Pediatric minimal invasive surgery—bile duct diseases
With the development of laparoscopy, surgical treatment for bile duct diseases enters into a new era. The magnified view and agile observation perspective provided by telescope update the knowledge of diseases, associated anomalies, and anatomical variations, improve the techniques, and facilitate individualized surgical strategies. The advantages includes: (I) it minimizes the surgical trauma, reduces complications, hence accelerates postoperative recovery; (II) umbilical-to-hepatic hilum observation provided by telescope is beneficial for maneuver at hepatic hilum; (III) magnified view facilitates precise dissection and anastomosis; (IV) the wide vision field allows observation of whole abdominal cavity, the laparoscope and/or cystoscope can inspect intrahepatic duct, common channel, pancreatic duct, hence resolve multiple anomalies with the same setting.
The laparoscopic techniques increasingly applied in children with the following bile duct diseases.
Choledochal cysts (CDC)
Evolution of laparoscopic cyst excision and Roux-Y hepaticojejunostomy
The laparoscopic CDC excision and hepaticojejunostomy was started since 1995 (1). A few small series of 1–3 CDC children were reported afterwards (2-8). Our center firstly published a larger series (n=35) in 2004 (9). Laparoscopic cyst excision and hepaticojejunostomy becomes an alternative surgical treatment for CDC children. Since 2011, we firstly started single-incision laparoscopic cyst excision and hepaticojejunostomy (SILH) (10,11), and extended this technique to complicated cases (12-15). To date, 1401 CDC children successfully underwent SILHs in our center.
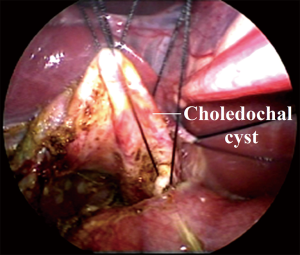
SILH is described as followings. A 2–2.5 cm umbilical longitudinal incision is made and stretched horizontally. A 5-mm telescope is placed at the midline of incision. Two 3-mm conventional laparoscopic instruments are placed at the ends of umbilical incision bilaterally. A series of trans-abdominal retraction sutures are placed through serosa of gallbladder fundus, proximal common hepatic duct (CHD), proximal to distal CDC to facilitate dissection and anastomosis (Figure 1).
The laparoscopic cyst excision and hepaticojejunostomy is performed according to different subtypes (16,17). In patient with stenotic distal common bile duct, dissection is carried out along lateral-anterior-distal-posterior direction. The distal CDC is transected at the level of stenotic segment. Distal stump ligation is optional to avoid pancreatic duct injury induced by excessive dissection. Ten percent of this subgroup associated hepatic duct stricture, requiring surgical correction. Intrahepatic ducts are inspected and irrigated by normal saline to remove stone debris. Thus, prevents post-operative stone formation and cholangitis. In patient with non-stenotic distal common bile duct, the cyst was transected at middle segment. Dissection was conducted circumferentially and continued distally to proximal biliary-pancreatic junction to prevent pancreatic duct injury. Distal stump should be ligated to avoid pancreatic leak. In this subgroup, 62.3% patients combined with protein plugs in common channel. Hence, clearance of protein plugs is necessary to prevent pancreatitis.
The advantages of SILH are (11):
- Trans-umbilical approach through the access that the gallbladder and cyst removal and Roux loop establishment;
- Using conventional laparoscopic instruments saves the cost of expensive curved instruments and special training. Hence, this technique can be promoted in each laparoscopic center;
- Similar operative time and post-operative complication rate compared with conventional laparoscopic hepaticojejunostomy;
- Immature muscle and fascial layer and relatively small surgical field in children allows more freedom of instrument movements and facilitates SILH with straight instruments.
Hence, SILH can be a viable option as a surgical treatment of CDC.
Robotic-assisted cyst excision and hepaticojejunostomy has been utilized in clinical practice since 2006. The 3D visualization and the additional degrees of freedom are major advantages. One camera port, three robotic instrument ports and one assistant port are required. The mean operative time is significantly longer than that of conventional laparoscopic procedures (18-23). Postoperative bile leak was reported (18). However, larger size, repeated launches of robotic system in different steps of one operation, lack of haptics, increased hospital cost restricts its application in CDC treatment. Recently, a hybrid of conventional laparoscopy and robotic technique, i.e., using robotic system in the CDC dissection and hepaticojejunostomy and conventional laparoscopy in the rest surgical steps, is adopted to avoid repeated launches of robotic platform. It maximizes the advantages of two approaches, minimizes the difficulty of robotic manipulation, and shortens operative time.
With the technical improvement, refined transumbilical robotic system and artificial intelligence will provide an optimal alternative to achieve the advanced goal of minimal invasive surgery, i.e., scarless, minimal surgical trauma, and steep learning curve.
General principles in laparoscopic hepaticojejunostomy
Individualized short Roux loop
Conventionally, adult’s standard 35–40 cm Roux loop is applied in CDC children. With increased age, the small bowel is significantly lengthened. A redundant Roux loop is prone to induce intestinal obstruction requiring redo surgery (24), bile stasis (25), bacterial overgrowth (26), cholangitis (26), stone formation, and mal-absorption of fat and fat-soluble vitamins (27). The Roux loop was tailored in our series according to the distance between umbilicus and hepatic hilum. The comparison study indicated that the individualized short Roux loop provides a comparable antireflux effect (28), and prevents Roux loop rotation and necrosis.
Laparoscopic clearance of protein plugs in the common channel
Post-operative pancreatic stone formation and pancreatitis are attributed to unresolved protein plugs/calculi in the common channels (29,30). In the era of open surgery, inspection and irrigation of the common channel are difficult, and increase the risk pancreatic duct injury (31).
We utilized laparoscopic-assisted irrigation through a catheter (32). Repeated intra-operative cholangiogram via the catheter can verify the clearance (32). The liver function and serum amylase in all the patients normalized in long-term follow-up (32). No pancreatic leak occurred (32,33). Our outcomes demonstrate that the catheter irrigation provides a comparable effect compared to flexible endoscope irrigation. The fine-caliber catheter is suitable for neonates and infants. It is an alternative option for protein plug removal in the centers where the flexible endoscopes are not available.
Management of single/multiple hepatic duct strictures
Our large-scale series verifies associated hepatic duct strictures in 11% CDC children (33,34), which often results in cholestasis, intrahepatic stone formation, and cholangitis. As an alternative of choledochoscope, laparoscope can be inserted into intrahepatic bile duct for careful inspection. The optimal proximal CDC resection level could be easily decided without hepatic duct injury or cyst remaining (35). The ductoplasty is carried out as followings.
Single hepatic duct stricture: the anterior wall of common/left/right hepatic duct is split. Ductoplasty and wide hepaticojejunostomy are carried out.
Multiple hepatic duct strictures: the stenotic segments should be split to the proximal dilated bile duct. After remove the intrahepatic duct calculi, a wide hepaticojejunostomy is conducted at this level to prevent post-operative biliary obstruction (33,34). No intrahepatic duct stone formation or cholangitis are developed in our mid- to long-term follow-up.
Management of aberrant hepatic duct (AHD)
Improper management of AHD often leads to bile leak or biloma after CDC operation, requiring further surgery (36-38). Recognizing different subtypes of AHDs and treating them accordingly is effective to prevent relevant complications.
We categorize AHD into 4 subtypes, and treat them individually:
- Type 1: AHD locates close to the conjunction of cystic duct and CHD. The AHD and CHD are combined as one stoma or sutured along their lateral walls to form an anastomotic stoma;
- Type 2: AHD locates in the mid-portion of cystic duct. Anastomosis of AHD and CHD to jejunum are carried out separately;
- Type 3: duplication of cystic duct. The duplicated cystic duct is ligated before being divided to prevent bile leak;
- Type 4: associated with aberrant right hepatic artery (RHA). In case of RHA anteriorly compressing CHD and AHD (type 4a). Aberrant RHA is repositioned behind. The AHD and CHD are combined as one anastomotic stoma. In case of RHA anteriorly compressing CHD alone, the connection between AHD and CHD is transected (type 4b). After repositioning the RHA behind CHD, the lateral walls of AHD and CHD are sutured to form an anastomotic stoma.
In our series, none of patients developed anastomotic stricture, cholangitis, or bile leak. Postoperative liver function tests and serum amylase level normalized within 1 year. Individualized laparoscopic ductoplasty and hepaticojejunostomy provides an efficacious surgical option for CDC children with AHDs.
Management of aberrant RHA
We firstly found that the aberrant RHA anteriorly compressing proximal CHD is a major factor attributed to the postoperative biliary re-obstruction (27%). To prevent postoperative biliary obstruction, the aberrant RHA is carefully mobilized from the proximal CHD, and repositioned behind the proximal CHD (39-41).
To prevent RHA injury, the assistant pulls up a retraction suture through proximal CHD to increase the space between RHA and CHD in dissection, and expose posterior wall and repositioned RHA in anastomosis.
Laparoscopic application in complicated CDCs
Neonatal CDCs
With the advents of ultrasonographic screenings, perinatally diagnosed CDCs significantly increase (42). In our center, the percentage of prenatally diagnosed CDC increased from 16% (2001 to 2010) to 37.7% (in 2017). Conventionally, postponed definitive surgery is postponed for 6 months because of difficulties of anaesthesia and surgery. In era of open surgery, anastomotic stricture and leakage have been reported (43,44). For neonates with giant CDCs, staged surgeries with external biliary drainages are utilized.
However, ultrasonographic studies found a large amount of sludge in CDC at birth in majority of antenatally diagnosed asymptomatic CDC patients (45,46). The accumulation of sludge often results in severe distal obstruction, even perforation (47). We randomized prenatally diagnosed CDC into early and late operation groups, i.e., underwent definitive surgery within and after the first month of life. The biliary obstruction and liver damage in the late operation group were significantly severer, and was similar to that in cystic biliary atresia. Liver fibrosis rate was significantly higher in the late operation group (45). Perforation and coagulopathy, including subdural hemorrhage requiring craniectomy frequently occurred and severer in infants younger than 1 year old (48-52). While, staged surgery often induced dehydration, electrolyte imbalance, infection, adhesion induced incidental injury. Hence, early definitive surgery in neonatal period is advocated.
Laparoscope with magnified vision and finer instruments increases the accuracy of anastomosis. It minimizes the anastomotic leakage and stricture, which are the main concerns in open surgery (43). Hence, more and more CDC neonates or antenatally diagnosed CDC patients undergo definitive surgery in the neonatal periods. In our series, the youngest CDC patients (cyst diameter: 10 cm) successfully underwent one-stage cyst excision and hepaticojejunostomy at Day 3 after birth, and recovered uneventfully.
Giant CDCs
Giant CDC occupies large space in abdominal cavity, particularly in neonates and younger infants. It significantly increases the difficulty and risk of incidental injury. Traditionally, it is thought to be a contra-indication of one-stage laparoscopy definitive surgery.
To create adequate working space, we puncture the cyst with a 20 G angiocatheter and evacuate the contents (13). A series of retraction sutures were placed from proximal to distal CBD to facilitate distal and posterior wall dissections (13). Severe CHD dilatation in these patients obscures the border of CHD and CDC. Transecting the proximal CDC to (I) identify the CHD orifice under direct vision to prevent injury of hepatic ducts; (II) clearly detect portal vein and hepatic artery from transection plane, and dissect posterior wall bi-directionally to gradually minimize the difficult dissection area (13).
Perforated CDCs
Conventionally, perforated CDC is thought to be a contraindication for laparoscopic treatment because of adhesions, deranged anatomy, and demanding techniques. Two-stage surgery is usually adopted. The one-stage definitive surgery via open approach has been utilized in case reports with selected patients (53,54). An attempt of laparoscopic one-stage cyst excision and hepaticojejunostomy was made for perforated CDC recently (55).
In our practice, placing a series of retraction suture and adjusting the tension and direction of suture retraction are helpful to facilitate dissection, particularly when severe inflammation obscures the margin between perforated site and surrounding tissues (15). The electro-hook and forceps are alternately used in cyst dissection. The forceps have a superior hemostasis effect for actively bleeding from intramural vessels of cyst wall (15). In case that severe inflammatory cyst wall was too fragile to place the retraction suture, blunt dissection is adopted. The intrapancreatic dissection area is closed by a double layer running suture to minimize oozing (15). The anterior cyst wall is incised to differentiate the border between perforated site and surrounding tissues in direct vision. Localized mucosectomy in the perforated area is recommended to prevent the injuries of surrounding tissues (15). In some patients, the perforated site sealed with surrounding tissues and forms a bile pseudocyst. The pseudocyst should be distinguished from CDC. Pseudocyst fenestration is recommended to prevent iatrogenic injury of normal anatomical structures (15).
Redo surgery
We categorize the cause of post-operative biliary re-obstruction into 2 groups: congenital and technical. Congenital factors include aberrant RHA anteriorly compressing proximal CHD, and unsolved single or multiple hepatic duct strictures. Technical factor is anastomotic stricture (14).
In our series, 27% re-obstruction originates from aberrant RHA. After dissection of adhesions, a series of sutures are placed through gallbladder fossa for liver retraction. The second suture is placed through anterior wall of proximal CHD to facilitate dissection and redo anastomosis. The vascular pulse should be noticed during dissection, particularly when adhesions cover the RHA and obscure vascular pulse. Aberrant RHA is carefully mobilized and repositioned behind CHD. Intrahepatic duct stones are removed. Ductoplasties and redo hepaticojejunostomy are carried out (14).
Thirty percent re-obstruction results from unrecognized single or multiple hepatic duct strictures. The stenotic segments should be split to the proximal dilated bile duct. A wide hepaticojejunostomy is then performed at this level to prevent biliary re-obstruction (14).
Forty-three percent re-obstructions are attributed to anastomotic stricture. Ductoplasty and redo hepaticojejunostomy are conducted (14).
Long-term follow-up results
Our large series demonstrated that after the learning curve, the operative time in laparoscopic group significantly decreased, and comparable to that in the open procedure (3.04 hours of laparoscopic group vs. 2.95 hours of open group, P=0.56) (33). By now, 1,855 CDC children successfully underwent laparoscopic definitive surgeries in our center. The laparoscopic definitive surgery could be accomplished within 1.5 hours for uncomplicated CDCs, and 3 hours for complicated CDCs. So far, 115 CDC patients have been followed up over 10 years. Compared with data in open group in previous reports and our series, patients in laparoscopic group have faster recovery, superior cosmetic outcomes, and lower morbidities (33). The results are accorded with those in Vietnam report (56). The application of laparoscope significantly decreases the morbidity in the era of open surgery. It attributed to meticulous dissection and anastomosis under the magnified view provided by telescope, technical improvement, and an accumulation of experience.
Biliary atresia
The biliary atresia is treated according to different subtypes (12): (I) correctable biliary atresia with proximal CHD diameter ≥1 cm: hepaticojejunostomy; (II) uncorrectable biliary atresia, including type I and II biliary atresia with proximal CHD diameter <1 cm, and type III biliary atresia: Kasai portoenterostomy.
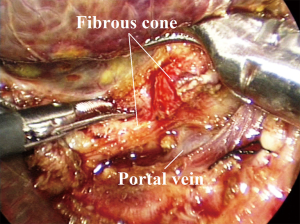
Laparoscopic cyst excision and hepaticojejunostomy for correctable biliary atresia are similar to those in CDC (12). Laparoscopic Kasai portoenterostomy are described in previous studies (Figure 2) (57-63). The fibrous cone is recommended to be dissected by a 3-mm laparoscopic scissors because the electro-cautery or ultrasonic scalpel may destroy the small bile duct, subsequently affect post-operative biliary drainage.
The outcomes of laparoscopic treatment for type III biliary atresia are debatable to date. Our short- to intermediate-term results of laparoscopic group (n=48), including jaundice clearance and native liver survival rates, were comparable to those of open group (n=47) (57). The operative time of laparoscopic group was longer than that of the open group (57). These results are similar to larger series reports from Japan (n=22) (58) and mainland China (n=80 and n=49 respectively) (59,60). While, small series from Germany (n=12) (61), Hong Kong (n=9, n=11 respectively) (62,63) showed that the native liver survival rates of laparoscopic group were lower than that of open group.
CO2 pneumoperitoneum is assumed to contribute to the poor results of laparoscopic Kasai procedure for biliary atresia patients with liver dysfunction and fibrosis because it temporarily altered the metabolism and function of hepatic macrophages (61). However, CO2 pneumoperitoneum does not affect the results of CDC children with liver dysfunctions and/or hepatic fibrosis who undergo laparoscopic surgeries. Poor results in previous small series of laparoscopic Kasai portoenterostomy may result from (I) difficult portal plate dissection, hemostasis, and anastomosis. Large case accumulation is required to improve techniques. Recent study reviewed outcomes of 80 patients with type III biliary atresia who underwent laparoscopic Kasai procedures. They divided the learning curve into 4 phases, each included 20 patients. After of the first 40 patients, the operative time and blood loss were remarkably reduced, and jaundice clearance and 2 years native liver survival rate remarkably increased (59); and (II) biliary atresia classification. There is no comparative study to evaluate the efficiency of laparoscopic versus open surgery in different subtypes of biliary atresia. A large sample RCT study with long-term follow-up is warranted.
Solitary liver cyst/cystic dilatation of main hepatic duct
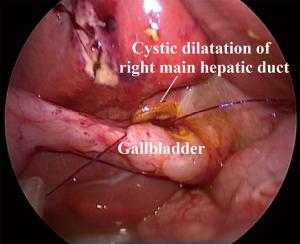
Solitary hepatic cysts with biliary communications (HC) and cystic dilatations of the main intrahepatic ducts (CIHD) are often associated biliary obstruction, causing cholestasis, stone formation, cholangitis, liver damage, and carcinoma in adulthood (64-66). Conventionally, Roux-Y cysto-jejunostomy is adopted. However, this procedure disrupts major biliary system (64,65). The bacteria migration increases the risk of cholangitis (67,68). Post-operative morbidities include anastomotic leak (67,68), anastomotic stenosis (69), hemorrhage (67), wound infection (67,68), intra-abdominal abscess (67,68), and Roux loop necrosis (70). We first employed laparoscopic cysto-cholecystostomy for children who have HC with biliary communication or CIHD (n=20 respectively) (71).
The cysts are dissected out and de-roofed. The orifice of intrahepatic ducts in HC can be detected by telescope. A longitudinal incision is made on the gallbladder based on the caliber of the de-roofed cysts. A side-to-side cysto-cholecystostomy is carried out (Figure 3) (71).
No bile leak, anastomotic stenosis, stone formation or cholangitis was detected in the intermediate-term follow-up (71). Liver function reversed to normal level after surgery (71). Laparoscopic cysto-cholecystostomy provides a safe, simpler, less disruptive and more physiological biliary drainage for HC with biliary communication and CIHD in children.
Progressive familial intrahepatic cholestasis (PFIC)
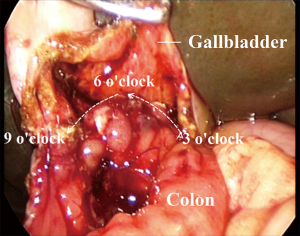
PFIC usually induces hepatic cirrhosis early childhood. Surgical treatments, including partial external biliary diversion (72,73), ileoileal bypass procedure (74), partial internal biliary diversion (75), and liver transplantation (76) are often associated chronic diarrhea, cholangitis, or lifelong immuno-suppression. Furthermore, recurrence is a major concern even after liver transplantation (77). We herewith developed a cholecystocolostomy with anti-reflux Y-loop. It diverts bile from the gallbladder to the descending colon (78).
The transverse colon is transected proximal to splenic flexure. To establish the Y-loop, an end-to-side anastomosis is carried out between the distal transverse colon and mid-descending colon extracorporeally. The Y-loop is individually tailored based on the distance between the umbilicus and the gallbladder. With assistance of laparoscope, a longitudinal incision is made on the gallbladder, and end-to-side cholecystocolostomy is performed. The diameter of the anastomosis ranged from 2.5 to 3.0 cm (Figure 4) (78).
The mean operative time was 2.02 hours. All patients were jaundice free after 7 to 20 days and pruritus subsided in 3 to 14 days. Liver function parameters significantly improved postoperatively. Success rate (normalization of serum bile acids at postoperative 12 months) (79) was 85%. No mortality, diarrhea, cholangitis, or intrahepatic reflux was observed.
A cholecystocolostomy with anti-reflux Y-loop simplifies the surgery, remains the integrity of the small intestine, decreases post-operative complication rate, and offers comparable even superior results with previous surgical interventions (80).
Symptomatic gallstone disease, hemolytic diseases, and biliary dyskinesia
Laparoscopic cholecystectomy is adopted in children with symptomatic gallstone disease, hemolytic diseases, and biliary dyskinesia (81,82). A review of 20,246 patients who underwent laparoscopic cholecystectomies showed that after learning curve, the postoperative recovery and cosmetic outcomes were superior to those in open cholecystectomies (83).
Conclusions
In summary, laparoscopic treatments for bile duct diseases in children are safe. The outcomes are comparable or superior to those of open surgical procedures. Both patients and pediatric surgeons have benefited from these revolutionary techniques.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Kenneth K. Y. Wong and Patrick Ho Yu Chung) for the series “Paediatric Minimally Invasive Surgery” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2018.11.07). The series “Paediatric Minimally Invasive Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Farello GA, Cerofolini A, Rebonato M, et al. Congenital choledochal cyst: video-guided laparoscopic treatment. Surg Laparosc Endosc 1995;5:354-8. [PubMed]
- Lee H, Hirose S, Bratton B, et al. Initial experience with complex laparoscopic biliary surgery in children: biliary atresia and choledochal cyst. J Pediatr Surg 2004;39:804-7. [Crossref] [PubMed]
- O’Rourke RW, Lee NN, Cheng J, et al. Laparoscopic biliary reconstruction. Am J Surg 2004;187:621-4. [Crossref] [PubMed]
- Tan HL, Shankar KR, Ford WD. Laparoscopic resection of type I choledochal cyst. Surg Endosc 2003;17:1495. [Crossref] [PubMed]
- Shimura H, Tanaka M, Shimizu S, et al. Laparoscopic treatment of congenital choledochal cyst. Surg Endosc 1998;12:1268-71. [Crossref] [PubMed]
- Watanabe Y, Sato M, Tokui K, et al. Laparoscope-assisted minimally invasive treatment for choledochal cyst. J Laparoendosc Adv Surg Tech A 1999;9:415-8. [Crossref] [PubMed]
- Liu DC, Rodriguez JA, Meric F, et al. Laparoscopic excision of a rare type II choledochal cyst: case report and review of the literature. J Pediatr Surg 2000;35:1117-9. [Crossref] [PubMed]
- Chowbey PK, Katrak MP, Sharma A, et al. Complete laparoscopic management of choledochal cyst: report of two cases. J Laparoendosc Adv Surg Tech A 2002;12:217-21. [Crossref] [PubMed]
- Li L, Feng W, Jing-Bo F, et al. Laparoscopic-assisted total cyst excision of choledochal cyst and Roux-en-Y hepatoenterostomy. J Pediatr Surg 2004;39:1663-6. [Crossref] [PubMed]
- Diao M, Li L, Dong N, et al. Single-Incision Laparoscopic Roux-en-Y Hepaticojejunostomy Using Conventional Instruments for Children with Choledochal Cysts. Surg Endosc 2012;26:1784-90. [Crossref] [PubMed]
- Diao M, Li L, Li Q, et al. Single-incision versus conventional laparoscopic cyst excision and Roux-Y hepaticojejunostomy for children with choledochal cysts: a case-control study. World J Surg 2013;37:1707-13. [Crossref] [PubMed]
- Diao M, Li L, Cheng W. Single-incision laparoscopic hepaticojejunostomy using conventional instruments for neonates with extrahepatic biliary cystic lesions. Surg Innov 2013;20:214-8. [Crossref] [PubMed]
- Diao M, Li L, Li Q, et al. Challenges and strategies for single-incision laparoscopic Roux-en-Y hepaticojejunostomy in managing giant choledochal cysts. Int J Surg 2014;12:412-7. [Crossref] [PubMed]
- Diao M, Li L, Cheng W. Laparoscopic redo hepaticojejunostomy for children with choledochal cysts. Surg Endosc 2016;30:5513-9. [Crossref] [PubMed]
- Diao M, Li L, Cheng W. Single-incision laparoscopic hepaticojejunostomy for children with perforated choledochal cysts. Surg Endosc 2018;32:3402-9. [Crossref] [PubMed]
- Diao M, Li L, Cheng W. Congenital biliary dilatation may consist of two disease entities. J Pediatr Surg 2011;46:1503-9. [Crossref] [PubMed]
- Diao M, Li L, Cheng W. Is it necessary to ligate distal common bile duct stumps after excising choledochal cysts? Pediatr Surg Int 2011;27:829-32. [Crossref] [PubMed]
- Chang EY, Hong YJ, Chang HK, et al. Lessons and tips from the experience of pediatric robotic choledochal cyst resection. J Laparoendosc Adv Surg Tech A 2012;22:609-14. [Crossref] [PubMed]
- Dawrant MJ, Najmaldin AS, Alizai NK. Robot-assisted resection of choledochal cysts and hepaticojejunostomy in children less than 10 kg. J Pediatr Surg 2010;45:2364-8. [Crossref] [PubMed]
- Kang CM, Chi HS, Kim JY, et al. A case of robot-assisted excision of choledochal cyst, hepaticojejunostomy, and extracorporeal Roux-en-Y anastomosis using the da Vinci surgical system. Surg Laparosc Endosc Percutan Tech 2007;17:538-41. [Crossref] [PubMed]
- Meehan JJ, Elliott S, Sandler A. The robotic approach to complex hepatobiliary anomalies in children: preliminary report. J Pediatr Surg 2007;42:2110-4. [Crossref] [PubMed]
- Woo R, Le D, Albanese CT, et al. Robot-assisted laparoscopic resection of a type I choledochal cyst in a child. J Laparoendosc Adv Surg Tech A 2006;16:179-83. [Crossref] [PubMed]
- Akaraviputh T, Trakarnsanga A, Suksamanapun N. Robotassisted complete excision of choledochal cyst type I, hepaticojejunostomy and extracorporeal Roux-en-Y anastomosis: a case report and review literature. World J Surg Oncol 2010;8:87. [Crossref] [PubMed]
- Wang HZ. Congenital biliary dilatation. In: Li Z, Wang HZ, Ji SJ (eds). Practical pediatric surgery. Beijing: People’s Health Publishing House, 2001:1060-75.
- Ducrotte P, Peillon C, Guillemot F, et al. Could recurrent cholangitis after Roux-en-Y hepaticojejunostomy be explained by motor intestinal anomalies? A manometric study. Am J Gastroenterol 1991;86:1255-8. [PubMed]
- Zhao YW, Huang DH. The relationship between choledochojejunostomy and post-operative cholangitis. Changchun: Norman Bethune Medical University, 1994.
- Suzuki M, Tanaka K, Ohtani K, et al. Estimation of postoperative fat absorption using the 13C mixed-triglyceride breath test in children with choledochal cyst. Eur J Pediatr 2009;168:35-8. [Crossref] [PubMed]
- Diao M, Li L, Zhang JS, et al. A shorter loop in Roux-Y hepatojejunostomy reconstruction for choledochal cysts is equally effective: preliminary results of a prospective randomized study. J Pediatr Surg 2010;45:845-7. [Crossref] [PubMed]
- Miyano T, Yamataka A, Kato Y, et al. Choledochal cysts: special emphasis on the usefulness of intraoperative endoscopy. J Pediatr Surg 1995;30:482-4. [Crossref] [PubMed]
- Miyano T, Yamataka A, Kato Y, et al. Hepaticoenterostomy after excision of choledochal cyst in children: a 30-year experience with 180 cases. J Pediatr Surg 1996;31:1417-21. [Crossref] [PubMed]
- Li SL, Zhang DR, Li YC, et al. Prevention and treatment for pancreatic duct injury during the excision of choledochal cyst. Chin J Pediatr Surg 2000;21:211-3.
- Diao M, Li L, Zhang JS, et al. Laparoscopic-assisted clearance of protein plugs in the common channel in children with choledochal cysts. J Pediatr Surg 2010;45:2099-102. [Crossref] [PubMed]
- Diao M, Li L, Cheng W. Laparoscopic versus open Roux-en-Y hepatojejunostomy for children with choledochal cysts: intermediate-term follow-up results. Surg Endosc 2011;25:1567-73. [Crossref] [PubMed]
- Li L, Liu SL, Hou WY, et al. Laparoscopic correction of biliary duct stenosis in choledochal cyst. J Pediatr Surg 2008;43:644-6. [Crossref] [PubMed]
- Miyano T, Li L, Yamada K. Choledochal cyst. In: Gupta D, Sharma S, Azizkhan R (eds). Pediatric surgery-diagnosis and management. New Delhi: Jaypee Brother Medical Publishers (P) Ltd., 2009:1013-25.
- Takahashi T, Shimotakahara A, Takahashi T, et al. Choledochal cyst associated with an accessory hepatic duct identified by intra-operative endoscopy: case report and literature review. Pediatr Surg Int 2008;24:1079-82. [Crossref] [PubMed]
- Goor DA, Ebert PA. Anomalies of the biliary tree: report of a repair of anaccessory bile duct and review of the literature. Arch Surg 1972;104:302-9. [Crossref] [PubMed]
- Momiyama T, Souda S, Yoshikawa Y, et al. Injury to a duplicated cystic duct during laparoscopic cholecystectomy. Surg Laparosc Endosc 1996;6:315-7. [Crossref] [PubMed]
- Diao M, Li L, Cheng W. Recurrence of Biliary Tract Obstructions after Primary Laparoscopic Hepaticojejunostomy in Children with Choledochal Cysts. Surg Endosc 2016;30:3910-5. [Crossref] [PubMed]
- Todani T, Watanabe Y, Toki A, et al. Coexisting biliary anomalies and anatomical variants in choledochal cyst. Br J Surg 1998;85:760-3. [Crossref] [PubMed]
- Lal R, Behari A, Hari RH, et al. Variations in biliary ductal and hepatic vascular anatomy and their relevance to the surgical management of choledochal cysts. Pediatr Surg Int 2013;29:777-86. [Crossref] [PubMed]
- Makin E, Davenport M. Understanding choledochal malformation. Arch Dis Child 2012;97:69-72. [Crossref] [PubMed]
- Redkar R, Davenport M, Howard ER. Antenatal diagnosis of congenital anomalies of the biliary tract. J Pediatr Surg 1998;33:700-4. [Crossref] [PubMed]
- Okada T, Sasaki F, Ueki S, et al. Postnatal management for prenatally diagnosed choledochal cysts. J Pediatr Surg 2004;39:1055-8. [Crossref] [PubMed]
- Diao M, Li L, Cheng W. Timing of surgery for prenatally diagnosed asymptomatic choledochal cysts: a prospective randomized study. J Pediatr Surg 2012;47:506-12. [Crossref] [PubMed]
- O’ Neill JA, Goran AG, Fonkalsrud E. Choledochal cyst. Mosby, St. Louis: Pediatric Surgery, 2006:1620-34.
- Howell CG, Templeton JM, Weiner S, et al. Antenatal diagnosis and early surgery for choledochal cyst. J Pediatr Surg 1983;18:387-93. [Crossref] [PubMed]
- Evans K, Marsden N, Desai A. Spontaneous perforation of the bile duct in infancy and childhood: a systematic review. J Pediatr Gastroenterol Nutr 2010;50:677-81. [Crossref] [PubMed]
- Ono S, Tokiwa K, Aoi S, et al. A bleeding tendency as the first symptom of a choledochal cyst. Pediatr Surg Int 2000;16:111-2. [Crossref] [PubMed]
- Chen TY, Wang HK, Yeh ML, et al. Subdural hemorrhage as a first symptom in an infant with a choledochal cyst: case report. J Neurosurg Pediatr 2012;9:414-6. [Crossref] [PubMed]
- Krstovski N, Janic D, Dokmanovic L, et al. Late vitamin K deficiency bleeding in an infant with choledochal cyst. Turk J Pediatr 2010;52:652-4. [PubMed]
- Fumino S, Iwai N, Deguchi E, et al. Bleeding tendency as a first symptom in children with congenital biliary dilatation. Eur J Pediatr Surg 2007;17:2-5. [Crossref] [PubMed]
- Fumino S, Iwai N, Deguchi E, et al. Spontaneous rupture of choledochal cyst with pseudocyst formation-report on 2 cases and literature review. J Pediatr Surg 2006;41:e19-21. [Crossref] [PubMed]
- Winkler RE, Lancry K, Velcek FT. Choledochal cyst with perforation- an unusual presentation. Case report and review of the literature. S Afr J Surg 2001;39:95-6. [PubMed]
- Ilyas MIM, Tieman J, Alkhoury F. Laparoscopic single stage procedure for perforated choledochal cyst. J Ped Surg Case Reports 2015;3:436-9. [Crossref]
- Liem NT, Pham HD, Vu HM. Is the laparoscopic operation as safe as open operation for choledochal cyst in children? J Laparoendosc Adv Surg Tech A 2011;21:367-70. [Crossref] [PubMed]
- Sun X, Diao M, Wu XZ, et al. A Prospective Study Comparing Laparoscopic and Conventioanl Kasai Portoenterostomy in Children with Biliary Atresia. J Pediatr Surg 2016;51:374-8. [Crossref] [PubMed]
- Cazares J, Koga H, Murakami H, et al. Laparoscopic portoenterostomy for biliary atresia: single-center experience and review of literatures. Pediatr Surg Int 2017;33:1341-54. [Crossref] [PubMed]
- Li Z, Ye Y, Wu Z, et al. Learning Curve Analysis of Laparoscopic Kasai Portoenterostomy. J Laparoendosc Adv Surg Tech A 2017;27:979-82. [Crossref] [PubMed]
- Li Y, Xiang B, Wu Y, et al. Medium-term Outcome of Laparoscopic Kasai Portoenterostomy for Biliary Atresia With 49 Cases. J Pediatr Gastroenterol Nutr 2018;66:857-60. [Crossref] [PubMed]
- Ure BM, Kuebler JF, Schukfeh N, et al. Survival with the native liver after laparoscopic versus conventional Kasai portoenterostomy in infants with biliary atresia: A prospective trial. Ann Surg 2011;253:826-30. [Crossref] [PubMed]
- Wong KK, Chung PH, Chan KL, et al. Should open Kasai portoenterostomy be performed for biliary atresia in the era of laparoscopy? Pediatr Surg Int 2008;24:931-3. [Crossref] [PubMed]
- Chan KWE, Lee KH, Wong HYV, et al. Ten-Year Native Liver Survival Rate After Laparoscopic and Open Kasai Portoenterostomy for Biliary Atresia. J Laparoendosc Adv Surg Tech A 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Charlesworth P, Ade-Ajayi N, Davenport M. Natural history and long-term follow-up of antenatally detected liver cysts. J Pediatr Surg 2007;42:494-9. [Crossref] [PubMed]
- Longmire WP Jr, Mandiola SA, Gordon HE. Congenital cystic disease of the liver and biliary system. Ann Surg 1971;174:711-26. [Crossref] [PubMed]
- Weimann A, Klempnauer J, Gebel M, et al. Squamous cell carcinoma of the liver originating from a solitary non-parasitic cyst case report and review of the literature. HPB Surg 1996;10:45-9. [Crossref] [PubMed]
- Nagino M, Nishio H, Ebata T, et al. Intrahepatic cholangiojejunostomy following hepatobiliary resection. Br J Surg 2007;94:70-7. [Crossref] [PubMed]
- Kapoor VK, Pradeep R, Haribhakti SP, et al. Intrahepatic segment III cholangiojejunostomy in advanced carcinoma of the gallbladder. Br J Surg 1996;83:1709-11. [Crossref] [PubMed]
- Cahow CE. Intrahepatic cholangiojejunostomy: a new simplified approach. Am J Surg 1979;137:443-8. [Crossref] [PubMed]
- Hashimoto D, Arita T, Kuroki H, et al. Acute afferent loop necrosis after Roux-en-Y cholangiojejunostomy. Clin J Gastroenterol 2010;3:165-7. [Crossref] [PubMed]
- Diao M, Li L, Cheng W. Cysto-cholecystostomy - A More Physiological Procedure for Hepatic Cysts with Biliary Communications and Cystic Dilatations of Main Intrahepatic Ducts. World J Surg 2018;42:2599-605. [Crossref] [PubMed]
- Whitington PF, Whitington GL. Partial external diversion of bile for the treatment of intractable pruritus associated with intrahepatic cholestasis. Gastroenterology 1988;95:130-6. [Crossref] [PubMed]
- Emond JC, Whitington PF. Selective surgical management of progressive familial intrahepatic cholestasis (Byler’s disease). J Pediatr Surg 1995;30:1635-41. [Crossref] [PubMed]
- Hollands CM, Rivera-Pedrogo FJ, Gonzalez-Vallina R, et al. Ileal exclusion for Byler’s disease: an alternative surgical approach with promising early results for pruritus. J Pediatr Surg 1998;33:220-4. [Crossref] [PubMed]
- Bustorff-Silva J, Sbraggia Neto L, Olimpio H, et al. Partial internal biliary diversion through a cholecystojejunocolonic anastomosis-a novel surgical approach for patients with progressive familial intrahepatic cholestasis: a preliminary report. J Pediatr Surg 2007;42:1337-40. [Crossref] [PubMed]
- Davit-Spraul A, Gonzales E, Baussan C, et al. Progressive familial intrahepatic cholestasis. Orphanet J Rare Dis 2009;4:1. [Crossref] [PubMed]
- Hori T, Nguyen JH, Uemoto S. Progressive familial intrahepatic cholestasis. Hepatobiliary Pancreat Dis Int 2010;9:570-8. [PubMed]
- Diao M, Li L, Zhang JS, et al. Laparoscopic Cholecystocolostomy: A Novel Surgical Approach for Treatment of Progressive Familial Intrahepatic Cholestasis. Ann Surg 2013;258:1028-33. [Crossref] [PubMed]
- Schukfeh N, Metzelder ML, Petersen C, et al. Normalization of serum bile acids after partial external biliary diversion indicates an excellent long-term outcome in children with progressive familial intrahepatic cholestasis. J Pediatr Surg 2012;47:501-5. [Crossref] [PubMed]
- Davis AR, Rosenthal P, Newman TB. Nontransplant surgical interventions in progressive familial intrahepatic cholestasis. J Pediatr Surg 2009;44:821-7. [Crossref] [PubMed]
- Svensson J, Makin E. Gallstone disease in children. Semin Pediatr Surg 2012;21:255-65. [Crossref] [PubMed]
- Halata MS, Berezin SH. Biliary dyskinesia in the pediatric patient. Curr Gastroenterol Rep 2008;10:332-8. [Crossref] [PubMed]
- Dagash H, Chowdhury M, Pierro A. When can I be proficient in laparoscopic surgery? A systematic review of the evidence. J Pediatr Surg 2003;38:720-4. [Crossref] [PubMed]
Cite this article as: Diao M, Li L. Pediatric minimal invasive surgery—bile duct diseases. Ann Laparosc Endosc Surg 2018;3:100.