How I do it: laparoscopic anatomical segment 8 resection
Introduction
For the liver cancer surgery especially hepatocellular carcinoma, anatomical resection of the cancerous segment or section, with the corresponding portal territories removed as appropriate, should theoretically be considered the gold standard surgical approach and can reduced the recurrence rate. While the results of anatomical or non-anatomical resection still is debated, the rational of anatomical resection is preferred in many centers (1,2).
Segment 8 is considered as the largest segment with the highest anatomical variation of the liver. The posterosuperior location of segment 8, its boundaries represented by the right and middle hepatic veins (MHVs) at the caval confluence, the complete absence of anatomical landmarks on the liver surface, particularly in the cirrhotic liver, and the vague boundary between segments 5 and 8 are the main reasons accounting for its complexity (3). Anatomical resection of segment 8 is generally considered a demanding procedure.
Since the first consensus of laparoscopic liver surgery, laparoscopic segment 8 resection was categorized in the most complicated and difficult procedure category (4). Nowadays, advancement of laparoscopic camera, instruments, surgical techniques and knowledge can make segment 8 resection more feasible and safer than in the past (5). It is important to emphasize that this procedure remain challenging and should only be attempted by surgeons with advanced experience in laparoscopic liver resection (LLR).
At our institution, LLR has been adopted since 2010. Although the essential procedures were similar to those in open segmentectomy, that is, anatomical area of segment was determined by Glissonian pedicle approach, and guided by intra-parenchymal hepatic veins (6,7). The aim of this study was to review detail of the knowledge and demonstrate the laparoscopic surgical techniques to complete anatomically accurate segment 8 resection.
Anatomy of segment 8
Anatomy of segment 8 consists with the complexity and controversial issues. According to the classification of Couinaud, segment 8 corresponds to the anterosuperior portion of right paramedian sector, which lies between the middle and the right hepatic veins (RHVs) (8). The exact limit of medial and lateral borders are not visible on the liver surface, while the inferior border adjacent to segment 5 is controversial due to the lack of a clear anatomical landmarks. For inflow of segment 8, there are many portal venous ramification patterns. Kogure reported that the third-order of the portal vein of the right paramedian sector could be divided into two distinct groups; ventral and dorsal branches, which were separated by a vertical fissure. The upper three-quarter area of this sector was fed by 6 to 8 third order portal branches ramifying from the tip of the second order portal branch, whereas the lower quarter area was fed with the unique third order portal branches ramifying from the root of the second order portal branch (9). Takayasu et al. described the division of the portal branch to segment 8 into four subsegmental branches that appear, counterclockwise, as ventral, dorsolateral, dorsal, and medial branches (10).
Instead of the craniocaudal segmentation introduced by Couinaud, in 1951, Hjortsjo proposed the concept of ventro-dorsal segmentation. The anterior section is divided into two segments that is ventral and dorsal segment by a vertical fissure (11). Hjortsjo indicated that a hepatic vein lying in it defines the vertical fissure. Some authors name this vein “anterior fissure vein (AFV)”. Base on Hjortsjo’s concept, the anatomical resection of segment 8 defined as combine resection of the superior part of both ventral and dorsal segments (12). This is significantly different concept of the segmental anatomy widely used today.
Kogure reported the study on cadaveric liver specimen and defined the concept of Hjortsjo can classification into ventral and dorsal section for 90% of cases (9). Recently Kobayashi, using 3-dimentional computer software, studied the anatomy of the anterior section. This paper reported that the classic Couinaud’s classification was observed in 53% of patients, the Hjortsjo’s concept seen in only 23% and the remaining were variations which did not match either Couinaud or Hjortsjo’s system (13).
Consequently, the anatomical resection of segment 8 could be difficult procedure to perform. By understanding the complexity of segmental anatomy and careful pre-operative evaluation of the patient’s segment 8 anatomy with its supplying portal branches using the high quality imaging of the liver and 3-dimentional simulation systems, the resection can be made more precise.
Patient selection and workup
All our patients undergo basic evaluation using the tool called the “Triple factors” inspection including patient factors, disease factors and operative factors. Patient factors such as age, underlying disease, laboratories, current medication are considered. The type of cancer, underlying liver disease, cirrhosis, Child’s score, previous treatment are examples of disease factors. As for operative factors, we look at the difficulty level and complexity of operation amongst other factors. From the basic evaluation, we excluded extremely aged patient, those with life-threatening underlying condition, poor liver function such as Child’s score B or C or patients with advanced cancer.
For the advance evaluation, each patient undergo high quality, thin-sliced computed tomography (CT) scan, and/or magnetic resonance imaging (MRI) with hepatocyte specific contrast injection, the test of indocyanine green retention at 15 minutes (ICG-R15) and liver volumetry to indicate future liver remnant volume (14). We used the 3-dimentional analyzing software to evaluate the variation of vascular anatomy of segment 8. This step is necessary for selection the approach to ligate all of segment 8 pedicles when perform the operation. There are two approaches to expose the segmental pedicles; the central approach (CA) and the anterior approach (AA). If the corresponding portal pedicles of segment 8 are branching near the hilar region, we prefer CA. On the other hand, if its pedicles are far from hilum, we choose AA (15-18). We will detail these approaches in procedure section.
Preoperative preparation
Patient should admitted one day before operation to re-check basic blood test and cross match for 2 unit of packed red cell and 4 unit of fresh frozen plasma. Platelet supplement are rarely required if the platelet count is more than 100,000/µL. Patient are fasted after midnight before operative day and fluid supplement is given as maintenance fluid balance on the morning before the operation. After standard general anesthesia, a small caliber nasogastric tube and urine catheter were inserted. The invasive monitoring such as central venous pressure line or intra-arterial line was used only in selected patient with concomitant heart, lung or kidney disease.
Equipment preference card
- ϖ High definition, two-dimension laparoscopic system;
- ϖ 10 mm, 30º angle camera;
- ϖ Carbon dioxide gas supplement system;
- ϖ 2 of 12 mm trocars;
- ϖ 3 of 5 mm trocars;
- ϖ Cavitron Ultrasonic Surgical Aspirator (CUSA; IntegraTM Life Sciences Corporation. NJ, USA);
- ϖ LigaSureTM Maryland (Covidien, Tokyo, Japan);
- ϖ The endo-clamp (B. Braun, Tuttlingen, Germany);
- ϖ Cord tape & Tiemann’s catheter for extracorporeal Pringle’s manoeuvre;
- ϖ Endo Retract Maxi & Endo Mini Retract (Covidien, Tokyo, Japan);
- ϖ Polymer locking clip and applier;
- ϖ Specimen retrieval bag.
Procedures
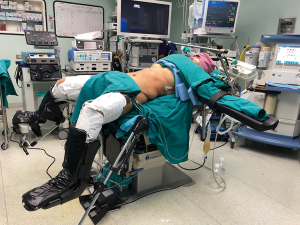
Patient was placed on low lithotomy with slightly right-side up position (Figure 1). The surgeon was standing between the patient’s legs, while camera operator and assistant were standing on the left side of the patient. The camera port is placed at supra-umbilical area. If the liver is shriveled and located high in the rib cage, the port should be placed higher. The 2 surgeon’s working ports were always placed at right upper quadrant area just beneath the line of costal margin. The 2 assistant’s working ports are placed at subxiphoid and left upper quadrant area.
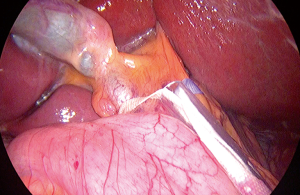
After general exploration, the round and falciform ligament were transected by bipolar sealing device until the supra-hepatic area was exposed and then dissected to identify inferior vena cava (IVC), RHV, MHV or common trunk (Figure 2). Intraoperative laparoscopic ultrasonography was used to detect the location of the tumor. Hepatoduodenal ligament was encircled with an umbilical tape for the extracorporeal Pringle’s manoeuvre (Figure 3). Cholecystectomy was done as standard for all cases.
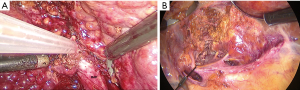
Caudal traction of the round ligament after releasing the right triangular ligament may facilitate exposure and transection. We use two approaches and two landmark veins, MHV and RHV, to determine the segment 8 territories. The first technique is CA which begins with taking down the hilar plate by gentle dissection using the tip of a suction device to expose the left edge of the right portal pedicle. The dissection at the junction between right anterior and right posterior portal pedicle was proceeded until the right anterior portal pedicle was apparent and then encircled (Figure 4). Tracking down the right anterior portal pedicle to expose the segment 8 pedicle inside the liver parenchyma, sometime partial hepatotomy using CUSA should be done for better exposure (Figure 5A,B). Dissection to identify ramification between segment 5 and segment 8 pedicle was proceeded then the segment 8 pedicle was encircled (Figure 6). We prefer to dissect the hilar area clearly under temporary inflow control by Pringle’s manoeuvre and take care not to injure the fine branches derived from the pedicle to avoid the bile leakage afterward. Segment 8 pedicle was temporarily clamped and was confirmed it was actually the segment 8 branch (Figure 7).
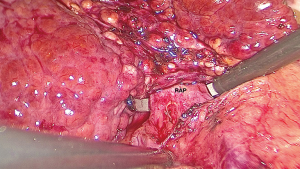
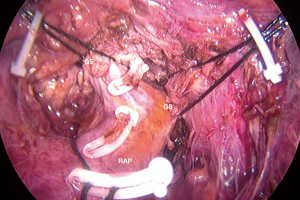
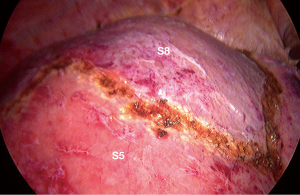
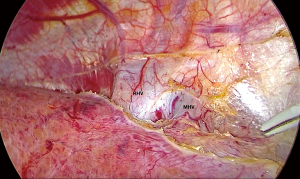
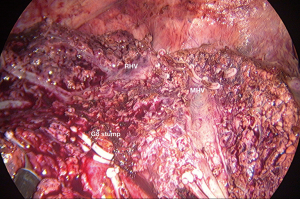
After the border of the segment 8 was identified, the parenchyma was transected firstly at the medial border using CUSA. To identify the intersegmental plane in the liver, dissection continues by tracing the thin hepatic vein from the superficial part of the liver until the main trunk is exposed (Figure 8). Once the trunk of MHV was identified, parenchymal transection was proceeding along the ventral surface of the MHV until its root was exposed. The thick drainage vein to MHV such as segment 8 vein (V8) or AFV were identified and ligated individually (Figure 9). Next, the parenchyma at the border between segment 5 and 8 was transected downward from demarcation line. Segment 8 pedicle was widely exposed during the parenchymal transection and was finally ligated and divided. The limit of the posterior dissection plane is the line between the caval confluence and segment 8 pedicle. We prefer to transect the parenchyma at the lateral border (plane between segment 7 and 8) from the root side of RHV and proceed caudally along the medial edge of its trunk. Finally, the segment 8 parenchyma was transected from RHV trunk to the demarcation line at the liver surface to complete the procedure (Figure 10).
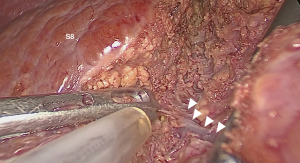
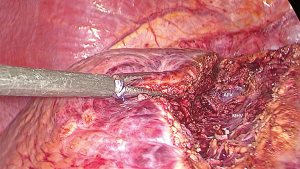
For the second technique, AA start with taking down the hilar plate and the right portal pedicle was identified same as CA. The endo-clamp was applied at the right portal pedicle. The demarcation of right liver was revealed and the line was marked. Liver parenchymal transection along this line should be done on upper part then carried down to identified MHV and its branches. After ligation of these branches, the ventral pedicle of segment 8 was identified and temporarily clamped to confirm the subsegmental demarcation then ligated and transected. At this step, the inferior border of segment 8 was partially revealed. The parenchymal transection along this line will facilitate to expose the dorsal pedicle of segment 8. After all superior dorsal branches were ligated, the whole segment 8 was demarcated then the resection can be done with the same manner as CA.
Specimen was retrieved via specimen retrieval bag and was removing by extended the umbilical incision for the small specimen. For the large specimen, suprapubic incision was made to remove the resected liver. Drainage tube was not routinely required.
Postoperative management
Intensive care is not routinely needed unless the patients have significant co-morbidities or advanced age. Early recovery after surgery (ERAS) protocol can be applied to all patients if there were no contraindication. The patient can be started on liquid diet on the first day after operation and progressed to soft diet the next day. The patient can be discharged home on the second or third day after the operation and scheduled for follow up for 1 week.
Tips, tricks and pitfalls
Operative table should be adjusted for head and right side up position for better exposure of segment 8. Due to the tilted position, the patient should be carefully restrained during the operation. The hemodynamics of the patient may be changed in elderly patients; the surgeon should adjust the table to desire position to check for this before starting the operation.
The working ports should be placed just below the costal margin because low lying ports may limit the operation due to the insufficient length of instruments during resection high up the rib cage. The use of extra-long instruments or trans-thoracic approach for tall or obese patients has been reported in the literature (19).
Pringle’s manoeuvre should be applied intermittently before starting the hilar dissection and until complete parenchymal transection. This procedure not only reduces bleeding during dissection but also can decrease the liver volume to better identify the Glissonian pedicles in the hilar area.
Pre-operative planning by 3-dimentional software is mandatory to select the appropriate approach. For segmental Glissonian pedicles control, identifying or encircling these branches at hilar region are technically demanding and may injure to the other important structures such as bile duct or hepatic vein. A Hepatotomy along the right anterior pedicle as CA or AA on liver surface along demarcation line should be performed to locate these segmental branches.
A laparoscopic CUSA can be very useful for meticulous perpendicular dissection as well as the parenchymal transection. It was used as the parenchymal dissector to trace the tiny vein to the main trunk and followed the main trunk to its root. It is critical that the main working port for CUSA should be placed as close as possible to the costal margin to minimize the distance between port and target. Caudal traction of the round ligament, traction and counter-traction of the parenchyma may facilitate the parenchymal dissection.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Chung Yip Chan, Ser Yee Lee and Adrian Kah Heng Chiow) for the series “Advanced Laparoscopic HPB Surgery in South East Asia” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2018.07.02). The series “Advanced Laparoscopic HPB Surgery in South East Asia” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Huang X, Lu S. A meta-analysis comparing the effect of anatomical resection vs. non-anatomical resection on the long-term outcomes for patients undergoing hepatic resection for hepatocellular carcinoma. HPB 2017;19:843-9. [Crossref] [PubMed]
- Tan Y, Zhang W, Jiang L, et al. Efficacy and safety of anatomic resection versus nonanatomic resection in patients with hepatocellular carcinoma: A systemic review and meta-analysis. PLoS One 2017;12:e0186930 [Crossref] [PubMed]
- Mazziotti A, Maeda A, Ercolani G, et al. Isolated resection of segment 8 for liver tumors: A new approach for anatomical segmentectomy. Arch Surg 2000;135:1224-9. [Crossref] [PubMed]
- Buell JF, Cherqui D, Geller DA, et al. The international position on laparoscopic liver surgery: The Louisville Statement, 2008. Ann Surg 2009;250:825-30. [Crossref] [PubMed]
- Wakabayashi G, Cherqui D, Geller D, et al. Recommendations for laparoscopic liver resection: A report from the second international consensus conference held in Morioka. Ann Surg 2015;261:619-29. [PubMed]
- Fiorentini G, Ratti F, Cipriani F, et al. Tips and tricks for a laparoscopic approach to paracaval liver segments. Ann Surg Oncol 2018;25:1695-8. [Crossref] [PubMed]
- Ho CM, Wakabayashi G, Nitta H, et al. Total laparoscopic limited anatomical resection for centrally located hepatocellular carcinoma in cirrhotic liver. Surg Endosc 2013;27:1820-5. [Crossref] [PubMed]
- Couinaud C. Liver anatomy: portal (and suprahepatic) or biliary segmentation. Dig Surg 1999;16:459-67. [Crossref] [PubMed]
- Kogure K, Kuwano H, Fujimaki N, et al. Reproposal for Hjortsjo’s segmental anatomy on the anterior segment in human liver. Arch Surg 2002;137:1118-24. [Crossref] [PubMed]
- Takayasu K, Moriyama N, Muramatsu Y. Intrahepatic portal vein branches studied by percutaneous transhepatic portography. Radiology 1985;154:31-6. [Crossref] [PubMed]
- Hjortsjo CH. The topography of the intrahepatic duct systems. Acta Anat (Basel) 1951;11:599-615. [Crossref] [PubMed]
- Taniai N, Machida T, Yoshida H, et al. Role of the anterior fissure vein in ventral or dorsal resection at segment 8 of liver. Eur J Surg Oncol 2018;44:664-9. [Crossref] [PubMed]
- Kobayashi T, Ebata T, Yokoyama Y, et al. Study on the segmentation of the right anterior sector of the liver. Surgery 2017;161:1536-42. [Crossref] [PubMed]
- Majno P, Mentha G, Toso C, et al. Anatomy of the liver: An outline with three levels of complexity – A further step towards tailored territorial liver resections. J Hepatol 2014;60:654-62. [Crossref] [PubMed]
- Ishizawa T, Gumbs AA, Kokudo N, et al. Laparoscopic segmentectomy of the liver: From segment I to VIII. Ann Surg 2012;256:959-64. [Crossref] [PubMed]
- Giuliani A, Aldrighetti L, Benedetto FD, et al. Total abdominal approach for postero-superior segments (7, 8) in laparoscopic liver surgery: a multicentric experience. Updates Surg 2015;67:169-75. [Crossref] [PubMed]
- Martínez-Cecilia D, Fontana M, Siddiqi NN, et al. Laparoscopic parenchymal sparing resections in segment 8: techniques for a demanding and infrequent procedure. Surg Endosc 2018;32:2012-9. [Crossref] [PubMed]
- Jang JY, Han HS, Yoon YS, et al. Three-dimentional laparoscopic anatomical segment 8 liver resection with glissonian approach. Ann Surg Oncol 2017;24:1606-9. [Crossref] [PubMed]
- Lee W, Han HS, Yoon YS, et al. Role of intercostal trocars on laparoscopic liver resection for tumors in segment 7 and 8. J Hepatobiliary Pancreat Sci 2014;21:E65-E68. [Crossref] [PubMed]
Cite this article as: Chanwat R, Bunchaliew C, Tanompetsanga R, Orannapalai N. How I do it: laparoscopic anatomical segment 8 resection. Ann Laparosc Endosc Surg 2018;3:58.