
Lateral release in the repair of large ventral hernia
Introduction
Incisional hernias are a frequent problem complicating 11–23% of patients who undergone laparotomy (1-3), by itself a complex condition often confounded by variety of factors (patient factors, technique variation, surgeon skill, mesh characteristics, etc.) that complicates its evolution, the repair, and outcome (4). Ventral hernias uncouple the abdominal wall muscles and over time results to lateral retraction of the muscles; concurrently disuse atrophy develops, irreversible muscle fibrosis sets in with the muscles becoming stiffer and less elastic (5). The anatomical transformations have resultant physiological alterations: the visceral herniation out of the abdominal cavity leads to reduction of intra-abdominal pressure causing diaphragmatic descent and respiratory disfunction, portal venous stasis causes mesenteric and bowel wall edema leading to bowel congestion, ischemic bowel, diarrhea, and abdominal pain (6); and the malalignment of the rectus muscles, atrophy of abdominal wall muscles, and reduced intra-abdominal pressure leads to an unsupported spine leading to chronic back pain (7).
A nationwide prospective study using the Danish Ventral Hernia Database, showed that of the patients for repair of incisional hernias, 89% had <15 cm defects and 11% had >15 cm defects; and that large hernias pose a significant problem and are important risk factor for poorer outcome compared to the repair of smaller hernias (8). Suture repairs of ventral hernia are associated with high failure rate of up to 50% (9) due to excessive tension, poor vascularity, and/or poor tissue quality (10). The introduction of the intraperitoneal onlay mesh (IPOM) repair offered a tension-free repair and significantly decreased recurrence; however, being a bridging repair results to an adynamic abdominal wall repair without restoring abdominal wall function, that is prone to developing mesh eventration or pseudo-hernia, and ultimately to poor patient satisfaction. Fascial approximation restores natural abdominal musculature, improves strength and stability and normalizes abdominal pressures (11); the development of the IPOM Plus repair was to address the shortcomings of the IPOM repair, however not all are amenable to suture approximation without tension.
“Lateral release” refers to division and dissection of specific lateral muscle elements of the anterior abdominal wall to facilitate repair of ventral hernias (generally a subset, between 10 and 20 cm defect width) (12). The muscle release allows substantial medial mobilization and approximation of fascial defect edges with resulting reestablishment of the anterior abdominal wall; consequentially it reduces the abdominal wall tension (13) and restores abdominal wall function (14,15). The concept of incising elements of the abdominal wall to facilitate repair of ventral hernias was envisioned as early as 1951 by Albanese (16) with the use of “liberating incisions” to treat a large supraumbilical eventration, and by Young in 1961 (17) with incisions to the anterior rectus sheath to facilitate the repair of an epigastric incisional hernia. The concept only gained significant interest after the publication by Ramirez et al. in 1990 and who coined the term “component separation” (CS) (18). Numerous modifications and application of Minimally Invasive Surgery technique have been proposed, aimed at reduction of associated surgical site morbidity and at improvement of outcome and recovery. We aim to present the evolution of the lateral release concept from open to the application of minimally invasive surgery technique, considerations in performing CS, and present some recent studies on them.
Methodology
A search for articles reporting on outcomes of CS techniques in the repair of midline ventral hernia was performed in the PubMed database. The following search terms were employed in varying combination: “component separation”, “component release”, “anterior component”, “external oblique”, “posterior component”, “transversus abdominis release”, “incisional hernia”, and “ventral hernia”. The output was limited to articles in English language. The articles obtained were examined and duplicate articles were eliminated, based on the title and authors. The remaining articles were scrutinized for relevancy based on their titles and abstracts. The reference lists of relevant articles were subsequently examined to glean additional relevant studies.
Anterior component separation (ACS)
Open ACS
The open ACS was envisioned by Ramirez (18) as a means to repair large ventral hernias using mobilized innervated, vascularized, autologous abdominal wall tissue; especially in conditions that preclude mesh use, and to outright avoid mesh use related risks. The ACS entails an extensive subcutaneous dissection over the anterior rectus sheath until just beyond the semilunar line. At which, the external oblique aponeurosis and muscle are divided lateral to the semilunar line which may be carried superiorly beyond the costal margin and inferiorly up to the inguinal ligament, and the external oblique muscle is dissected off the underlying internal oblique from the division towards as far lateral as the posterior axillary line. Additional medial mobilization of the defect edge may be achieved with incision of the posterior rectus sheath and dissection of the rectus muscle off the posterior sheath.
The ACS repair successfully allows for reconstruction of the abdominal wall without the use of a prosthetic mesh, however it is hindered by substantial incidence of wound-related complications specifically infection as high as 50% (19,20) and reported recurrence rate of 30% (21). The extensive subcutaneous dissection disrupts perforator vessels supplying the skin, causing skin necrosis and dehiscence (22).
Perforator-sparing anterior component separation (PS-ACS)
In 2002, Saulis and Dumanian (23) proposed that preservation of perforator vessels to decrease the incidence of post-operative wound complications. The technique entails preservation of the periumbilical rectus abdominis perforator vessels by avoiding subcutaneous dissection within a 3cm radius from the umbilicus. Result of their retrospective study of 66 consecutive patients with comparable demographics (of which 41 patients underwent the PS-ACS), the conventional ACS group had significantly higher incidence of post-operative wound-related complications 5/25 (20%) than the PS-ACS group 1/41 (2.4%), P<0.05; the recurrence rate remained comparable in both groups (7% vs. 8%). Similar results were obtained by Espinosa-de-Los-Monteros et al. (24), they showed significantly lower wound complications with the PS-ACS (11% vs. 48%, P=0.003) against conventional ACS, after a mean follow-up of 14 months.
Endo-laparoscopic ACS
In view of the significance of perforator preservation in minimizing wound-related complications, Rosen et al. (25) proposed the application of minimally invasive surgical technique that aimed to completely eliminate the need for a broad subcutaneous dissection yet achieve lateral release. The access into the space between the external and internal oblique muscles starts with incision about 1cm lateral to the rectus edge and near the costal margin carried down to the external oblique muscle which is then opened and bluntly separated off the internal oblique muscle, until an adequate area is achieved to allow placement of the camera port. The intermuscular space dissection may be initially facilitated by the use of a dissecting balloon and subsequently carried on by careful blunt dissection using the scope, with the extent of dissection carried as far lateral as the posterior axillary line, superiorly may go above the costal margin, and caudal up to the level of the inguinal ligament. A 5 mm working port may then be inserted over the lateral area, to allow additional instrument for the release phase. With lateral edge of the rectus sheath identified, the external oblique aponeurosis is incised about 1cm lateral to the edge of the rectus sheath and carried both cephalad and caudal, depending on the extent of the hernia defect. The cephalad portion of the external oblique is more muscular than aponeurotic and its release would be facilitated by the use of advanced surgical energy devices. It is suggested to carry the release up into the deep subcutaneous fascia over the lower abdomen to allow better mobilization of the resultant medial flap. After which, the contralateral side may be released in a similar fashion. The repair of the ventral hernia may be performed via the laparoscopic or open technique, and the mesh placed in varying positions (retro-rectus, preperitoneal, or as IPOM) and fixed accordingly.
Retrospective studies comparing endoscopic ACS to the conventional ACS have reported significantly lower incidence of wound complications (26-28), reduction in length of hospital stay (28,29), and even improved recurrence rate (27,29). Two meta-analyses revealed similar findings: Jensen et al. in 2014 (30) reviewed 222 articles and eventually included five retrospective studies for analysis; these reported on a total of 163 patients, the incidence of wound complications comprising surgical site infection, skin necrosis, subcutaneous abscess, seroma, skin dehiscence, cellulitis, and fistula was significantly less after endoscopic CS (OR =0.27; 95% CI, 0.12–0.58; P<0.001). The incidence of recurrent hernia was comparable: 13% after endoscopic CS vs. 16% (OR =0.76; 95% CI, 0.29–1.98; P=0.57). That by Switzer et al. (31) reviewed 63 studies and included 7 controlled studies in its analysis which included total of 387 patients: overall wound complication was lower for endoscopic CS (20.6%) against open CS (34.6%), as was the recurrence rate (11.1% vs. 15.1%).
The cohort study by Thomsen et al. in 2015 (32) revealed significant improvement in self-rated QoL after ventral hernia repair using the endoscopic ACS technique, with 19 consecutive patients completed the questionnaire before and 16 months after the index repair. The overall QoL improvement was based on the following assessed outcome and perception: hernia gravity, pain, discomfort, fatigue, impairment of QoL, physical health, mental health, and over-all health.
Posterior component separation (PCS)
Open PCS
In view of the high incidence of wound complications in open ACS associated with the extensive subcutaneous dissection, Carbonell et al. (33) proposed in their 2008 a modification of the retro-muscular hernia repair described by Rives and Stoppa, which he termed “posterior component separation” (PCS). It aimed to expand the retro-rectus space by incising the PRS and dissecting into the space between the transversus abdominis and the internal oblique muscle, consequently permits the deployment of a larger prostheses to repair large hernia defects with diameter greater than 15 cm. They successfully achieved anterior rectus sheath closure in 85% (17/20 patients), for mean defect area of 223 cm2 and mean horizontal defect size of 11.9 cm. Wound complication occurred in 3/17 and recurrence of 1/17 during a mean follow-up of 10 months (range, 1–27 months). They were unable to approximate the defect of three patients, these had horizontal fascial defect sizes of 15, 22, and 25 cm. They cited technique’s advantage of eliminating extensive subcutaneous dissection, of producing a wide inter-parietal space ideal for mesh deployment and eliminating necessity for a specialized composite mesh to prevent visceral adhesion; however, pointed out a possible technical weak point, that of potential dissection injury to the lateral cutaneous nerve branches that runs between the transversus abdominis and internal oblique muscles. In their study, none of their patients reported any long-term pain nor abdominal wall dysfunction.
A 2012 retrospective study by Krpata et al. (34) comparing PCS against ACS, involving 111 cases of ventral hernias (56 ACS, 55 PCS), both groups had 5 cases repaired by bridging; the PCS group had significantly lower wound complication (25.5% vs. 48.2%, P=0.01) and trend toward lower recurrence rate (3.6% vs. 14.3%, P=0.09).
Posterior component separation with transversus abdominis release (PCS-TAR)
Novitsky et al. (35) in 2012, proposed an alternative PCS technique: which differs from that of Carbonell (33) by the dissection of the divided transversus abdominis muscle off the underlying transversalis fascia and peritoneum. Division of the transversus abdominis muscle can be performed either “top to bottom” or “bottom to top” manner. The “top to bottom” method proposed by Novitsky et al. (35) is based on the anatomic feature that the transversus abdominis muscle extends medially beyond the semilunar line at the upper third of the abdomen (Figure 1A). At the upper abdomen, incision of the PRS just medial to the neuro-vascular bundle permits visualization of the transversus abdominis muscle underneath. The PRS incision is carried cranially to the subcostal area and caudally till the arcuate line. The transversus abdominis muscle underneath may be teased off the underlying transversalis fascia and peritoneum, and subsequently divided vertically along its attachment using the electrosurgical hook or ultrasonic shears. The “bottom to top” TAR technique of Robin-Lersundi et al. (36), starts at the landmark arcuate line of the posterior rectus sheath which represents the end of transversus abdominis aponeurosis contribution to the posterior rectus sheath (Figure 1B) and starts contributing entirely to the anterior rectus sheath (Figure 1C). Dissection of the PRS off the underlying transversalis fascia and peritoneum can be initiated at this area and thereafter division of the PRS in a cephalad direction just medial to the neuro-vascular bundles/perforators. As the division of the PRS reaches the upper abdomen, the transversus abdominis aponeurosis gives way to the transversus abdominis muscle; this is lifted off and divided until the subcostal area.
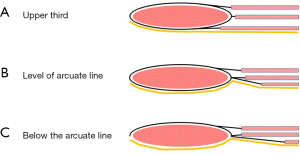
The Novitsky study (35), PCS-TAR repair was successfully performed on 42 patients with massive ventral hernial defects, post-operative wound complications occurred in ten patients (28.8%), and during the mean follow-up of 26.1 months had recurrence of 4.7%. In their update published in 2016 (37), they accumulated a total of 428 patients, the mean defect size repaired was 606 cm2; disclosed improved wound complication rate of 18.7% (80/428) and recurrence rate of 3.7%, during mean follow-up of 31.5 months.
The meta-analysis comparing open ACS against PCS-TAR recently published by Hodgkinson et al. in 2018 (38), included 12 studies in its analysis with total of 281 patients. The pooled data on hernia recurrence rate for PCS-TAR (from 7 studies) was lower than ACS (from 6 studies) at 5.7% vs. 9.5%; no statistical difference was noted between the two groups for: hernia recurrence, superficial and deep wound complication, re-operation rate, use of bridging mesh, and length of hospital stay. They concluded that both techniques have comparable outcomes for adverse events, recurrence rates, and use of bridging mesh; but pointed out that there is need for more comparative studies and randomized trials, and the presence of heterogeneity observed among the studies likely influenced the resulting outcome.
Endo-laparoscopic posterior component separation—TAR
Laparoscopic transabdominal posterior component separation with transversus abdominis release (L-TAR)
Belyansky et al. in 2015 (39) reported their initial experience on the application of minimally invasive technique to PCS-TAR. The method entailed access into the peritoneal cavity with set of three ports (a camera port and two working ports) on either side of the ventral hernia, preferentially initiated on the left-side. These permits inspection of the intra-abdominal cavity and subsequent performance of a thorough visceral adhesiolysis from the peritoneum. The TAR was thereafter performed starting with incision of the posterior rectus sheath in the manner described by Novitsky (35). Three patients underwent the procedure, hernia defect sizes ranged between 6–14 cm, loss of domain limit of ≤20%, and without active intraabdominal infection nor chronic wounds. Release and dissection of the PRS achieved 3 cm medial mobilization of the defect edge, an additional 7 cm was achieved with a unilateral TAR. The procedure was undeniably technically challenging resulting in longer mean operative time (329 vs. 238 min) compared to their historical data of open PCS-TAR approach. The PCS-TAR was associated with lower estimated blood loss (91.7 vs. 148 mL) and shorter hospital stay (4.7 vs. 6 days), with no wound-related complications nor recurrence noted during the six weeks of follow-up. The PCS-TAR permits retro-muscular mesh deployment which allowed use of less expensive uncoated mesh, prevents mesh migration, and hypothetically allows both sides of the mesh to integrate, potentially providing better strength of repair.
Enhanced view-totally extraperitoneal transversus abdominis release (eTEP-TAR)
The enhanced view-totally extraperitoneal (e-TEP) technique was initially promoted by Jorge Daes in 2012 (40) for the repair of large inguinal hernias. Two articles reporting on eTEP-TAR repair of ventral hernias were found: Belyansky et al. (41), the first to extend eTEP application to the repair of ventral hernias, and that of Baig and Priya (42). The eTEP-TAR technique (41,42) entailed an initial access into the retro-rectus space, with entry site dependent on location of the ventral hernia: upper midline defects or lower midline defects. The bilateral retro-rectus spaces are developed; including release and dissection of the medial edge of the PRS and rectus muscle, respectively. The peritoneal cavity is entered at a safe distance from the hernia sac, to inspect the contents and perform a thorough adhesiolysis. The PCS-TAR may then be performed, the fascial defect approximated, mesh deployed, and fixation follows.
The multicenter series by Belyansky et al. (41) had 79 patients that underwent eTEP technique for ventral hernia repair, of which 38 (48%) underwent eTEP-Rives-Stoppa (eTEP-RS) and 41 (51%) underwent the eTEP-TAR repair; for the following reasons: wide defect (>10 cm), tension on the posterior layer, narrow retrorectus space (<5 cm), or poorly compliant abdominal wall. Those who underwent eTEP-TAR, had mean defect width of 11.1±7.6 cm and length of stay of 2.7±1.3 days after repair. The over-all wound-related complications was 3.8%, no readmission within 30 days were noted, one case of recurrence in the eTEP-TAR group detected at 1-year follow-up. The QoL was likewise analyzed from data of one institution (Anne Arundel Medical Center) that routinely collect Carolina’s Comfort Scale surveys, it showed significant improvement (against pre-operative scores) for pain and for movement limitation at 1-month follow-up (60%, P<0.002 and 66%, P<0.004, respectively), and further improvement at 6-month follow-up reporting complete resolution (68%, P<0.007 and 87%, P<0.004, respectively).
The series by Baig and Priya (42) with 21 patients (9 eTEP-RS and 12 in eTEP-TAR); the over-all mean defect size repaired was 6.65±1.95 cm, with mean hospital stay of 2.67±0.79 days. They reported limiting hernia width to 4–12 cm; in view of the difficulty of suturing wider defects, and preference for IPOM-plus for smaller defects due to ease and shorter operative time.
Both studies (41,42) found eTEP-TAR to be safe and feasible, but associated with drawbacks of steep learning curve and longer operative time. The fascial defect approximation was the technically demanding and the critical step of the procedure.
Considerations in component separation
Predicting successful fascial approximation
Concern exists on predicting successful fascial approximation with CS; when closure cannot achieve without excessive tension, will necessitate the use of bridging mesh repair, this occurs in 18–33% of patients undergoing CS (43,44). Franklin et al. (10) in their 2013 retrospective study iterated that even with defect less than that advocated for by Ramirez et al., closure with CS is not always achieved. They reviewed pre-operative CT scans of 54 patients who underwent CS (48 successful fascial closure and 6 bridged repair); the CS with bridged repair group had significantly higher mean transverse defect size (19.8 vs. 10 cm, P<0.05), mean defect area (420 vs. 184.2 cm2, P<0.05) and quotient for transverse defect size against abdominal wall circumference—“percent abdominal wall defect” (18.9% vs. 10.6%, P<0.05). They concluded that these variables may be used as predictors of abdominal wall closure with CS, however no cut off values were suggested due to the small sample size. Poruk et al. (45) in their retrospective study on the effect of hernia size on repair and outcome after an open repair, suggested that those with hernia defect area 200–300 cm2 were amenable to CS repair, <200 cm2 can be managed by primary closure with mesh reinforcement, >300 cm2 were unlikely to benefit from CS alone and were addressed by sandwich mesh repair.
Christy et al. (46) suggested a new value “Component Separation Index” (CSI) as predictor of fascial approximation success in CS; it takes into account hernial defect size in relation to the remaining tissue in the abdominal wall, and serves as a biometric assessment of the abdominal wall defect rather than an absolute value of defect size or area. The CSI is the “angle of diastasis” (AD) divided by 360; with AD taken at the CT image identified with the maximal transverse defect size, with aorta as the vertex, and the arms of the angle taken from the vertex to the medial edges of the defect. They reviewed pre-operative CT scans of 18 patients who underwent successful CS and similar number of patients who had CS with bridged mesh repair; the CSI was significantly higher among those that require mesh bridging (0.21±0.04 vs. 0.11±0.06, P<0.0001). They caution on the limitation of CSI not accounting for scar tissue formation, that are likely to affect abdominal wall compliance and fascial medialization with CS.
A systematic review on loss of domain (LOD) by Parker et al. (47), found that LOD is ordinarily used to predict operative difficulty and success, and is pertinent as it describes volumetric relationship of hernial sac to the abdominopelvic cavity. It however observed inconsistent definition of the term, even with volumetric definition different methods [Tanaka et al. (48) or Sabbagh et al. (49) method] used, or simply stated the two volumes without relationship to the other. The clinically significant thresholds cited appear to be anecdotal and based on clinical expertise; the mentioned values ranged from 10–50%, with 20% LOD being the most commonly cited (47). The observational study by Azar et al. (50) reported an institution’s experience on repair of patients with LOD; which involved 21 patients with LOD calculated using the Tanaka method from preoperative CT imaging; using threshold of >30% to define giant ventral hernias. 81% of patients were managed using the sandwich mesh repair, four patients repaired with CS; of note, one CS was performed among the giant hernia group. This observation seems to concur with the popular estimation the threshold may be around 20% LOD.
Adhesiolysis
The complete lysis of visceral adhesions to the anterior abdominal wall is an imperative step. The motivations behind this comprehensive adhesiolysis are the adherent visceral may be prone to unintended injury during the course of tissue release, the adhesions may contribute to peritoneal or posterior sheath tears during myofascial component release or advancement, or it may hinder mobilization of the components. Adhesiolysis of inter-loop adhesions is usually avoided, unless there are pre-operative sign of obstruction or need for intestinal intervention.
Mesh reinforcement and choice of mesh in component separation repair
A study (51) into recurrence after purely tissue CS repair, followed 75 patients for a mean period of 40.9 months, noted significantly higher incidence of recurrence than that reported in literatures (38.7% vs. 14%, P<0.01). The series of O’Halloran et al. (52) on 85 patients who underwent ventral hernia repair using CS (with/without mesh reinforcement) and followed up for mean of 14.4 months; reinforcement with mesh lowered the incidence of recurrence (11.1% vs. 14.1%) compared to the overall recurrence rate. Ko et al. (53) reported on their experience with 200 PS-ACS: significantly higher overall recurrence of 22.8% without mesh reinforcement compared to mesh reinforced (16.7% using heavy-weight and 0% using medium-weight polypropylene mesh, P=0.04); subset analysis of non-contaminated cases revealed similar higher recurrence of 26.4% for without mesh reinforcement versus that with mesh reinforcement (20% heavy-weight and 0% medium weight, P=0.02). They noted there was significant difference in hernial defect widths between groups; however, regression analysis found it had no effect on hernia recurrence. Use of mesh reinforcement does not influence development of major (25.3% without mesh vs. 16.7–22.2% with mesh) and minor (19.0% without mesh vs. 16.7–33.3% with mesh) complications.
Study into the mechanisms of failure and recurrence pattern in ventral hernia mesh repair by Warren et al. in 2017 (54) noted the majority of mechanism of failure after repair were attributed to central mesh breakdown (39.6%), with 78.9% of which occurring after using light-weight polypropylene mesh and 18.8% due to central recurrence after repair with biologic or bio-absorbable mesh. The use of medium-weight polypropylene mesh was associated with lower recurrence rate than with biologic (P<0.001) or bio-absorbable (P<0.006) mesh.
Adjunctive tissue lengthening with CS
Meta-analysis by Alam et al. in 2016 (55) on utility of various abdominal wall expansion methods for incisional hernia repair included 21 studies, identified 2 studies on Botulinum toxin A (BTA) injection in 29 patients achieving 100% fascial closure with 15 (51.7%) repaired in combination with CS. BTA had no reported complication, recurrence, nor mortality. All the tissue lengthening methods were found to be safe and feasible and were able to provide the necessary extra tissue mobility to achieve fascial closure. Weissler et al. in 2017 (56) and Rodriguez-Acevedo et al. (57) similarly noted significant mean abdominal wall length gain after BTA injection: 3.33 cm per side, P<0.001 and 4 cm per side, P<0.0001, respectively, and all patients subsequently underwent successful abdominal wall closure. An observational study by Bueno-Lledo et al. (58) on pre-operative use of combination tissue lengthening procedures (progressive pneumoperitoneum and BTA) in 70 patients with at least 20% LOD (ranging 20.5–65.1% prior to intervention) followed by abdominal wall repair. The tissue lengthening procedures triggered significant increase in mean abdominal cavity volume (9,045±3,345 vs. 10,999±3,490 cc, P=0.02) and caused significant reduction of the LOD percentage (to 13.2%±11.0%, P=0.02 after intervention); subsequent abdominal wall restoration was successful in all patients (77.1% with ACS, 20% with PCS-TAR, 2.9% with Rives-Stoppa technique).
Summary
Currently, lateral release shows encouraging results in the repair of large ventral hernias. Modifications to the original technique have improved outcome by markedly improving wound complication rates and some incremental reduction of recurrence rate. Use of synthetic mesh reinforcement with the CS repair results in lower recurrence rate over than with purely tissue CS repair. Reinforcement with medium-weight mesh is recommended over light-weight mesh, to minimize the risk of mesh failure and subsequent recurrence. The use of biologic or bio-absorbable mesh may be considered in contaminated field, but at the cost of higher recurrence rate. The application of MIS technique in CS ventral hernia repair is feasible and have significantly lowered wound-related complications but is associated with increased technical difficulty. Pre-operative assessment of the anterior abdominal wall and defect will likely provide beneficial information predicting likelihood of defect approximation with CS, however current studies are unable to provide a definitive threshold values due to small study size. In cases with LOD, the use of adjunct tissue lengthening techniques have shown to permit successful abdominal wall reconstruction using the CS repair technique.
The current studies show encouraging results for the various CS repairs, but studies are limited, small size, with short-term follow-up, and wide heterogeneity between studies that does not inspire robust conclusions. This trend is understandable as the technique has only recently become popular and is currently still in the furor of development. Larger studies, longer follow-up periods, and focused outcome investigation will likely help the CS technique to consolidate and to mature and provide conclusive evidence of its benefit in the repair of large midline ventral hernias.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Davide Lomanto and Anil Sharma) for the series “Ventral Hernia” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2019.02.05). The series “Ventral Hernia” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bucknall TE, Cox PJ, Ellis H. Burst abdomen and incisional hernia: a prospective study of 1129 major laparotomies. Br Med J (Clin Res Ed) 1982;284:931-3. [Crossref] [PubMed]
- Mudge M, Hughes LE. Incisional hernia: a 10 year prospective study of incidence and attitudes. Br J Surg 1985;72:70-1. [Crossref] [PubMed]
- Le Huu Nho R, Mege D, Ouaissi M, et al. Incidence and prevention of ventral incisional hernia. J Visc Surg 2012;149:e3-14. [Crossref] [PubMed]
- Bittner R, Bingener-Casey J, Dietz U, et al. Guidelines for laparoscopic treatment of ventral and incisional abdominal wall hernias (International Endohernia Society (IEHS)—Part 1. Surg Endosc 2014;28:2-29. [Crossref] [PubMed]
- Kirkpatrick AW, Nickerson D, Roberts DJ, et al. Intra-Abdominal Hypertension and Abdominal Compartment Syndrome after Abdominal Wall Reconstruction: Quaternary Syndromes? Scand J Surg 2017;106:97-106. [Crossref] [PubMed]
- Valezi AC, de Melo BGF, Marson AC, et al. Preoperative progressive pneumoperitoneum in obese patients with loss of domain hernias. Surg Obes Relat Dis 2018;14:138-42. [Crossref] [PubMed]
- Rives J, Lardennois B, Pire JC, et al. Large incisional hernias. The importance of flail abdomen and of subsequent respiratory disorders. Chirurgie 1973;99:547-63. [PubMed]
- Helgstrand F, Rosenberg J, Kehlet H, et al. Nationwide prospective study of outcomes after elective incisional hernia repair. J Am Coll Surg 2013;216:217-28. [Crossref] [PubMed]
- George CD, Ellis H. The results of incisional hernia repair: a twelve year review. Ann R Coll Surg Engl 1986;68:185-7. [PubMed]
- Franklin BR, Patel KM, Nahabedian MY, et al. Predicting abdominal closure after component separation for complex ventral hernias: maximizing the use of preoperative computed tomography. Ann Plast Surg 2013;71:261-5. [Crossref] [PubMed]
- Shell DH, de la Torre J, Andrades P, et al. Open repair of ventral incisional hernias. Surg Clin North Am 2008;88:61-83. viii. [Crossref] [PubMed]
- Heller L, McNichols CH, Ramirez OM. Component separations. Semin Plast Surg 2012;26:25-8. [Crossref] [PubMed]
- Afifi AM, Hartmann E, Talaat A, et al. Quantitative Assessment of Tension Reduction at the Midline Closure During Abdominal Component Separation. J Am Coll Surg 2017;224:954-61. [Crossref] [PubMed]
- Criss CN, Petro CC, Krpata DM, et al. Functional abdominal wall reconstruction improves core physiology and quality-of-life. Surgery 2014;156:176-82. [Crossref] [PubMed]
- De Silva GS, Krpata DM, Hicks CW, et al. Comparative radiographic analysis of changes in the abdominal wall musculature morphology after open posterior component separation or bridging laparoscopic ventral hernia repair. J Am Coll Surg 2014;218:353-7. [Crossref] [PubMed]
- Albanese AR. Liberating incisions in the treatment of large supraumbilical eventrations. Prensa Med Argent 1966;53:2222-7. [PubMed]
- Young D. Repair of epigastric incisional hernia. Br J Surg 1961;48:514-6. [Crossref] [PubMed]
- Ramirez OM, Ruas E, Dellon AL. "Components separation" method for closure of abdominal-wall defects: an anatomic and clinical study. Plast Reconstr Surg 1990;86:519-26. [Crossref] [PubMed]
- Borud LJ, Grunwaldt L, Janz B, et al. Components separation combined with abdominal wall plication for repair of large abdominal wall hernias following bariatric surgery. Plast Reconstr Surg 2007;119:1792-8. [Crossref] [PubMed]
- de Vries Reilingh TS, van Goor H, Charbon JA, et al. Repair of giant midline abdominal wall hernias: "components separation technique" versus prosthetic repair: interim analysis of a randomized controlled trial. World J Surg 2007;31:756-63. [Crossref] [PubMed]
- de Vries Reilingh TS, van Goor H, Rosman C, et al. "Components separation technique" for the repair of large abdominal wall hernias. J Am Coll Surg 2003;196:32-7. [Crossref] [PubMed]
- Mazzocchi M, Dessy LA, Ranno R, et al. "Component separation" technique and panniculectomy for repair of incisional hernia. Am J Surg 2011;201:776-83. [Crossref] [PubMed]
- Saulis AS, Dumanian GA. Periumbilical rectus abdominis perforator preservation significantly reduces superficial wound complications in "separation of parts" hernia repairs. Plast Reconstr Surg 2002;109:2275-80; discussion 81-2. [Crossref] [PubMed]
- Espinosa-de-Los-Monteros A, Avendano-Peza H, Gomez-Arcive Z, et al. Total Abdominal Wall Reconstruction with Component Separation, Reinforcement, and Vertical Abdominoplasty in Patients with Complex Ventral Hernias. Aesthetic Plast Surg 2016;40:387-94. [Crossref] [PubMed]
- Rosen MJ, Williams C, Jin J, et al. Laparoscopic versus open-component separation: a comparative analysis in a porcine model. Am J Surg 2007;194:385-9. [Crossref] [PubMed]
- Albright E, Diaz D, Davenport D, et al. The component separation technique for hernia repair: a comparison of open and endoscopic techniques. Am Surg 2011;77:839-43. [PubMed]
- Ghali S, Turza KC, Baumann DP, et al. Minimally invasive component separation results in fewer wound-healing complications than open component separation for large ventral hernia repairs. J Am Coll Surg 2012;214:981-9. [Crossref] [PubMed]
- Giurgius M, Bendure L, Davenport DL, et al. The endoscopic component separation technique for hernia repair results in reduced morbidity compared to the open component separation technique. Hernia 2012;16:47-51. [Crossref] [PubMed]
- Harth KC, Rosen MJ. Endoscopic versus open component separation in complex abdominal wall reconstruction. Am J Surg 2010;199:342-6; discussion 6-7. [Crossref] [PubMed]
- Jensen KK, Henriksen NA, Jorgensen LN. Endoscopic component separation for ventral hernia causes fewer wound complications compared to open components separation: a systematic review and meta-analysis. Surg Endosc 2014;28:3046-52. [Crossref] [PubMed]
- Switzer NJ, Dykstra MA, Gill RS, et al. Endoscopic versus open component separation: systematic review and meta-analysis. Surg Endosc 2015;29:787-95. [Crossref] [PubMed]
- Thomsen CØ, Brøndum TL, Jørgensen LN. Quality of Life after Ventral Hernia Repair with Endoscopic Component Separation Technique. Scand J Surg 2016;105:11-6. [Crossref] [PubMed]
- Carbonell AM, Cobb WS, Chen SM. Posterior components separation during retromuscular hernia repair. Hernia 2008;12:359-62. [Crossref] [PubMed]
- Krpata DM, Blatnik JA, Novitsky YW, et al. Posterior and open anterior components separations: a comparative analysis. Am J Surg 2012;203:318-22; discussion 22. [Crossref] [PubMed]
- Novitsky YW, Elliott HL, Orenstein SB, et al. Transversus abdominis muscle release: a novel approach to posterior component separation during complex abdominal wall reconstruction. Am J Surg 2012;204:709-16. [Crossref] [PubMed]
- Robin-Lersundi A, Blazquez Hernando L, Lopez-Monclus J, et al. How we do it: down to up posterior components separation. Langenbecks Arch Surg 2018;403:539-46. [Crossref] [PubMed]
- Novitsky YW, Fayezizadeh M, Majumder A, et al. Outcomes of Posterior Component Separation With Transversus Abdominis Muscle Release and Synthetic Mesh Sublay Reinforcement. Ann Surg 2016;264:226-32. [Crossref] [PubMed]
- Hodgkinson JD, Leo CA, Maeda Y, et al. A meta-analysis comparing open anterior component separation with posterior component separation and transversus abdominis release in the repair of midline ventral hernias. Hernia 2018;22:617-26. [Crossref] [PubMed]
- Belyansky I, Zahiri HR, Park A. Laparoscopic Transversus Abdominis Release, a Novel Minimally Invasive Approach to Complex Abdominal Wall Reconstruction. Surg Innov 2016;23:134-41. [Crossref] [PubMed]
- Daes J. The enhanced view-totally extraperitoneal technique for repair of inguinal hernia. Surg Endosc 2012;26:1187-9. [Crossref] [PubMed]
- Belyansky I, Daes J, Radu VG, et al. A novel approach using the enhanced-view totally extraperitoneal (eTEP) technique for laparoscopic retromuscular hernia repair. Surg Endosc 2018;32:1525-32. [Crossref] [PubMed]
- Baig SJ, Priya P. Extended totally extraperitoneal repair (eTEP) for ventral hernias: Short-term results from a single centre. J Minim Access Surg 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Butler CE, Campbell KT. Minimally invasive component separation with inlay bioprosthetic mesh (MICSIB) for complex abdominal wall reconstruction. Plast Reconstr Surg 2011;128:698-709. [Crossref] [PubMed]
- Bluebond-Langner R, Keifa ES, Mithani S, et al. Recurrent abdominal laxity following interpositional human acellular dermal matrix. Ann Plast Surg 2008;60:76-80. [Crossref] [PubMed]
- Poruk KE, Farrow N, Azar F, et al. Effect of hernia size on operative repair and post-operative outcomes after open ventral hernia repair. Hernia 2016;20:805-10. [Crossref] [PubMed]
- Christy MR, Apostolides J, Rodriguez ED, et al. The component separation index: a standardized biometric identity in abdominal wall reconstruction. Eplasty 2012;12:e17 [PubMed]
- Parker SG, Halligan S, Blackburn S, et al. What Exactly is Meant by "Loss of Domain" for Ventral Hernia? Systematic Review of Definitions. World J Surg 2019;43:396-404. [Crossref] [PubMed]
- Tanaka EY, Yoo JH, Rodrigues AJ Jr, et al. A computerized tomography scan method for calculating the hernia sac and abdominal cavity volume in complex large incisional hernia with loss of domain. Hernia 2010;14:63-9. [Crossref] [PubMed]
- Sabbagh C, Dumont F, Robert B, et al. Peritoneal volume is predictive of tension-free fascia closure of large incisional hernias with loss of domain: a prospective study. Hernia 2011;15:559-65. [Crossref] [PubMed]
- Azar FK, Crawford TC, Poruk KE, et al. Ventral hernia repair in patients with abdominal loss of domain: an observational study of one institution's experience. Hernia 2017;21:245-52. [Crossref] [PubMed]
- Slater NJ, van Goor H, Bleichrodt RP. Large and complex ventral hernia repair using "components separation technique" without mesh results in a high recurrence rate. Am J Surg 2015;209:170-9. [Crossref] [PubMed]
- O'Halloran EB, Barwegen CJ, Dombrowski JM, et al. Can't have one without the other: component separation plus mesh for repairing difficult incisional hernias. Surgery 2014;156:894-9. [Crossref] [PubMed]
- Ko JH, Wang EC, Salvay DM, et al. Abdominal wall reconstruction: lessons learned from 200 "components separation" procedures. Arch Surg 2009;144:1047-55. [Crossref] [PubMed]
- Warren JA, McGrath SP, Hale AL, et al. Patterns of Recurrence and Mechanisms of Failure after Open Ventral Hernia Repair with Mesh. Am Surg 2017;83:1275-82. [PubMed]
- Alam NN, Narang SK, Pathak S, et al. Methods of abdominal wall expansion for repair of incisional herniae: a systematic review. Hernia 2016;20:191-9. [Crossref] [PubMed]
- Weissler JM, Lanni MA, Tecce MG, et al. Chemical component separation: a systematic review and meta-analysis of botulinum toxin for management of ventral hernia. J Plast Surg Hand Surg 2017;51:366-74. [Crossref] [PubMed]
- Rodriguez-Acevedo O, Elstner KE, Jacombs ASW, et al. Preoperative Botulinum toxin A enabling defect closure and laparoscopic repair of complex ventral hernia. Surg Endosc 2018;32:831-9. [Crossref] [PubMed]
- Bueno-Lledó J, Torregrosa A, Jimenez R, et al. Preoperative combination of progressive pneumoperitoneum and botulinum toxin type A in patients with loss of domain hernia. Surg Endosc 2018;32:3599-608. [Crossref] [PubMed]
Cite this article as: Buenafe AA, Lee-Ong A. Lateral release in the repair of large ventral hernia. Ann Laparosc Endosc Surg 2019;4:24.


