
A double-channel cap-assisted closure of a gastric defect after endoscopic submucosal dissection
Introduction
Recently endoscopic submucosal dissection (ESD) has been widely accepted as a standard of care for early gastric cancer (EGC), which has little risk of lymph-node metastasis, because ESD allows for high en-bloc resection rates with minimal invasiveness (1). However, post ESD bleeding is one of the adverse events after ESD procedure, particularly for patients with antithrombotic therapy (2). Most post ESD bleeding can be controlled by endoscopic hemostasis, however, sometimes massive gastrointestinal bleeding with hemorrhage shock can occur (3,4), so it is clinically important to prevent delayed bleeding, especially in at-risk patients.
The prophylactic closure of mucosal defects after endoscopic resection is attempted to prevent postoperative bleeding. According to previous reports, some closure methods reported are endoloop (5), 8-ring (6) and loop clip (7), but these methods require specialized equipment. For example, the endoloop method requires a double channel endoscope (GIF-2TQ260M; Olympus, Tokyo, Japan) that is not commonly available, particularly in the West.
The impact shooter (TOP Co, Tokyo, Japan) is an innovative device consisting of a transparent hood with a catheter fitted to a standard scope. It forms a second accessory channel on the outside of the scope and allows use of a standard endoscope as double channel endoscope. It is typically used for tissue retraction during ESD (8). We believed that the impact shooter could also be useful for closure of large mucosal defects in difficult location after ESD, using a standard gastroscope. Herein, we report the details of our closure technique using impact shooter for post gastric ESD defect.
Case presentation (Figure 1)
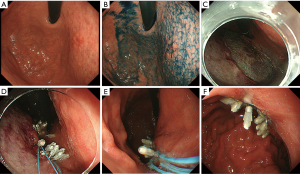
A 73-year-old male underwent esophagogastroduodenoscopy (EGD) for screening for gastric cancer at outside institution, and a gastric lesion was detected. He was taking cilostazol (100 mg/day) for ischemic heart disease.
Preoperative EGD was performed in our institution. Antiplatelet therapy was continued based on Japanese guideline (9). The lesion was seen as depressed area 20 mm in size with slightly marginal elevation located at anterior wall of upper gastric body near the cardia (Figure 1A). Chromoendoscopy with indigo carmine enhanced the margin of the lesion (Figure 1B). The biopsy specimen revealed moderately differentiated adenocarcinoma. ESD was performed for this lesion, and en-bloc resection was achieved without perforation.
A large post ESD mucosal defect was seen, and size was approximately 50 mm in diameter (Figure 1C). First, we attached the impact shooter to a standard endoscope (GIF-Q260J; Olympus, Tokyo, Japan). Next, we inserted the endoloop through the impact shooter, and an endoclip through the accessory channel of endoscope (Figure 2). We grabbed the endoloop with the endoclip and then used the endoclip to anchor the endoloop to the gastric mucosa. Several clips are required to performed complete the closure. Additional endoclips were used to fix the endoloop along the edge of the mucosal defect (Figure 3). As a result, complete closure was achieved with two endoloops (Figure 1D). A second look EGD, 4 days after ESD, demonstrated sustained complete closure of post ESD defect (Figure 1E,F). He was discharged 7 days after ESD without post ESD bleeding


Histopathological examination showed well differentiated adenocarcinoma 15 mm in size confined to mucosa with negative vertical and horizontal margins, without lymphovascular invasion, consistent with curative resection
Discussion
We successfully demonstrated our new technique for closure of mucosal defects after gastric ESD with the use of impact shooter. Oda et al. reported that delayed bleeding after ESD is 5.7% (4). Koh et al. reported that antithrombotic drugs are risk factors for delayed bleeding after gastric ESD (12).
Complete closure of mucosal defect after ESD has a potential to prevent post ESD bleeding. However, complete closure of gastric mucosa is difficult because of the thicker gastric mucosa, compared to other areas of the gastrointestinal tract (esophagus, colon and duodenum). Delayed bleeding rates of gastric ESD is higher than colon ESD (4,13), and sometimes massive gastrointestinal bleeding with hemorrhagic shock can occur, so complete closure is necessary for closure of gastric mucosal defect in at risk patients.
Some favorable results of complete closure of gastric mucosal defect after gastric ESD have been reported (14,15). However, these methods are technically challenging. Abe et al. reported complete closure of a gastric ESD mucosal defect with endoloop and endoclips (16). This method requires a two-channel endoscope to insert both the endoclip and endoloop through accessory channels. Two-channel endoscopes are rarely available in most of countries, particularly in the west, and it is difficult to approach narrow area such as upper gastric body and cardia in retroflexion. Thus, the method with a two-channel scope is not suitable for this case. Therefore, we modified this method by using an impact shooter.
We used the impact shooter as another accessory channel of use of an endoloop. We closed the defect in the almost the same way of previous report using the double channel endoscope. This closure method is a good option for closure of gastric defect mucosa after ESD. Advantages of our method is it is safe and simple and can be performed without a double channel endoscope.
Conclusions
We reported the details of our closure technique using impact shooter for post gastric ESD defect. This method allowed for complete closure of even large defect at narrow portion without two-channel endoscope.
Acknowledgments
Funding: This paper was partially funded by the National Cancer Center Research and Development Fund (25-A-12, 28-K-1 and 29-A-13).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2018.03.15). YS serves as an unpaid editorial board member of Annals of Laparoscopic and Endoscopic Surgery from Dec 2016 to Nov 2018. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gotoda T. Endoscopic resection of early gastric cancer. Gastric Cancer 2007;10:1-11. [Crossref] [PubMed]
- Takeuchi T, Ota K, Harada S, et al. The postoperative bleeding rate and its risk factors in patients on antithrombotic therapy who undergo gastric endoscopic submucosal dissection. BMC Gastroenterol 2013;13:136. [Crossref] [PubMed]
- Takizawa K, Oda I, Gotoda T, et al. Routine coagulation of visible vessels may prevent delayed bleeding after endoscopic submucosal dissection--an analysis of risk factors. Endoscopy 2008;40:179-83. [Crossref] [PubMed]
- Oda I, Gotoda T, Hamanaka H, et al. Endoscopic submucosal dissection for early gastric cancer: technical feasibility, operation time and complications from a large consecutive series. Dig Endosc 2005;17:54-8. [Crossref]
- Matsuda T, Fujii T, Emura F, et al. Complete closure of a large defect after EMR of a lateral spreading colorectal tumor when using a two-channel colonoscope. Gastrointest Endosc 2004;60:836-8. [Crossref] [PubMed]
- Fujii T, Ono A, Fu KI, et al. A novel endoscopic suturing technique using a specially designed so-called “8-ring” in combination with resolution clips (with videos). Gastrointest Endosc 2007;66:1215-20. [Crossref] [PubMed]
- Osada T, Sakamoto N, Ritsuno H, et al. Process of wound healing of large mucosal defect areas that were sutured by using a loop clip-assisted closure technique after endoscopic submucosal dissection of a colorectal tumor. Gastrointest Endosc 2013;78:793-8. [Crossref] [PubMed]
- Motohashi O, Nishimura K, Nakayama N, et al. Endoscopic submucosal dissection (two-point fixed ESD) for early esophageal cancer. Dig Endosc 2009;21:176-9. [Crossref] [PubMed]
- Fujimoto K, Fujishiro M, Kato M, et al. Guidelines for gastroenterological endoscopy in patients undergoing antithrombotic treatment. Dig Endosc 2014;26:1-14. [Crossref] [PubMed]
- Ichijima R, Abe S, Oda I, et al. An endoclip through the accessory channel of endoscope. Asvide 2018;5:395. Available online: http://www.asvide.com/article/view/24270
- Ichijima R, Abe S, Oda I, et al. Additional endoclips were used to fix the endoloop along the edge of the mucosal defect. Asvide 2018;5:396. Available online: http://www.asvide.com/article/view/24271
- Koh R, Hirasawa K, Yahara S, et al. Antithrombotic drugs are risk factors for delayed postoperative bleeding after endoscopic submucosal dissection for gastric neoplasms. Gastrointest Endosc 2013;78:476-83. [Crossref] [PubMed]
- Saito Y, Uraoka T, Yamaguchi Y, et al. A prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video). Gastrointest Endosc 2010;72:1217-25. [Crossref] [PubMed]
- Goto O, Sasaki M, Akimoto T, et al. Endoscopic hand-suturing for defect closure after gastric endoscopic submucosal dissection: a pilot study in animals and in humans. Endoscopy 2017;49:792-7. [Crossref] [PubMed]
- Kobara H, Mori H, Fujihara S, et al. Outcomes of gastrointestinal defect closure with an over the scope clip system in a multicenter experience: An analysis of a successful suction method. World J Gastroenterol 2017;23:1645-56. [Crossref] [PubMed]
- Abe S, Oda I, Mori G, et al. Complete endoscopic closure of large gastric defect with endoloop and endoclips after complex endoscopic submucosal dissection. Endoscopy 2015;47:E374-5. [Crossref] [PubMed]
Cite this article as: Ichijima R, Abe S, Oda I, Nonaka S, Suzuki H, Yoshinaga S, Bhatt A, Saito Y. A double-channel cap-assisted closure of a gastric defect after endoscopic submucosal dissection. Ann Laparosc Endosc Surg 2018;3:38.


