Laparoscopic ventral hernia repair: extraperitoneal repair
Introduction
The optimal operative management of primary ventral hernia and incisional hernia is still debatable. No single treatment has been able to tackle all ventral and incisional hernias. LeBlanc and Booth in 1993 first reported application of intra-peritoneal onlay mesh (IPOM) for ventral and incisional hernia (1). It is a relatively straight forward procedure which in comparison to open mesh repair has been found to reduce chances of surgical site and mesh infection (2-4). However the technique requires expensive fixation devices which may cause acute and chronic pain.
The laparo endoscopic groin hernia repair using synthetic mesh in TEP or TAPP are acceptable surgical techniques today (5,6). These techniques are rarely associated with mesh induced complications, the reason being extraperitoneal placement of synthetic mesh. It is apparent that despite great progress in mesh technology, nearly all types of meshes have been found to produce a varying level of adhesion or tissue reaction, regardless of the material and coating used. Preoperatively unpredictable, a mesh-induced visceral complication may occur in some patients to produce severe reaction or major mesh-related adverse events (7). The incitation to develop certain novel minimally invasive techniques that enables researchers to bring the mesh out of abdominal cavity has been an exciting trend in laparoscopic hernia repair. Various extraperitoneal techniques available for ventral hernia repair in literature are:
- Transabdominal pre peritoneal repair (TAPP);
- Transabdominal retromuscular repair;
- Transabdominal partially extraperitoneal repair (TAPE);
- Enhanced-view totally extraperitoneal repair (eTEP);
- Endoscopic mini/less open sublay technique/repair (EMILOS);
- Robotic transabdominal preperitoneal repair (rTAPP).
TAPP
TAPP refers to the laparoscopic ventral hernia and incisional hernia repair whereby mesh is placed in the preperitoneal space, similar to TAPP and TEP for inguinal hernia repair. It involves use of a transabdominal pre peritoneal approach and can be used for midline and lateral hernias.
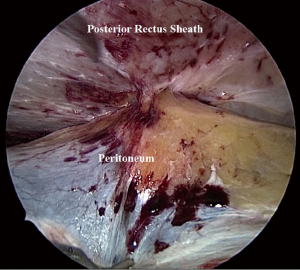
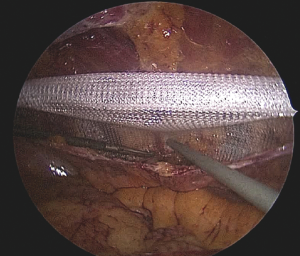
After positioning the camera and working ports, an important consideration is to create a peritoneal pocket or flap for accommodating a mesh. The working ports and camera port are placed at a distance of 3–4 cm from the planned peritoneal incision. Using a reverse 30° laparoscope, the peritoneum is incised and preperitoneal space opened with blunt and sharp dissection (Figure 1). Dissection of preperitoneal plane is technically easier in the medial compartment of upper abdomen due to preperitoneal fat.
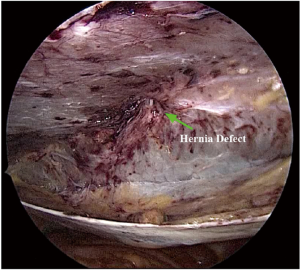
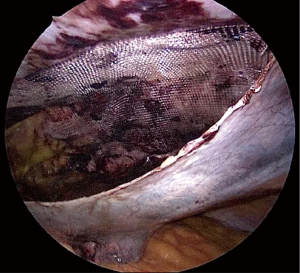
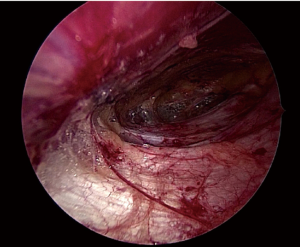
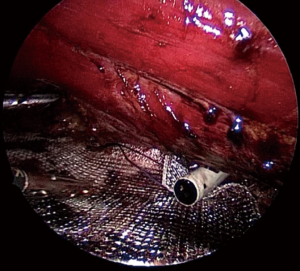
Damage to the overlying skin is particularly avoided at the umbilical region, if there is just hernial sac and fat below the skin. The optimal ergonomics is important and this is facilitated by positioning of the patient to have a contralateral downward tilt. The hernia sac may be dissected last after the peritoneal flap creation has been completed on both sides (Figures 2,3). A synthetic polypropylene mesh is inserted and positioned in preperitoneal space that has been created and positioned such that central part of the mesh stays at the centre of the hernial defect. It is recommended to place two stay sutures, one at the 6 o’clock and other at the 12 o’clock position before inserting mesh into the operative space (Figure 4). Lesser fixation of mesh to the abdominal wall may be required with this technique. Iatrogenically created peritoneal defects are to be sutured. The available omentum is spread out on the surface of bowel to act as a protective barrier. Having fewer fixation points in TAPP repair may reduce the trauma to nerves by tackers used in IPOM and thus reduces postoperative pain (8-10).
The drawbacks of this procedure have been its technical difficulty, longer operative time and poor reproducibility. The peritoneal dissection often results in multiple peritoneal tears which may result in bowel getting exposed to the underlying mesh.
Transabdominal retromuscular repair
It is a well-known fact that by creating a potential space behind the rectus muscle, a vascularised pocket for mesh can be achieved. Stoppa et al. (11) have introduced the retromuscular hernia repair which became popular and is widely performed by surgeons. Laparoscopic transabdominal retromuscular repair is a minimally invasive approach to the open Rives Stoppa retromuscular sublay repair for ventral hernia. One 10-mm port and two intraperitoneal 5-mm ports and placed at least 10 cm away from the hernial defect in the left flank region. The content of the hernial sac is reduced and limited adhesiolysis performed.
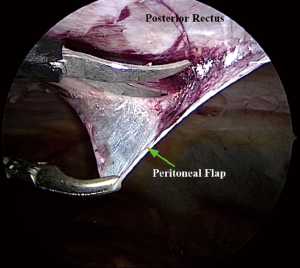
The peritoneum and posterior rectus are opened by a longitudinal incision made through a 5-mm port, nearly 5 cm from the defect of hernia. The plane (beneath the rectus abdominis) is then created by sharp dissection to form a flap of fascia, posterior rectus sheath (wherever present), and peritoneum. The hernial sac is pulled down and transected close to abdominal wall leaving the distal sac in situ. Midline crossover is performed close to the linea alba to enter into the contralateral retromuscular space. The composite peritoneal flap is developed to accommodate an adequately sized polypropylene mesh, which is placed in the retromuscular space to cover the hernial defect along with previous scar with a minimum surrounding margin of 4–5 cm.
The polypropylene mesh fits snugly in the created retrorectus space and may be fixed at few places with transabdominal sutures or metallic fixation devices. Alternatively fibrin sealant may be used to fix the mesh. The composite peritoneal flap is then closed by means of a continuous suture or application of metallic fixation devices.
Though an effective procedure, again technical difficulties have limited its spread among the surgeons. Moreover, retromuscular space is not easily accessible in the flank regions of upper abdomen.
TAPE
This technique is suitable for laparoscopic repair of Suprapubic hernias where lower margin is within 5 cm from pubic arch. These hernias are known to occur after transverse or low vertical incisions in gynecological, urological or bowel related surgery (12). The repair of suprapubic hernias is as such difficult due to absent posterior rectus sheath, proximity to urinary bladder and essential neurovascular structures (13).
After catheterization and creation of pneumoperitoneum the port placement is chosen on the basis of nature and position of the previous surgical scar. Two additional ports are made on the lateral abdominal wall, mainly on the left flank to assess the hernial defect, previous scar and perform adhesiolysis. This allows space for adequate prosthetic overlap. The hernial content is completely reduced to expose the fascial borders of the defect.
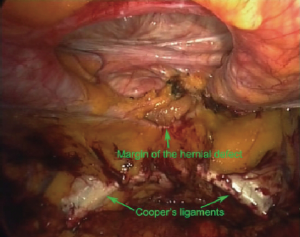
A peritoneal flap is dissected starting close to one anterior superior iliac spine (ASIS) and extended to the contralateral ASIS (Figure 5). The flap is inferiorly dissected till space of Retzius so that pubic arch and Cooper’s ligaments on either side are completely exposed (Figure 6). Medially, dissection is meticulous and carefully done avoiding any sort of iatrogenic injury to the urinary bladder.
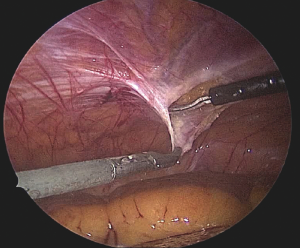
A composite mesh of the appropriate size is chosen so that an overlap of at least 5 cm is achieved around the defect. The lower margin of the mesh must extend below the pubic arch by 1–2 cm to a low firm fixation to Cooper’s ligaments bilaterally (Figure 7).
The rolled mesh is passed through a 10-mm or 12-mm port is spread inside the abdomen in a manner so that that the surface with adhesion barrier faces the abdominal viscera. The mesh is pulled up against the abdominal wall using four transabdominal sutures. Spiral tacks are used to fix the mesh at two points on the Cooper’s ligaments on both sides. Circumferential fixation is also done at the mesh margins and around the margins of the hernial defect in the manner of double crowning. The peritoneal flap raised initially is made to reperitonealize the maximum possible part of the mesh. For wide hernial defects and Swiss-cheese defects, two or more meshes may be required.
The main advantage of TAPE technique is suitable mesh overlap of more than 5 cm from the distal margin of the hernial defect. The fixation of the lower mesh margin to the Cooper’s ligaments on either side increases the strength of repair. The procedure does allow part of the mesh to remain in the extraperitoneal space. Since polypropylene meshes can be safely used, it allows to minimize the cost of procedure.
TAPE technique in few series has also been applied for lumbar hernia repairs (14).
eTEP approach
The eTEP approach has been described previously for inguinal hernia repair (15,16). In ventral hernia repair, it relies on initiation of dissection in one retrorectus space and then crossover to the contralateral retrorectus space. The initial port set-up and point of crossover depends on the location of defect. Patients with long midline laparotomy scar are a relative contraindication to this technique.
While dealing with upper midline defects, crossover is performed below the level of the umbilicus, to develop a pre-peritoneal and retromuscular space. The first incision is just medial to linea semilunaris approximately 2 cm below a horizontal line drawn through umbilicus. The anterior rectus sheath is sharply incised (Figure 8). With the help of balloon dissector a retrorectus space is developed in cephalad and caudal directions avoiding any sort of over-inflation which may cause injury to the rectus muscle. The Retzius space once entered, two additional ports are placed under direct vision in the lower abdomen (Figure 9). A 30-degree scope enters through third port and dissection is carried out in the retrorectus plane in the cephalad direction. The posterior rectus sheathes once identified are released from below upwards to connect the retrorectus spaces.
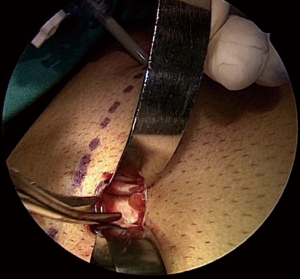
For lower midline defects, the dissection is initiated in the upper portion of left retrorectus space. Using balloon dissector at the first port position left retrorectus space is developed, and further ports are placed into the created space. Blunt dissection is then performed in caudal direction in left retrorectus space to identify the pubis. Incision is made in medial aspect of left posterior rectus sheath and preperitoneal space is entered superficial to falciform ligament. After identifying posterior rectus on right side, medial aspect of it is incised and released from above downwards followed by blunt dissection into the space. Fourth port is made under direct vision through the upper aspect of right rectus abdominis muscle for its further usage as camera port. Retrorectus dissection finally goes in the caudal direction to complete the release of posterior rectus sheaths bilaterally. Hernial sac once encountered is sharply dissected and made to release the distal attachments of the sac. Any defects in posterior layer are repaired. Finally a polypropylene mesh is deployed into the widely created space (Figure 10). The mesh is secured with tacks, transfacial sutures or a fibrin sealant.
For challenging defects (>10 cm) that require large mesh placement, transverse abdominal muscle release (TAR) procedure may be added.
EMILOS technique
This technique is basically a reversed total extraperitoneal (TEP) procedure which has been designed for midline, epigastric, umbilical or incisional hernia with coexisting rectal diastasis. It utilizes the original MILOS concept (mini/less open sublay) which has been introduced by Reinpold (17). A large mesh (20×30) is implanted in the retromuscular space via a small skin incision (2–8 cm) without any fixation.
The patient placement is in French position and during endoscopic part of the operation and the surgeon stands between patient’s legs. A 3–6-cm transverse skin incision is made over the hernia sac followed by its dissection and clear exposition of hernial ring. The rim of the fascial defect is lifted while the peritoneum and hernia sac including the preperitoneal fat are pushed down on both sides from the posterior wall of the rectus sheath at a distance of about 1–2 cm. The endoscopic part (E) of the MILOS operation starts by incising one side of the posterior rectus sheath. The opened fascial rims are held by sutures.
An indigenous balloon like in TEP is prepared and pushed into the extraperitoneal space anterior to the symphysis to create a space meant for introduction of a 12 mm camera port which is also utilized later to introduce a mesh. Initially introduced 10-mm port through the wound is removed and operation continues momentarily like in MILOS technique (17). The opposite side of the posterior sheath is incised on both sides and continued caudally and cranially. Care is taken to preserve the linea alba. Blunt detachment of the posterior rectus sheath is performed using the curved sponge forceps. Provisionally skin incision is tightly closed and 10-mm optic (30°) introduced via a 12-mm port into the preperitoneal space proximal to the symphysis pubis. The operating surgeon standing between the patient’s legs and the monitor lies behind the head of patient for endoscopic visualization of retromuscular space. The endoscopic dissection i.e., reversed TEP is carried out after introduction of 5-mm working trocars on each side lateral to the midline in medio-clavicular line nearly about 3–5 cm above the umbilicus.
Incision of posterior rectus sheath continues upwards till costal margin and xiphoid process. The space behind the costal margin and the sternum is opened for later mesh placement. Complete blunt detachment of the fascia from the rectus muscle is carried out while preserving the nerves and vessels laterally perforating the fascia.
Ten-mm optic trocar is introduced through the rectus under view about 5–7 cm superior to the working trocars. Incision of the posterior rectus sheath continues downwards to the arcuate line. The Retzius space is opened and dissected down till pubic bone. A large macroporous mesh (20 cm × 30 cm) is prefixed with 4–6 holding loops placed close to the rim to facilitate its positioning. The mesh remains placed flat within the retromuscular space.
The disadvantage of this approach is its technical difficulty, learning curve and prolonged operative time.
rTAPP
Hernia repair by robotic approach is an emerging minimal access technique which utilizes set principles of open as well as conventional laparoscopic techniques. The popularity of this technique is growing in the west, attributed to its enhanced precision, 3D vision and surgeon ergonomics. The robotic platform allows exploration of the individual abdominal wall layers (18) and subsequent mesh placement in a preperitoneal, retromuscular, and onlay position.
Robotic surgery alleviates the challenges presented by laparoscopic surgery. Inherent limitations of conventional rigid laparoscopic instruments make operating high on the anterior abdominal wall difficult. The articulating instruments and high degree of freedom offered by robotic platform can help to compensate for arduous maneuvers required to perform an extraperitoneal ventral hernia repair. The experience with robotic TAPP is in evolution and has been demonstrated to be safe and feasible in small retrospective case series (19).
Generally, TAPP repair for primary ventral hernias like epigastric and umbilical hernias can be completed using single docking; though for incisional hernias it is preferable to employ double‐docking technique.
The technique is best utilized for small or medium size hernias (<6 cm) that do not demand component separation in order to reconstitute the linea alba. Hernia in atypical locations like flank, suprapubic, and subxiphoid defects can be readily repaired by this approach.
A concern regarding the use of robotic surgery is its cost.
Studies in literature
In an experimental study, Díaz-Pizarro Graf et al. (20) showed that the laparoscopic TAPP approach to place a polypropylene mesh in a swine model for repair of ventral hernia was technically feasible with less adhesion formation with the mesh (88.81% vs. 27.27%, P=0.032) and less interloop adhesion formation (81.81% vs. 9.09% P=0.003). Macroscopically, the adhesions were less firm when the mesh was placed preperitoneally than intraperitoneally (P=0.001). They further observed that preperitoneal polypropylene mesh does induce fibrous reaction by its direct contact with the muscle fibres that enhances its integration with the adjacent tissues.
Chowbey et al. (21) in a series of 34 patients who had a median duration of follow up as 16.5 months demonstrated that placement of a polypropylene mesh in preperitoneal space is technically feasible with an advantage over laparoscopic IPOM repair for primary ventral and incisional hernia in selected group of patients.
Sharma et al. (22) retrospectively studied 72 patients of suprapubic hernia. Mean diameter of the hernial defect was 5.2 cm and overall complication rate as 27.8%. No recurrences occurred in their series. They demonstrated that a low cost polypropylene mesh can be safely used as larger part of the mesh remains in the extraperitoneal space.
Recently Belyansky et al. (23) in 79 patients conducted a retrospective review of laparoscopic retromuscular hernia repair cases using eTEP approach from five hernia centres. They concluded that eTEP approach offers flexible port set-up which is optimal for defect closure, along with wide coverage of mesh in the retromuscular space with limited transfascial fixation.
Schwartz et al. (24) developed EMILOS technique utilizing MILOS concept (mini/less open sublay) in 25 out of 33 cases and showed that mesh can be placed in retromuscular space without entering the abdominal cavity and making a large skin incision. They showed its benefits in patients of ventral hernia with coexisting rectus diastasis.
The experience with robotic TAPP is still evolving, though small retrospective case series have shown it to be a safe and feasible procedure.
Conclusions
The mesh placement in extraperitoneal or preperitoneal space allows an even distribution of forces along the surface area of mesh enabling adequate abdominal wall reinforcement and extensive tissue growth. In addition, extraperitoneal mesh implantation reduces the need for extensive surgical fixation and therefore avoids complications related to the intra peritoneal position of the mesh as well as need for fixation devices, especially in the suprapubic or subxiphoid region. Moreover, placement of mesh in extraperitoneal space obviates the need for more expensive coated meshes required in laparoscopic intraperitoneal onlay repair. The approaches are technically demanding than laparoscopic intraperitoneal mesh repair, at least for the beginners. However extraperitoneal techniques deserve to be compared in a prospective multicentric randomized trial with open incisional as well as laparoscopic intraperitoneal ventral hernia repair to establish their long term benefits.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Laparoscopic and Endoscopic Surgery for the series “Ventral Hernia”. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2018.09.07). The series “Ventral Hernia” was commissioned by the editorial office without any funding or sponsorship. AS served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- LeBlanc KA, Booth WV. Laparoscopic repair of incisional abdominal hernias using expanded polytetrafluoroethylene: Preliminary findings. Surg Laparosc Endosc 1993;3:39-41. [PubMed]
- Barbaros U, Asoglu O, Seven R, et al. The comparison of laparoscopic and open ventral hernia repairs: A prospective randomized study. Hernia 2007;11:51-6. [Crossref] [PubMed]
- Forbes SS, Eskicioglu C, McLeod RS, et al. Meta-analysis of randomized controlled trials comparing open and laparoscopic when the compartment is disrupted by lateral dissection in ventral and incisional hernia repair with mesh. Br J Surg 2009;96:851-8. [Crossref] [PubMed]
- Kaoutzanis C, Leichtle SW, Mouawad NJ, et al. Prospective surgical site infections after ventral/incisional hernia repair: A comparison of open and laparoscopic outcomes. Surg Endosc 2013;27:2221-30. [Crossref] [PubMed]
- Golani S, Middleton P. Long-term follow-up of laparoscopic total extraperitoneal (TEP) repair in inguinal hernia without mesh fixation. Hernia 2017;21:37-43. [Crossref] [PubMed]
- Muschalla F, Schwarz J, Bittner R. Effectivity of laparoscopic inguinal hernia repair (TAPP) in daily clinical practice. Early and long term result. Surg Endosc 2016;30:4985-94. [Crossref] [PubMed]
- Tung KLM, Cheung HYS, Tang CN. Non-healing enterocutaneous fistula caused by mesh migration. ANZ J Surg 2018;88:E73-74. [Crossref] [PubMed]
- Colak E, Ozlem N, Kucuk GO, et al. Prospective randomized trial of mesh fixation absorbable versus nonabsorbable tacker in laparoscopic ventral incisional hernia repair. Int J Clin Exp Med 2015;8:21611-6. [PubMed]
- Sajid MS, Parampalli U, McFall MR. A meta-analysis comparing tacker mesh fixation with suture mesh fixation in laparoscopic incisional and ventral hernia repair. Hernia 2013;17:159-66. [Crossref] [PubMed]
- Bansal VK, Misra MC, Kumar S, et al. A prospective randomized study comparing suture mesh fixation versus tacker mesh fixation for laparoscopic repair of incisional and ventral hernias. Surg Endosc 2011;25:1431-8. [Crossref] [PubMed]
- Stoppa RE, Warlaumont CR, Verhaeghe PJ, et al. Prosthetic treatment in the repair of groin hernias. Int Surg 1986;71:154-8. [PubMed]
- Carbonell AM, Kercher KW, Matthews BD, et al. The laparoscopic repair of suprapubic ventral hernias. Surg Endosc 2005;19:174-7. [Crossref] [PubMed]
- Varnell B, Bachman S, Quick J, et al. Morbidity associated with laparoscopic repair of suprapubic hernias. Am J Surg 2008;196:983-7; discussion 987-8. [Crossref] [PubMed]
- Palanivelu C, Rangarajan M, John SJ, et al. Laparoscopic transperitoneal repair of lumbar incisional hernias: a combined suture and ‘double-mesh’ technique. Hernia 2008;12:27-31. [Crossref] [PubMed]
- Daes J. The enhanced view-totally extraperitoneal technique for repair of inguinal hernia. Surg Endosc 2012;26:1187-9. [Crossref] [PubMed]
- Daes J. The extended-view totally extraperitoneal e-TEP technique for inguinal hernia repair. In: Novitsky YW. editor. Hernia surgery, current principles. New York: Springer, 2016:467-72.
- Reinpold W. Endoskopisch total extraperitonealer transhernialer sublay Bauchwand-Hernienverschluss in single port-technik. In: Schumpelick V, Arlt G, Conze J, et al. editors. Hernien, 5th edn. Stuttgart: Thieme, 2015:301-4.
- Ballantyne GH, Hourmont K, Wasielewski A. Telerobotic laparoscopic repair of incisional ventral hernias using intraperitoneal prosthetic mesh. JSLS 2003;7:7-14. [PubMed]
- Sugiyama G, Chivukula S, Chung PJ, et al. Robot-assisted transabdominal preperitoneal ventral hernia repair. JSLS 2015;19:e2015.00092.
- Díaz-Pizarro Graf JI, Moreno Portillo M, Cardenas Lailson LE, et al. Laparoscopic transabdominal preperitoneal approach to place a polypropylene mesh on the abdominal wall: an experimental swine model of a technique that can be used for incisional hernia repair. Surg Endosc 2005;19:990-5. [Crossref] [PubMed]
- Chowbey PK, Sharma A, Khullar R, et al. Laparoscopic ventral hernia repair with extraperitoneal mesh: surgical technique and early results. Surg Laparosc Endosc Percutan Tech 2003;13:101-5. [Crossref] [PubMed]
- Sharma A, Dey A, Khullar R, et al. Laparoscopic repair of suprapubic hernias: transabdominal partial extraperitoneal (TAPE) technique. Surg Endosc 2011;25:2147-52. [Crossref] [PubMed]
- Belyansky I, Daes J, Radu VG, et al. A novel approach using the enhanced-view totally extraperitoneal (eTEP) technique for laparoscopic retromuscular hernia repair. Surg Endosc 2018;32:1525-32. [Crossref] [PubMed]
- Schwarz J, Reinpold W, Bittner R. Endoscopic mini/less open sublay technique (EMILOS)—a new technique for ventral hernia repair. Langenbecks Arch Surg 2017;402:173-80. [Crossref] [PubMed]
Cite this article as: Shahdhar M, Sharma A. Laparoscopic ventral hernia repair: extraperitoneal repair. Ann Laparosc Endosc Surg 2018;3:79.