Robotic-assisted ipsilateral adrenalectomy after robotic-assisted partial nephrectomy: a case report
Introduction
Partial nephrectomy has become the standard procedure for small renal tumors (less than 4 cm, T1a) (1,2). Open and laparoscopic approaches have been used until the da Vinci robotic system (Intuitive Surgical, Sunnyvale, CA, USA) was FDA-approved over a decade ago. Since then the robotic-assisted partial nephrectomy (RPN) has become a safe and feasible procedure for the treatment of renal tumors (3,4). After the first robotic-assisted adrenalectomy (RA) was reported in 2001 by Horgan et al. (5), it has been increasingly utilized. The advantages of using robotic technology include three-dimensional vision, the Endowrist® freedom of movement and surgical precision. Such technologic advantages significantly enhance the surgeon’s skill in an ergonomically efficient manner. The wrist-like movements of the robotic instruments allow the surgeon to dissect anatomical structures and suture more easily.
Ipsilateral adrenal recurrence is uncommon after partial nephrectomy. The incidence of adrenal metastases from renal cell carcinoma (RCC) is 2–10% from clinical diagnosis (6,7). A previous study showed that adrenalectomy should not be routinely performed during partial nephrectomy, even for upper pole tumors because adrenal involvement is uncommon in the absence of radiographic findings (8). Adrenalectomy may be performed if radiographically suspicious adrenal lesions are found on surveillance imaging after partial nephrectomies (9). Re-operative adrenalectomy may be complicated because of inflammatory changes and adhesions around the adrenal and upper renal pole. Therefore, exposure of the ipsilateral adrenal gland and dissection of surrounding organs may be difficult by open or laparoscopic approaches. To our knowledge there aren’t any previous studies describing the RA post RPN. Here we present a patient with RA using da Vinci Xi after upper pole RPN suspicious for metastatic disease of the ipsilateral adrenal gland.
Patient
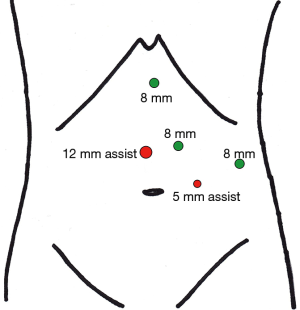
A 43-year-old male with a BMI of 32.1 kg/m2 presented with a history of RCC a year ago. MRI demonstrated an incidental 3.3 cm left upper pole (Figure 1A) enhancing mass with internal septations and hyperintensity on T2. He underwent robotic-assisted left partial nephrectomy. Pathology revealed clear cell, RCC, FG 2, pT1a, with negative margins. Seven months later, routine surveillance CT demonstrated a 2.09 cm enhancing left adrenal nodule (Figure 1B). Percutaneous biopsy of the adrenal was not a diagnostic. The case was presented in multi-disciplinary tumor board and the decision was made to proceed with adrenalectomy. A robotic approach was selected.
Surgical procedure (Figure 2)

For the procedure the patient was positioned in the right lateral decubitus (left-flank-up) position. The table was gently flexed and the kidney rest was raised slightly.
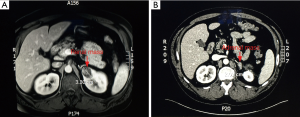
Access to the abdomen was obtained using a cut-down technique. A 12 mm midline incision was made 2 cm above the umbilicus. A 10/12 mm Ethicon Xcel™ blunt tip Hassan trocar (Ethicon Endo-surgery, Cincinnati, OH, USA) was placed. The abdomen was insufflated to 15 mmHg. There were minimal adhesions to the anterior wall. Three robotic 8 mm trocars were placed, triangulated to access the left upper quadrant. A 5 mm AirSeal assistant trocar (ConMed Corporation, Utica, New York, USA) was placed inferior to the camera port (Figure 3). After trocar placement, the robot was docked (Intuitive Surgical, Sunnyvale, CA, USA). The 8 mm robotic Xi instruments (fenestrated bipolar forceps, monopolar curved scissors and permanent cautery hook) were used during the procedure.
We began our dissection by taking down the white line of Toldt and reflecting the colon medially. We then incised the lienorenal attachments and reflected the spleen medially. Due to the prior partial nephrectomy, the colon, spleen and pancreas were extremely adherent to Gerota’s fascia of the upper pole of the kidney (Figure 4A), a great deal of care was involved during the mobilization. The pancreas and colon were reflected medially, and we could visualize the contour of the kidney and the adrenal mass superior to it. We carefully dissected all of the tissue from the sidewall laterally, to the aorta medially, from the spleen superiorly to the renal artery inferiorly and all the way to the posterior wall. This included the renal capsule of the upper pole of the kidney and a rim of renal parenchyma. The left adrenal vein was circled with the robotic hook and clipped with 5 mm green Hem-o-lok clips (Teleflex Medical, Morrisville, NC, USA) (Figure 4B).
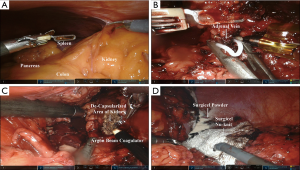
It appeared to be a gross total resection. The adrenal en bloc with surrounding tissue was removed and bagged in a 10 mm Endopouch Retriever (Ethicon Endo-surgery, Cincinnati, OH, USA). We over-sewed an area of bleeding on the kidney with 4-0 PDS. We then used the argon beam coagulator to cauterize the de-capsulized area of the kidney (Figure 4C). Surgicel powder and Surgicel Nu-knit were placed over the upper pole of the kidney and in the resection bed (Figure 4D). The hemostasis was excellent and there was no apparent injury to the adjacent structures.
Results
Total operation time was 249 minutes. Total console time was 134 minutes. Blood loss was 250 mL and there were no complications. He was discharged on post-operative day one. Pathology showed adrenal gland with inflammatory changes, but no evidence of tumor. The gland measured 8.3 cm × 7.6 cm × 2.8 cm and weighed 63.2 grams. The post-operative course was unremarkable.
Discussion
Partial nephrectomy is the preferred extirpative procedure for small renal tumors (less than 4 cm, T1a) (1,2). Metachronous ipsilateral adrenal metastasis uncommon, but both ipsilateral and contralateral recurrences have been described, even as long as 38 years after the original surgery (11-13).
Ipsilateral adrenalectomy at the time of partial nephrectomy is indicated at the time of partial nephrectomy on if the renal tumor is closely adherent to or growing into the adrenal gland intraoperatively, or there is evidence of involvement on preoperative imaging (8). Even for upper pole tumors, routine adrenalectomy is not recommended during partial nephrectomy (8). Instead, monitoring is performed with periodic imaging, and adrenalectomy may be performed radiographically suspicious adrenal lesions are identified (9). Although ipsilateral adrenalectomy was not recommended during the procedure of partial nephrectomy (8), their study determined that if the renal tumor was found closely adherent or entered the adrenal gland intraoperatively, the adrenal gland should be removed.
In this particular case, the patient underwent left RPN for an upper pole 3.3 cm pT1a clear cell RCC. There was no evidence of adrenal involvement on preoperative imaging or at the time of the original surgery. Nonetheless, he was suspected to have a metastatic recurrence to the ipsilateral adrenal gland seven months later based on findings on a routine surveillance CT. The intent of the surgery was to get complete pathologic information and potentially render the patient free of disease by removing the only site suspicious for recurrence. The post-operative pathology report was negative for involvement by carcinoma on the adrenal gland and renal capsular margin. Thus, the imaging turned out to be falsely positive.
Repeat surgeries (such as radical or partial nephrectomy and adrenalectomy) will be performed if there is evidence of metastasis diseases or local recurrence (9). In this case, we selected a robotic-assisted laparoscopic approach, rather than a large open incision or traditional laparoscopic approach, in order to provide the benefits of minimally-invasive surgery while having the necessary surgical precision for re-operative surgery. Since the da Vinci robotic system (Intuitive Surgical) was FDA-approved over 10 years ago, the RPN has become a safe and feasible procedure for treatment of renal tumors (3,4) compared to open and laparoscopic approaches. After the first RA was reported in 2001 by Horgan et al. (5), it has been increasingly used. The advantages of using robotic technology include three-dimensional vision, the Endowrist freedom of movement and surgical precision. The wrist-like movements of the robotic instruments allow the surgeon to dissect anatomical structures and suture more easily.
Since the da Vinci Xi (Intuitive Surgical) was launched in spring of 2014, more institutions have acquired this model. The biggest advantage of this Xi system includes the ability to attach the endoscope to any arm, flexibility for visualizing the surgical site, smaller and thinner arms with newly designed joints that offer a greater range of motion and longer instrument shafts designed for greater operative reach.
Conclusions
Preoperative planning prior to proceeding with surgery in re-operative fields it is critical. Obtaining an adrenal mass biopsy preoperatively for suspected ipsilateral adrenal recurrence should be considered. If the biopsy is inconclusive, obtaining consensus opinion prior to proceeding with surgery from a multidisciplinary tumor board is valuable.
RA is a safe and feasible procedure for adrenal masses after RPN. Surgeons attempting the procedure should be comfortable with the inflammation and adhesions encountered in re-operative surgery and should work with an experienced team to avoid damage to surrounding organs (such as the spleen, pancreas, colon and stomach) and major vessels (such as the aorta, renal artery and vein).
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2019.02.08). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lee CT, Katz J, Shi W, et al. Surgical management of renal tumors 4 cm. or less in a contemporary cohort. J Urol 2000;163:730-6. [Crossref] [PubMed]
- McKiernan J, Simmons R, Katz J, et al. Natural history of chronic renal insufficiency after partial and radical nephrectomy. Urology 2002;59:816-20. [Crossref] [PubMed]
- Abdel Raheem A, Sheikh A, Kim DK, et al. Da Vinci Xi and Si platforms have equivalent perioperative outcomes during robot-assisted partial nephrectomy: preliminary experience. J Robot Surg 2017;11:53-61. [Crossref] [PubMed]
- Giberti C, Gallo F, Schenone M, et al. Simultaneous bilateral robotic partial nephrectomy: Case report and critical evaluation of the technique. World J Clin Cases 2014;2:224-7. [Crossref] [PubMed]
- Horgan S, Vanuno D. Robots in laparoscopic surgery. J Laparoendosc Adv Surg Tech A 2001;11:415-9. [Crossref] [PubMed]
- Siemer S, Lehmann J, Kamradt J, et al. Adrenal metastases in 1635 patients with renal cell carcinoma: outcome and indication for adrenalectomy. J Urol 2004;171:2155-9; discussion 2159. [Crossref] [PubMed]
- Paul R, Mordhorst J, Leyh H, et al. Incidence and outcome of patients with adrenal metastases of renal cell cancer. Urology 2001;57:878-82. [Crossref] [PubMed]
- Lane BR, Tiong HY, Campbell SC, et al. Management of the adrenal gland during partial nephrectomy. J Urol 2009;181:2430-6; discussion 2436-7. [Crossref] [PubMed]
- Acar Ö, Şanlı Ö. Surgical Management of Local Recurrences of Renal Cell Carcinoma. Surg Res Pract 2016;2016:2394942 [Crossref] [PubMed]
- Feng DP, Feng Z, Luckenbaugh AN, et al. Robotic-assisted ipsilateral adrenalectomy after robotic-assisted partial nephrectomy. Asvide 2019;6:069. Available online: http://www.asvide.com/article/view/30505
- Dieckmann KP, Wullbrand A, Krolzig G. Contralateral adrenal metastasis in renal cell cancer. Scand J Urol Nephrol 1996;30:139-43. [Crossref] [PubMed]
- Lau WK, Zincke H, Lohse CM, et al. Contralateral adrenal metastasis of renal cell carcinoma: treatment, outcome and a review. BJU Int 2003;91:775-9. [Crossref] [PubMed]
- Featherstone JM, Bass P, Cumming J, et al. Solitary, late metastatic recurrence of renal cell carcinoma: two extraordinary cases. Int J Urol 2006;13:1525-7. [Crossref] [PubMed]
Cite this article as: Feng DP, Feng Z, Luckenbaugh AN, Avulova S, Barocas DA. Robotic-assisted ipsilateral adrenalectomy after robotic-assisted partial nephrectomy: a case report. Ann Laparosc Endosc Surg 2019;4:32.