
- Glehen O, Gilly FN, Boutitie F, et al. Toward curative treatment of peritoneal carcinomatosis from nonovarian origin by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy: a multi-institutional study of 1,290 patients. Cancer 2010;116:5608-18. [Crossref] [PubMed]
- Sugarbaker PH. Improving oncologic outcomes for colorectal cancer at high risk for local-regional recurrence with novel surgical techniques. Expert Rev Gastroenterol Hepatol 2016;10:205-13. [Crossref] [PubMed]
- Sugarbaker PH, Glehen O. Management of unexpected peritoneal metastases with primary colorectal cancer using second-look surgery with HIPEC. Can Surg 2015;1:101.
- Jacquet P, Sugarbaker PH. Current methodologies for clinical assessment of patients with peritoneal carcinomatosis. J Exp Clin Cancer Res 1996;15:49-58.
- Hirano M, Yonemura Y, Canbay E, et al. Laparoscopic Diagnosis and Laparoscopic Hyperthermic Intraoperative Intraperitoneal Chemotherapy for Pseudomyxoma Peritonei Detected by CT Examination. Gastroenterol Res Pract 2012;2012:741202 [Crossref] [PubMed]
- Esquivel J, Averbach A, Chua TC. Laparoscopic cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in patients with limited peritoneal surface malignancies: feasibility, morbidity and outcome in an early experience. Ann Surg 2011;253:764-8. [Crossref] [PubMed]
- Fish R, Selvasekar C, Crichton P, et al. Risk-reducing laparoscopic cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for low-grade appendiceal mucinous neoplasm: early outcomes and technique. Surg Endosc 2014;28:341-345. [Crossref] [PubMed]
- Badgwell B, Blum M, Das P, et al. Phase II Trial of Laparoscopic Hyperthermic Intraperitoneal Chemoperfusion for Peritoneal Carcinomatosis or Positive Peritoneal Cytology in Patients with Gastric Adenocarcinoma. Ann Surg Oncol 2017;24:3338-44. [Crossref] [PubMed]
- Facchiano E, Scaringi S, Kianmanesh R, et al. Laparoscopic hyperthermic intraperitoneal chemotherapy (HIPEC) for the treatment of malignant ascites secondary to unresectable peritoneal carcinomatosis from advanced gastric cancer. Eur J Surg Oncol 2008;34:154-8. [Crossref] [PubMed]
- Patriti A, Cavazzoni E, Graziosi L, et al. Successful palliation of malignant ascites from peritoneal mesothelioma by laparoscopic intraperitoneal hyperthermic chemotherapy. Surg Laparosc Endosc Percutan Tech 2008;18:426-8. [Crossref] [PubMed]
- Valle M, Van der Speeten K, Garofalo A. Laparoscopic hyperthermic intraperitoneal peroperative chemotherapy (HIPEC) in the management of refractory malignant ascites: A multi-institutional retrospective analysis in 52 patients. J Surg Oncol 2009;100:331-4. [Crossref] [PubMed]
- Nowacki M, Alyami M, Villeneuve L, et al. Multicenter comprehensive methodological and technical analysis of 832 pressurized intraperitoneal aerosol chemotherapy (PIPAC) interventions performed in 349 patients for peritoneal carcinomatosis treatment: An international survey study. Eur J Surg Oncol 2018;44:991-6. [Crossref] [PubMed]
- Nelson H, Sargent DJ, Wieand HS, et al. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med 2004;350:2050-9. [Crossref] [PubMed]
- Kim HH, Hyung WJ, Cho GS, et al. Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: an interim report – a phase III multicenter, prospective, randomized trial (KLASS Trial). Ann Surg 2010;251:417-20. [Crossref] [PubMed]
- Croome KP, Farnell MB, Que FG, et al. Pancreaticoduodenectomy with major vascular resection: a comparison of laparoscopic versus open approaches. J Gastrointest Surg 2015;19:189-94. [Crossref] [PubMed]
- Facchiano E, Risio D, Kianmanesh R, et al. Laparoscopic hyperthermic intraperitoneal chemotherapy: indications, aims, and results: a systematic review of the literature. Ann Surg Oncol 2012;19:2946-50. [Crossref] [PubMed]
- Jacquet P, Jelinek JS, Steves MA, et al. Evaluation of computer tomography in patients with peritoneal carcinomatosis. Cancer 1993;72:1631-6. [Crossref] [PubMed]
- Koh JL, Yan TD, Glenn D, et al. Evaluation of preoperative computed tomography in estimating peritoneal cancer index in colorectal peritoneal carcinomatosis. Ann Surg Oncol 2009;16:327-33. [Crossref] [PubMed]
- Sugarbaker PH, Sardi A, Brown G, et al. Concerning CT features used to select patients for treatment of peritoneal metastases, a pictoral essay. Int J Hyperthermia 2017;33:497-504. [Crossref] [PubMed]
Cite this article as: Sugarbaker PH. Laparoscopy in the diagnosis and treatment of peritoneal metastases. Ann Laparosc Endosc Surg 2019;4:42.


