
Minimally invasive treatment of early gastrointestinal cancers
Introduction
Cancer is a leading cause of death worldwide (1). For many years, surgery was the cornerstone treatment of gastrointestinal malignancies, including those at an early stage. Advances in surgical technique and post-operative care have significantly reduced the historically high perioperative mortality and morbidity but they nonetheless remain significant. This has led to exploration of less invasive and subsequently less morbid alternatives (2-4). Endoscopic resection emerged as an organ-preserving option with comparable cancer-free survival rates, and lower morbidity rates than surgical treatment in early cancers (5-8). The first descriptions of injection-assisted endoscopic mucosal resection (EMR) date back to the 1950s, when it was first used for removal of sigmoid polyps through rigid sigmoidoscopy (9). Nowadays EMR is one of the most widely used techniques for neoplasms limited to the superficial layers of the gastrointestinal tract (10). En bloc resection using EMR is limited to lesions smaller than 20 mm, without fibrosis (11). Larger lesions are usually removed in a piecemeal fashion, limiting appropriate histological staging, and jeopardizing the rates of curative resection (11). ESD emerged in the late 1990s as an alternative to overcome this limitation by achieving en bloc resection regardless of size or presence of fibrosis (12-14). It potentially allows definitive histological staging of early gastrointestinal neoplasms, as well as curative treatment. The technique has been widely adopted in the East, with excellent outcomes reported in high-volume centers. However, adoption in the West has been limited, primarily by epidemiological differences, longer procedure times and limited training opportunities (15,16).
The potential for endoscopic therapy has recently extended to include resection of tumors arising from the muscularis propria (17). This technique, known as submucosal tunneling endoscopic resection (STER), explores the submucosal space to provide a non-invasive option for safer removal of submucosal tumors (17).
The accepted indications for endoscopic resection of early gastrointestinal tumors are aligned along the rates of metastasis, the available experience according to geographical distribution, and the perceived risk of the procedure. Regardless of the technique chosen, the optimal method for resection of gastrointestinal tumors should be safe, cost-effective, and achieve complete removal of the lesion and oncologically sound. In this article, we will review the role of EMR, ESD and STER as minimally invasive approaches for removal of early esophageal, gastric and colorectal malignancies.
Techniques
EMR
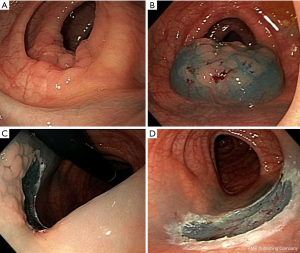
It consists of elevation of the lesion, with either submucosal injection of a solution, or with cap suction, followed by removal using a snare (Figure 1) (18).
ESD
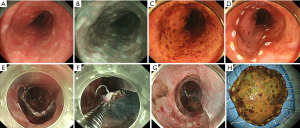
It consists of elevation of the lesion by submucosal injection; circumferential mucosal incision, dissection of the submucosa, and en bloc resection (Figure 2) (19).
Circumferential marking
Before starting the procedure, careful identification and demarcation of the lesion is crucial (20,21). Cautery, argon plasma coagulation, or the tip of a needle-type can be used to mark at 3 to 5 mm from the edge of the lesion. This is performed in order to recognize the borders of the lesion, which can be distorted after submucosal injection. Circumferential marking should be carefully performed to avoid perforation of the thin wall of the esophagus. In most colorectal ESD, this step is unnecessary as margins are properly visualized after chromoendoscopy. Often times, markings can be useful for IIc or LST-NG colorectal lesions.
Submucosal injection
Proper submucosal lifting is essential for safe and efficient ESD. An adequate lifting solution should be long-lasting, safe and non-expensive (22). In Asia, sodium hyaluronate 0.4% (MucoUp; Boston scientific, Tokyo, Japan) and glycerol (Chugai Pharmaceutical Co., Ltd., Tokyo, Japan) have been widely described (23,24). Use of hydroxypropyl methylcellulose 0.4% has been reported in the West (25,26). Addition of indigo carmine to any of these solutions can facilitate differentiation of tissue planes (27). Recently, a blinded randomized controlled trial in an ex-vivo porcine model comparing different submucosal injection solutions demonstrated the superior long-lasting lifting effect of Eleview™ (Cosmo Technologies Ltd, Dublin, Ireland), which is a polymer- and methylene blue-containing solution, when compared with all the submucosal injection fluids available in the West (28).
Circumferential incision
Circumferential incision is performed lateral to the mucosal markings to allow for normal tissue margins. In esophageal and colon ESD, partial circumferential incision is preferred to prevent the fluid leakage from the submucosal layer (29,30).
Submucosal dissection
After exposure of the submucosal layer, the lesion is lifted with injection of a lifting solution. The submucosa can be dissected with an IT knife 2 (KD-611L; Olympus) for gastric ESD, or IT nano (KD-612L/U; Olympus) for esophagus and colon or hook knife (KD-620LR / KD-620UR; Olympus) by hooking and cutting the submucosa, or by contact with the tip of a dual knife (KD-650L/KD-650U; Olympus). The stag beetle knife (MD-47707; Sumitomo Bakelite Co., Ltd), and Mucosectom2 (HOYA Pentax,) have also been used for dissection.
More recently, the clip line traction technique has been commonly used for submucosal dissection in esophageal and gastric ESD (31,32). It allows for improved exposure of submucosa allowing easier identification of the edges of exposed submucosa to direct dissection. One prospective study showed clip line traction contributed to significantly shorten the procedure time (33).
STER
STER procedures are performed under general anesthesia and endotracheal intubation. CO2 at the minimal pressure is highly recommended for insufflation given its fast absorption by the GI tract (34,35). Muscle relaxants are recommended to decrease muscle contraction and facilitate removal of large lesions through the esophagopharyngeal junction (36). Prophylactic antibiotics are administered 30 minutes prior to the procedure. A standard, single accessory channel gastroscope with water jet function and/or a dual-channel gastroscope are used in most procedures. A transparent cap is often times attached at the tip of the endoscope.
Identification of the tumor site
Injection of methylene blue or indigo carmine between the lesion and the site of mucosal incision is performed to provide a guide when creating the submucosal tunnel (35).
Creation of a submucosal tunnel
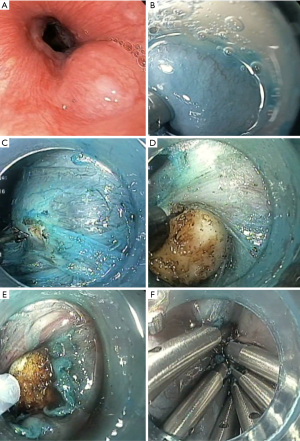
Submucosal injection at 5 cm proximal to the submucosal tumor is first performed to achieve mucosal lifting. This distance seems to provide the greatest leak resistance, when compared to direct incision (37). An epinephrine in saline (1:100,000) solution, with or without indigo carmine, is generally used. A hook (KD-620LR, Olympus) or non-insulating needle knife is used to make a 2-cm longitudinal incision in the mucosa as the entry point (17). Using an insulated-tip knife, a hook knife, or a hybrid knife, a tunnel between the submucosa and muscularis propria is then created by ways of endoscopic submucosal dissection (ESD) (17). Submucosal tunneling should extend toward and at least 2 cm beyond the tumor, to allow for an appropriate endoscopic view of the tumor, as well as for easier maneuverability (Figure 3) (17,36). This step has to be carefully performed in order to prevent mucosal injury that can result in leakage of GI contents in to the peritoneal cavity (17). Direct vision of endoscopic knives during this step reduces the risk of bleeding and perforation (17).
Endoscopic enucleation of the subepithelial tumor
Enucleation of the tumor is carefully performed by dissection from the tumor from muscle fibers using an insulated-tip knife, hybrid knife, or hook knife. It is of paramount importance to avoid disruption of the tumor capsule. Lifting solution can be injected in the surrounding tissue to differentiate the muscularis propria from the tumor, allowing for easier excavation of the tumor, and preventing capsule rupture (38). If the tumor involves muscle fibers of the muscularis propria, the needle of hook knives, or a snare can be used to perform a circumferential full-thickness muscularis propria resection. The tumor is removed via the tunnel (39,40). The long dimension of the tumor should be parallel to the long axis of the esophagus to facilitate extraction through the tunnel orifice (36). After removing the tumor, if the esophageal adventitia or gastric serosa is intact the wound is often times washed to reduce the possibility of residual tumor cells (36). Careful hemostasis of the resection edge is required to prevent bleeding into the abdominal cavity.
Closure of mucosal incision
Endoclips and endoscopic sutures are used to close the tunnel entry site (35,41).
Esophagus
Esophageal cancer is the eighth most common malignancy worldwide (42). It carries a dismal prognosis if not diagnosed early, as demonstrated by 5-year survival rates of survival rates of less than 20% for locally advanced esophageal cancer (43-45). Esophageal surgery historically was associated with major morbidity and high mortality rates (3,4,6,46,47). A recent analysis from the society of thoracic surgery database from 2011–2014 showed that esophagectomy had a major morbidity of 33% and much improved but still significant mortality of 3.1% among 164 participating centers (47). Studies have suggested that endoscopic resection has less morbidity than surgery, although no prospective trials have directly compared the outcomes of both techniques. The major histological types of esophageal cancer are squamous cell carcinoma (SCC) and esophageal adenocarcinoma (EAC). The former subtype accounts for 80 to 90% of cases worldwide (48). However, the incidence of EAC has increased dramatically in the West, surpassing the rates of SCC (49-53). Along those lines, signet ring cell histology and lymphovascular invasion are markers for increased risk of local-regional spread and more extensive disease (54,55). In this setting, more extensive resection such as esophagectomy should be considered in medically fit patients.
Barrett’s esophagus
Esophageal EMR followed by radiofrequency ablation of the remaining flat dysplasia remains the treatment of choice of high-grade dysplasia (HGD) and mucosal EAC associated with Barrett’s esophagus (56). It is considered safe and effective with complete remission in 98% of patients after 40 months of follow-up (5,57,58). However, EMR can only achieve en bloc resection of lesions smaller than 15 to 20 mm (59-62). Larger lesions require piecemeal resection, which is a well-known risk factor for recurrence of EAC (63). In light of this evidence, the American Society of Gastrointestinal Endoscopy (ASGE) recommends ESD for excision of lesions larger than 20 mm if expertise is available, and the European Society of Gastrointestinal Endoscopy (ESGE) recommends it for lesions larger than 15 mm, with fibrosis, or as a staging procedure if superficial submucosal invasion is suspected (64,65). Additionally, the high density of submucosal lymphatics in the esophagus results in high rates of lymph node involvement (25%), even in superficial cancers such as T1b submucosal tumors, may favor ESD over EMR as it allows for precise histopathologic analysis for determine depth of submucosal invasion (66).
Indications for endoscopic resection of Barrett’s esophagus and EAC
Endoscopic resection is indicated for treatment of HGD and EAC associated with Barrett’s esophagus (64). EMR is preferred for those lesions likely to be removed en bloc (67-71). ESD is preferred for lesions suspicious for superficial submucosal invasion, large lesions (≥15 mm) with intramucosal carcinoma that would otherwise be removed piecemeal, and poorly lifting lesions (Table 1) (64). Treatment should be supplemented with an endoscopic ablation technique, such as radiofrequency ablation, in order to decrease the risk of metachronous lesions from the remaining Barrett’s epithelium, irrespective of the endoscopic resection technique (63). Once eradication of abnormal mucosa has been achieved, it is important to continue surveillance as late recurrence can occur, and occasionally in the setting of persistent disease under normal appearing squamous mucosa (72).
Table 1
| Characteristic | Indication |
|---|---|
| Depth of invasion | sm1 (≤500 μm) |
| Size | >15 mm |
| Lifting | Poor |
*Based on the guidelines of the European Society of Gastrointestinal Endoscopy (64).
Histologic outcomes of endoscopic resection of Barrett’s esophagus and EAC
A recent meta-analysis, which included 11 studies, evaluated the safety and efficacy of ESD in the treatment of early BE neoplasia (73). The pooled en bloc resection rate was 92.9% (95% CI, 90.3–95.2%), and the R0 and curative resection rates were 74.5% (95% CI, 66.3–81.9%) and 64.9% (95% CI, 55.7–73.6%), respectively. Significant heterogeneity in R0 and curative resection rates was found, which was attributed to infiltrate lateral margins that were not diagnosed before endoscopic resection (73). This highlights the importance of detailed pre-procedural evaluation. This meta-analysis reported highly favorable outcomes and safety profiles, comparable to those in gastric and colorectal ESD from Asia and Europe (73).
Recently, two recent multicenter studies demonstrated the efficacy and safety of ESD in the West for resection of BE-HGD and EAC. A multicenter retrospective analysis in the United States reported en bloc and curative resection rates of 96% and 70%, respectively. Early bleeding was noted in 6% of the patients, perforation in 2.1%, and strictures in 15% (59). A multicenter European study, which included large (≥2 cm), nodular or fibrotic lesions revealed similar outcomes. En bloc resection rate was 90.8% and curative resection rate 65.8%. The learning curve portraying en bloc resection revealed that it plateaued after 30 procedures, providing evidence of better outcomes with experience. Rate of bleeding was 1.4%, perforation 0%, and stricture 2.1% (74). These findings highlight the potential role of ESD for the assessment and management of neoplastic lesions associated with BE, and provide reassurance on the safety of the technique when performed by experts in high-volume centers.
SCC
Most of the data regarding this subtype comes from Asia, where the prevalence of SCC and the experience with ESD are higher. ESD is currently the standard of care for removal of superficial esophageal SCC because of its optimal histologic outcomes, and better morbidity profile when compared to surgery.
Indications for endoscopic resection of SCC
According to the Japan Esophageal Society guidelines for treatment of esophageal cancer, endoscopic resection is indicated in lesions limited to the mucosa (T1a, m1–m2). Additionally, indications have expanded to include lesions with superficial infiltration of the submucosa (T1b-SM1, m3–sm1) (Table 2) (75). The ESGE recommends ESD as the first option for resection of superficial esophageal squamous cell cancers (m1 or m2), due to its ability to provide en bloc resection (64).
Table 2
| Characteristic | Absolute indications | Relative indications |
|---|---|---|
| Depth of invasion | m1, m2 | m3, sm1 (≤200 μm) |
*Based on the guidelines of the Japan Esophageal Society (75).
Histologic outcomes of endoscopic resection for superficial SCC
A meta-analysis comparing ESD and EMR for resection of early SCC showed significantly higher en bloc resection rates in the ESD group than in the EMR group regardless of lesion size (97.1% vs. 49.3%), as well as higher curative resection rates (92.3% vs. 52.7%), and lower recurrence rates (0.3% vs. 11.5%) (76). In a study of 70 patients with SCC ≥20 mm, recurrence rates were higher in patients undergoing piecemeal resection (0% for en bloc resection, 15% for lesions resected in 2–4 pieces, and 47% for those resected in ≥5 pieces) (77). Supporting these results, en bloc resection rates of ESD have been reported to be 100% compared with 53% for EMR. Local recurrence has been reported to be 1% in ESD, and 10% in patient undergoing EMR (78). A Western prospective trial confirmed these findings, revealing en bloc resection rates of 90% in 20 lesions resected by ESD (79).
Adverse events of esophageal endoscopic resection
Successful endoscopic resection requires proficiency with management of its potential complications (30). The perceived rate of adverse events is higher for ESD than EMR, because of the longer procedure times, and its challenging technique. However, a significant difference in the complication rates has been noted only for esophageal strictures (67,77,80,81).
Bleeding
Bleeding, defined as ≥2 g/dL drop in hemoglobin, has been noted in 0–22.8% of esophageal ESD case series, with a mean of 2.5% (13,20,29,59,67,68,71,78,79,82-90). It usually presents during the procedure, or within the first 24 hours. According to a recent systematic review, bleeding was controlled conservatively in 95% of cases, and required intervention in less than 10% of cases (81). Delayed bleeding after esophageal ESD is rare, being reported in 0–5.2% of patients.
Perforation
Perforation has been noted in 0–4% of ESD procedures for resection of SCC and HGD and EAC associated with BE (13,20,29,59,67,68,71,78,79,82-89). Small perforations can be successfully treated with endoscopic clipping or stent placement, while larger perforations may require urgent surgical intervention (64,80). In those patients who develop mediastinal emphysema without a recognizable perforation, it might be beneficial to provide conservative treatment (78).
Stricture
An esophageal stricture after endoscopic resection is defined as a stenosis that limits the passage of a gastroscope. A circumferential extent larger ≥75% of the lumen, and greater invasion depth (>T1m2) have been associated with occurrence of strictures (12). It can develop in 12–17% of patients (90,91). When complete endoscopic resection is attempted, the rate can be as high as 30% (57). In light of its high prevalence, several interventions have been proposed to prevent this complication (92). Currently, the first-line options are oral or locally injected steroids (92-97). Alternatives include prophylactic endoscopic balloon dilation, self-expandable metal stents, local injection of botulinum toxin, and oral tranilast (93,98,99). Promising approaches are currently under investigation, including tissue-shielding resection sites with carboxymethyl cellulose, polyglycolic acid sheets followed by fibrin glue, and autologous cell sheet transplantation (100-105).
A post procedure stricture may add challenges to additional treatment should the pathology ultimately require adjuvant therapy, such as radiation, which can also exacerbate strictures.
Submucosal gastrointestinal tumors
Although upper gastrointestinal submucosal tumors are mostly benign (106), some of them, especially GI stromal tumors, have malignant potential (41). Traditionally, patients with upper GI tumors <3 cm are faced to choose between resection and endoscopic surveillance. Surgery is associated with a high morbidity rate (107). On the other hand, the most effective method and follow-up interval for endoscopic surveillance have not been clearly established, potentially leading to non-compliance, stress, missed cases of malignancy, and financial burdens (108,109). STER arose as a safe and minimally invasive method with the potential of providing accurate histopathology evaluation.
Pre-procedural assessment
Endoscopic ultrasound and computed tomography should be routinely performed to assess the size, layer of origin, margins, and growth pattern. Liu et al described the use of inflatable esophageal CT scan, which involves insufflation of air through a nasoesophageal tube while CT images are obtained, allowing for appreciation of the relation between the tumor and esophageal wall (35).
Indications
Traditionally, STER of gastric lesions has been limited to lesions <3.5 cm due to the limited submucosal space and poor endoscopic visualization with larger tumors. Tan and colleagues recommended STER as an appropriate alternative if EGD shows an intact mucosal surface; EUS does not reveal features of malignancy, including irregular borders, internal heterogeneity, echogenic foci, heterogeneous enhancement or lymphadenopathy; and there are no signs of distant metastasis on CT imaging (36).
Lesions originating from the deep layer of the muscularis propria might not be amenable for STER because of the higher risk of perforation, fistula formation and infection (38).
STER can be performed for resection of tumors located in the muscularis propria of the esophagus and cardia. Because submucosal dissection is difficult to perform in the deep fundus or lesser curvature of the gastric body, direct endoscopic full-thickness resection is preferred in these locations (110).
Post-procedure care
Post-operatively, patients are generally kept nil per os for the first day (35). Some endoscopists prefer resuming a liquid diet before gradually advancing to a regular diet over 2 weeks (34,111,112). Patients also receive proton pump inhibitors for 3 days to 4 weeks, as well as antibiotics for 3 days to prevent postoperative infections (34,35,38,113).
Surveillance
Upper standard endoscopy is the preferred modality for confirmation of adequate healing and identification of metachronous lesions. The majority of studies reported follow up after 3 and 6 months, and then annually thereafter. Some authors reported endoscopic ultrasound as a complement for surveillance (35,38,39,114). Additionally, certain endoscopists recommend indefinite evaluation of distant metastasis using abdominal CT or US, and chest radiograph every 12 months for patients with GISTs (38,113). There is no consensus regarding the ideal follow-up strategy (109).
Outcomes
A recent review of the experience with STER of upper GI submucosal tumors showed a composite complete resection rate of 99.8%. The composite en bloc resection rate, which is traditionally defined as resection of the tumor with an intact capsule, of STER for esophageal, esophagogastric junction, and gastric submucosal tumors was found to be 98.36%, 96.2%, and 97.9%, respectively (115). Xu and colleagues developed the technique and reported the first experience available. It included 100% rate of both en bloc and R0 resection (Table 3). Average lesion size was 19 mm (range, 12–30 mm). Mean procedure time was 78.7 min (range, 25–130 min). Nine lesions were leiomyomas, 5 were gastrointestinal stromal tumors (GISTs), and 1 was a glomus tumor (17). The majority of studies evaluating this technique come from China.
Table 3
| Authors | N (tumors) | Location | Mean size [range], mm | Pathology | En bloc resection rate, n (%) | Complete resection rate, n (%) | Complications | Mean follow-up (months) and recurrence |
|---|---|---|---|---|---|---|---|---|
| Xu et al. (17) | 15 | 9 esophagus; |
19 [12–25] | 9 leiomyomas; |
15 (100.0) | NA | 1 pneumoperitoneum; |
3.9, no recurrence |
| Liu et al. (35) | 12 | 7 esophagus; |
18.5 [10–30] | 9 leiomyomas; |
12 (100.0) | 12 (100.0) | 8 subcutaneous emphysema; |
7.1, no recurrence |
| Ge et al. (116) | 17 | 17 esophagus | 24 [12–50] | 16 leiomyomas; |
NA | 17 (100.0) | 2 subcutaneous emphysema and pneumomediastinum | 7, no recurrence |
| Gong et al. (117) | 12 | 8 esophagus; |
20 [10–40] | 7 GISTs; |
10 (83.0) | 12 (100.0) | 2 subcutaneous emphysema and pneumomediastinum; |
NA |
| Wang et al. (34) | 57 | 57 esophagogastric junction | 21.5 [6–35] | 46 leiomyomas; |
57 (100.0) | 57 (100.0) | 12 pneumomediastinum and subcutaneous emphysema; |
12, no recurrence |
| Ye et al. (38) | 85 | 60 esophagus; |
19.2 [10–30] | 65 leiomyomas; |
85 (100.0) | 85 (100.0) | 8 subcutaneous emphysema; |
8, no recurrence |
| Lu et al. (110) | 45 | 29 esophagus; |
12 [8–16] | 42 leiomyomas; |
NA | 44 (97.8) | 6 perforation; 1 subcutaneous emphysema | 8.7, no recurrence |
| Li et al. (118) | 32 | 12 gastric corpus; |
23 [10–50] | 18 leiomyomas; |
32 (100.0) | 32 (100.0) | 6 pneumoperitoneum; |
28, no recurrence |
| Zhou et al. (39) | 21 | 21 EGJ | 23 [10–40] | 15 leiomyomas; 6 GIST | 18 (85.7) | 21 (100.0) | 9 perforations* | 6 months, no recurrence |
| Tan et al. (36) | 18 | 18 esophagus | 41 [35–53] | 18 leiomyomas | 16 (88.9) | NA | 1 subcutaneous emphysema; |
18.9, no recurrence |
| Inoue et al. (119) | 7 | 4 stomach; |
18.5 [12–30] | 5 leiomyomas; |
7 (100.0) | 7 (100.0) | None | 5.5, no recurrence |
| Chen et al. (113) | 180 | 124 esophagus; |
26 [20–50] | 146 leiomyomas; |
163 (90.6) | NA | 2 Bleeding; 2 mucosal injury; |
36, no recurrence |
| Du et al. (120) | 89 | 89 Esophagus | 16 [10–60] | 87 leiomyomas; |
70 (78.7) | NA | 5 subcutaneous emphysema; |
6 months, no recurrence |
*Defined as subcutaneous emphysema, pneumothorax, pneumoperitoneum, or retroperitoneal gas.
Piecemeal resection has been found to be associated with irregular tumors (OR =6.0, P<0.001), and tumor size >30 mm (OR =7.5, P<0.001). A recent study compared STER with video-assisted thoracoscopic surgery (VATS) for resection of larger lesions thought not to be good candidates for STER (35–55 mm) (36). Patients who underwent STER were found to have shorter operation time, a shorter length of hospital stay and lower cost. There was no significant difference in the rate of en bloc resection (88.9% for STER vs. 100% for VATS, P=0.49) (36).
A recent meta-analysis including 28 studies, comprising 1,041 patients and 1085 lesions, found a complete resection rate of 97.5% (95% CI, 96.0–98.5%), and an en bloc resection rate of 94.6% (95% CI, 91.5–96.7%) (121).
Complications
Although STER is a minimally invasive technique, it still carries a risk of complications such as perforation, bleeding, and incomplete resection, especially in tumors with extraluminal growth and those attached to the muscularis propria (106,118).
Complications of STER mainly derive as consequences from the technique itself, and include subcutaneous emphysema, pneumomediastinum, pneumothorax, pneumoperitoneum, and pleural effusion (119). The most common complication has been found to be subcutaneous emphysema, with a pooled prevalence of 14.8% (95% CI, 10.5–20.5%) (121). The pooled prevalence of pneumothorax and pneumoperitoneum are 6.1% (95% CI, 4.0–9.0%) and 6.8% (95% CI, 4.7–9.6%), respectively (121). No local recurrence has been described in any of the studies reported to date (17,34-36,38,39,111-113,115,118,119,121-124).
Most complications can be successfully managed conservatively. Management of subcutaneous emphysema and pneumothorax generally includes subcutaneous puncture and chest-tube drainage using a central venous catheter at the third or fourth intercostal space. Insufflation with CO2 rather than air reduces post-procedural mediastinal emphysema (125). Hemostasis should be performed during the procedure in order to reduce postoperative bleeding and bleeding during second look endoscopy (126).
Risk factors of intraprocedural complications, as described by Ye and colleagues, include origin of the lesion in the deeper muscularis propria layer (70% vs. 1.3% for superficial involvement, P<0.001), as well as GIST on histopathology (26.3% for GIST, 4.6% for leiomyoma, 0% for calcifying fibrous tumor, P=0.016) (38). Chen and colleagues also identified tumor size ≥30 mm (OR =7.3, P<0.001), irregular tumor shape (OR =6.5, P<0.001) and operative time ≥60 min (OR =6.7, P<0.001) as features associated with STER-related complications (113).
Limitations of STER
Some parts of the gastrointestinal tract are not suitable for STER. First, lesions in the upper esophagus are challenging because of the lack of spare length for tunneling. Additionally, use in the stomach is limited by the difficulty of achieving creation of a submucosal tunnel because of its large space, extensibility, large vessels in the submucosa, and mucosal hypertrophy. Also, orientation or the submucosal tunnel can be challenging. This can be overcome by injection of methylene blue or indigo carmine before performing the submucosal dissection (40). Maintenance of mucosal integrity while creating the submucosal tunnel can be challenging in the stomach. Careful case selection is required to assure a safe and effective procedure.
The majority of evidence comes from retrospective, single-center studies conducted in China; hence the abovementioned outcomes may not be generalizable to the world. In addition, there is no long-term follow up data precluding conclusions in regards to recurrence. Prospective studies comparing STER with other surgical and endoscopic approaches are needed.
Stomach
Early gastric cancer has a distinctive biological behavior (127). Margins are usually not distinct, and there are lymphatics within the mucosa, which makes EGC a potentially aggressive malignancy. The high incidence of gastric cancer in Japan and Korea has resulted in organized screening programs, and an increased rate of diagnosis of early gastric cancer (128,129). The introduction of endoscopic therapy has allowed radical treatment of these neoplasms by ways of minimally invasive techniques that avoid resection of the stomach and D-2 lymph node dissection (130). ESD is well accepted as treatment of ECG due to its capability to achieve accurate histological staging and provide a cure (49).
Indications of endoscopic resection
It is of paramount importance to limit endoscopic therapies to lesions with a null risk of lymph node metastasis (131). Histopathologic evaluation of samples from patients with gastric cancer who underwent standard gastrectomy showed that tumor grading, size, macroscopic appearance, lymphovascular invasion, and submucosal invasion were risk factors for lymph node metastasis (14). Along these lines, the absolute indications for endoscopic resection (EMR or ESD) were proposed, and include a differentiated-type adenocarcinoma without ulcerative findings, with a pre-procedural depth of invasion diagnosed as T1a and a size ≤2 cm (131). The Japanese Gastric Cancer Association extended these criteria for curative endoscopic resection based on histology to include larger tumor size, presence of ulceration or submucosal invasion, and selected undifferentiated tumors to endoscopic therapy, based on the very low risk of lymph node metastasis that outweighs the risk of surgery (Table 4) (131). Only ESD can be considered under these expanded criteria (131). Furthermore, widespread application of these expanded criteria is limited to investigational use due to the lack of data on long-term outcomes, and the lack of validation studies outside East Asia (132).
Table 4
| Characteristic | Absolute indications for EMR or ESD | Expanded criteria only for ESD | ||
|---|---|---|---|---|
| A | B | C | ||
| Histologic type | Differentiated | Differentiated | Differentiated | Undifferentiated |
| Tumor size (cm) | ≤2 | >2 | ≤3 | ≤2 |
| Ulcerative component | No | No | Yes | No |
| Depth of invasion | T1a | T1a | T1a | T1a |
*Based on the Japanese Gastric Cancer Association guidelines for treatment of gastric cancer (131).
Surveillance after endoscopic resection
- Resection is considered curative if all of the following criteria are met: en bloc resection, tumor size ≤2 cm, intestinal-differentiated type, pT1a, negative lateral margin, negative vertical margin, and absence of lymphovascular invasion (131). Follow-up with upper endoscopy every 6 or 12 months is recommended (131). The presence of Helicobacter pylori should be evaluated, and if positive, eradication therapy should be provided (133).
- For lesions resected under the expanded indications, resection is considered curative if en bloc resection is achieved, with negative vertical and horizontal margins, negative lymphovascular invasion, and (131):
- Size >2 cm, differentiated-type, pT1a, without ulceration, or
- Size ≤3 cm, differentiated-type, pT1a, with ulceration, or
- Size ≤2 cm, undifferentiated-type, pT1a, without ulceration, or
- Size ≤3 cm, differentiated-type, pT1b (<500 µm).
- Follow-up with upper endoscopy every 6 or 12 months as well as abdominal CT is recommended (131). The presence of Helicobacter pylori should be evaluated, and if positive, eradication therapy should be provided (133).
- If the resection does not meet the criteria mentioned above, it is considered non curative, and surgical intervention is recommended (131).
Histologic outcomes of resection after endoscopic resection of early gastric cancer
Experience from large Asian series has shown en bloc resection rates as high as 86–97%, and curative resection rates of 88–93% for removal of early gastric cancer by ways of ESD (127,134-137). In the largest series of patients who underwent curative ESD for early gastric cancer, the 5-year survival rate was 92.6%, the 5-year disease-specific survival rate was 99.9%, and the 5-year relative survival rate was 105% (7). Similarly, in a Japanese population-based survival analysis, the relative 5-year survival rate for localized gastric cancer was 94.4% (138). Rates of en bloc resection and complete resection with ESD are higher than those with EMR (92% vs. 52%, and 82–92% vs. 42–43%, respectively), resulting in a lower risk of local recurrence in selected patients who undergo ESD (0.8% vs. 5–6.4%, respectively) (135). Western studies have shown similar outcomes (69,70).
A recent meta-analysis, which included 13 studies, evaluated the short- and long-term outcomes of ESD under the absolute and expanded indications (139). Patients on the expanded indication group had lower rates of en bloc resection (93.6% vs. 97.0%, P<0.0001) and complete resection (87.8% vs. 95.8%, P<0.00001) compared with the group of patients with absolute indications (139). Local recurrence was higher in the expanded indication group (1.5% vs. 0.6%, P=0.03) (139). There were no significant differences in regards to gastric-cancer specific mortality (P=0.22) and overall mortality (P=0.37). In light of the favorable long term outcomes, the authors recommend ESD as an effective approach of early cancer gastric under the expanded indications (139).
Compared to surgery, ESD has shown shorter procedure time (90 vs. 260 min), lower complication rates (5% vs. 15%), and shorter hospital stay (3–7 vs. 9–14 days). Furthermore, both techniques have shown to achieve similar R0 resection, and recurrence rates (140,141). Delayed bleeding was reported to be 6%, and perforation 1%.
Colon and rectum
In the United States, colorectal cancer is the fourth most common diagnosed cancer with an incidence of 132,700 cases per year (43). It is the consequence of accumulation of genetic alterations in which epithelial cells turn into adenomas, and then adenocarcinomas (142). Widespread screening programs have led to increased detection and therapy of adenomas, potentially preventing advanced cancer (143). Endoscopic therapy should aim to achieve en bloc resection because of increasing risk of local recurrence with a greater number of resected pieces (144,145). Furthermore, the carcinomatous area should not be sectioned because of the need to evaluate invasion depth and lymphovascular invasion (146). Although the size limit for en bloc resection by polypectomy or snare EMR is 2 cm, EMR remains the minimally invasive procedure of choice for removing colorectal adenomas (147). ESD has emerged as an endoscopic technique to remove large colorectal lesions, especially those that would require piecemeal EMR (146,148-150).
Endoscopic versus surgical treatment
Surgery has shown to have a 5-year survival rate of 94.3% for stage 0 and 90.6% for stage 1 colorectal carcinoma (151). Rates of cure with endoscopic therapy have been reported to be 92.7% (151). Comparable results can be achieved with surgical and endoscopic therapies (151).
The recurrence rate of high-risk T1 colorectal cancer after EMR has been reported to be 20.1%, while the recurrence rate after radical resection has been found to be 3.7% (152). Therefore, endoscopic resection is recommended only for low-risk colorectal cancer (152,153).
For early large rectal adenomas, higher recurrence rates have been reported after EMR compared with transanal endoscopic microsurgery (TEMS) (31% vs. 10%, P<0.001), with the advantage of lower postoperative complications in patients undergoing EMR (13% vs. 24%, P=0.038) (154).
A systematic review by Arezzo and colleagues comparing TEMS and ESD for large noninvasive rectal lesions revealed that the en bloc resection rate for patients undergoing ESD was lower than that of patients on the TEMS group (87.8% vs. 98.7%, P<0.001), resulting in an increased need of further abdominal interventions (8.4% vs. 1.8%, P<0.001), but decreased recurrence rate (2.6% vs. 5.2%, P<0.001) (155).
Preoperative assessment
First, determining whether a lesion is malignant or benign is of paramount importance before attempting EMR or ESD. The pit-pattern and the structure of the crypts have been found to correlate with malignant transformation (156). Magnifying colonoscopy along with white-light high definition endoscopy and narrow-band imaging can be used to classify the lesions and evaluate for depth of invasion according to the Kudo’s classification (157).
It is recommended to avoid taking biopsies to make a preoperative diagnosis (146). First, it may cause fibrosis in the submucosa of superficial-type lesions, leading to a non-lifting sign. Second, for large lesions such as LST, a single biopsy may not have adequate diagnostic yield (158).
Second, estimating the depth of submucosal invasion before the procedure is essential, given it correlates with the degree of lymph node metastasis (159). Pit pattern diagnosis with dye-spraying magnifying endoscopy has an accuracy of 90% if VN-type is observed. Magnifying endoscopy with narrow-band imaging and blue laser imaging can also be used, although these carry a slightly inferior accuracy than pit pattern analysis (160,161). The accuracy of endoscopic ultrasonography varies according to the morphology of the lesion, and reaches approximately 80% (162,163).
In order to perform successful colorectal ESD, endoscopists should have the skill to carry out a smooth and accurate insertion technique of the colonoscope, as well as experience with the techniques of polypectomy, EMR, hemostasis, and clip suture (146). Prior experience with upper tract ESD is helpful before performing colorectal cases (146).
Indications for endoscopic treatment
Lesions amenable for en bloc resection, with negligible risk of lymph node metastasis are candidates for endoscopic resection (Table 5). Additional criteria for endoscopic resection include invasion limited to the mucosa or superficial submucosa, of any macroscopic type. Of note, size is not a limitation. Carcinomas with submucosal invasion ≥1,000 µm should be treated surgically. En bloc ESD is required for LST-NGs larger than 2 cm, because their submucosal invasion rate is as high as 35% (164,165). Homogeneous-type LST-G <3 cm without invasive features can be potentially removed with piecemeal EMR, given their lower possibility of invasive cancer (165). Those LST-G lesions >3 cm show multifocal submucosal invasion; therefore, ESD is recommended (165).
Table 5
| Lesions not amenable for en bloc removal with endoscopic mucosal resection |
| Laterally spreading tumor-non-granular type |
| Tumors with a V1-type pit pattern |
| Carcinoma with shallow T1 invasion |
| Large depressed-type tumors |
| Large protruded-type tumors suspected to be carcinoma |
| Mucosal tumors with submucosal fibrosis |
| Localized tumors in conditions of chronic inflammation |
| Local residual or recurrent early carcinomas after endoscopic resection |
*Based on the guidelines of the Japan Gastroenterological Endoscopy Society (146).
Criteria for additional treatment after endoscopic resection
Histologic examination determines the need of additional therapy after endoscopic resection.
If negative margins are found, with papillary or tubular adenocarcinoma, depth of invasion <1,000 µm, no vascular invasion and G1 budding, surveillance is recommended (19).
If vertical margins are negative, and at least one of the following characteristics is present, intestinal resection with lymph node dissection is suggested (19):
- Poorly differentiated adenocarcinoma;
- Signet-ring cell or mucinous carcinoma;
- Depth of invasion ≥1,000 µm;
- Presence of vascular invasion;
- G2/G3 budding at the site of deepest invasion.
If positive vertical margins are found, intestinal resection with lymph node dissection is needed (19).
Careful preoperative endoscopic diagnosis and consideration of the endoscopist’s skill are vital to determine whether resection by EMR, piecemeal EMR, or ESD is indicated (19).
In Japan, colorectal ESD for lesions between 2 and 5 cm has been approved for implementation under health insurance since April 2014 (19).
The incidence of recurrence for stage I colorectal cancer was found to be 3.7%, with 68.6% happening in the first 3 years, and 96.1% within the first 5 years following resection (19).
The 3- and 5-year rates of endoscopic recurrence for lesions with curative resection (n=344) were 0.4% and 0.4%, respectively (164). The 3- and 5-year endoscopic recurrence rates in lesions with non-curative resection were 13% and 17%, respectively. During the follow up period no colorectal cancer related to ESD deaths were identified (164). Recurrence was associated with piecemeal resection (OR =8.5; 95% CI, 1.2–59.7) and T1b cancer (OR =5.8; 95% CI, 1.8–18.5) (164).
ESD has solid clinical outcomes of low recurrence rates if curative resection is achieved. In a recent multicenter prospective study, Oka and colleagues found that piecemeal resection is the most important risk factor for local recurrence, regardless of the endoscopic resection technique used (166).
Outcomes
Retrospective series comparing ESD to EMR for large colonic laterally spreading tumors have shown higher rates of en bloc resection (84–95% vs. 33–57%), and lower rates of local recurrence (0–2% vs. 12–26%) in patients undergoing ESD than those undergoing EMR (167). These benefits came at the cost of longer procedures time (108 vs. 29 min) and higher rates of perforation (5–8% vs. 1.3–3%) (167). Difference in lesion size (29–37 vs. 22–28 mm) might partially account for these disadvantages. When rates of perforation for ESD and EMR among similar-sized lesions were compared, no difference was found (1.6 vs. 0.8, P<0.05) (168).
A recent meta-analysis by Yamada and colleagues, included 423 lesions with a mean size of 37 mm. Complete (R0) resection was achieved in 344 (81%), with perforation happening in 14 (3%), and delayed bleeding in 4 (1%) (164). After a median follow-up of 4.9 years, 3-year overall cumulative endoscopic recurrence was 2.9% (95% CI, 1.2–43.7%). The cancerous recurrence rate was 1.1% (95% CI, 0–2.1%). The 5-year overall cumulative endoscopic recurrence rate was 3.8% (95% CI, 1.7–5.9%), and the cancerous recurrence rate was 1.6% (95% CI, 0.1–3.0%) (164).
ESD has been showed to be an effective alternative treatment for surgery, as suggested by one of the largest Japanese multicenter cohort studies by Saito and colleagues. The study included 1,111 large superficial colorectal tumors (35±18 mm) and showed en bloc and curative resection rates of 88% and 89%, respectively (169). The difference in experience of ESD and EMR in the East and the West was described in a recent meta-analysis by Fuccio and colleagues. The R0 resection rate was 71.3% in non-Asian countries versus 85.6% in Asian ones. Furthermore, a lower recurrence was noted after ESD (2.0%) at 12 months when compared with EMR (13.8%). These findings confirm the limited availability of ESD expertise in Western countries (170).
The advantages of ESD over surgical approaches for the treatment of early colorectal cancer have been reported extensively in Asian literature. Kiriyama and colleagues reported comparable en bloc and curative resection rates, and lower complication rates for ESD when compared with laparoscopic-assisted colorectal surgery (LAC) (171). Additionally, Nakamura reported the advantages of ESD over LAC in regards to decreased length of hospital stay (5 vs. 10 days), mean procedure time (90 vs. 185 min), complications (7% vs. 15%), and quality of life (172,173). ESD has also shown to lower recurrence rates than transanal resection for rectal cancer (174).
Surveillance
Surveillance after EMR or ESD aims to detect local residual or recurrent lesions (146). Patients with curative resection are traditionally followed up with colonoscopy at 1, 3 and 5 years after ESD (164). Patients with positive lateral margins generally undergo follow up at 3 to 6 months after ESD. Early endoscopic follow up within 6 months is suggested for lesions ≥10 mm resected by piecemeal EMR. Based on the findings reported by Yamata and colleagues, early endoscopic follow-up and long term surveillance should be done only in those cases with non-curative ESD (164).
Recurrence
Benign neoplastic recurrence can be successfully treated by additional endoscopic treatment in the form of EMR or ESD (175-177). Cancerous recurrence, defined as invasive or distant recurrence requires surgery (164).
Bourke and colleagues recently developed the Sydney EMR recurrence tool (SERT), a scoring tool to stratify the risk of residual or recurrent adenoma based on the characteristics of the index EMR. Tumor size larger than 40 mm (OR =2.47, P<0.001), intraprocedural bleeding (OR =1.78; P=0.024), and HGD (OR =1.72, P=0.029) were found to be associated with endoscopic recurrence. Based on these parameters, the authors state that follow-up can be deferred up to 18 months for lesions without any of the abovementioned characteristics (178).
Complications
Although the cure rates of ESD are very high for removing colorectal tumors, several studies have shown the safety of ESD is inferior to that of EMR. A recent meta-analysis showed the rate of perforation of ESD was significantly higher than that of EMR (5.7% and 1.4%, respectively, OR =4.96; 95% CI, 2.79–8.85) (168).
Perforation, defined as a complete mural defect in the colorectal wall, has been reported to occur in 0.05% in polypectomy, 0.58–0.8% in EMR, and 2–14% in ESD (19,148,179,180). The thinner colonic wall is more prone to perforation than that of the stomach. It is vital to ensure good maneuverability of the scope, as well as to select the most appropriate devices, carbon dioxide insufflation, and injection agents in order to carry out a successful procedure (181). If perforation occurs, clips should be placed regardless of the location. If closure can be achieved with endoscopic therapies, antibiotics and fasting should also be implemented (148,182). If closure is incomplete, emergent surgery should be performed to decrease the risk of peritonitis (146). In rectal lesions, perforation results in retroperitoneal, mediastinal or subcutaneous emphysema, and not perforation into the abdominal cavity because of the peritoneal reflection (183).
Serious bleeding rarely occurs in the colon compared with the stomach. It has traditionally been defined as a decrease in hemoglobin >2 mg/dL, or the need for blood transfusion. It has been reported in 1.6% cases of polypectomy, 1.15–1.7% for EMR, and 0.7–2.2% for ESD (19,148,179,180).
For ESD, minor bleeding can be controlled with contact coagulation with the tip of a knife. If bleeding arises from a larger vessel, the point of bleeding should be grasped with hemostatic forceps, while minimal electrocoagulation is applied to minimize thermal damage (146). Evidence evaluating the role of prophylactic clipping for prevention of post-polypectomy hemorrhage is controversial (184,185). A randomized trial reported it did not decrease the rate of delayed bleeding in small lesions, while a retrospective study revealed clipping was effective for lesions >2 cm (184,185). In lack of inconsistent evidence, the Japan Gastroenterological Endoscopy Society recommends prophylactic clipping in EMR for patients with large lesions or those on antithrombotic therapy (146). Fujiya and colleagues reported similar rates of delayed bleeding after ESD (3.5%) and EMR (2.0%; OR =0.85; 95% CI, 0.45–1.60) (168).
The most frequent reason for additional surgery after EMR or ESD is massive submucosal invasion (168). This finding stresses the importance of an adequate preoperative invasion of depth prediction to avoid delaying proper therapy. Another cause of additional surgery found in the meta-analysis was uncertain mucosal margins in specimens removed by EMR (168). This observation confirms the benefit of ESD over EMR as far as providing adequate specimens for histopathologic evaluation.
Limitations
Longer procedure times have been associated with the degree of difficulty of ESD. This is particularly notable for gastric neoplasms in the upper and middle thirds (186). The submucosa of lesions in these locations tends to collapse under the weight of the lesion (187). This limitation has been theoretically overcome with clip traction (187).
Conclusion remarks
The diagnostic and therapeutic paradigm of early esophageal, gastric and colorectal cancer is shifting from a surgical approach to a minimally invasive alternative. EMR, ESD, and the recently described STER provide accurate histologic diagnosis, as well as curative resection for early gastrointestinal malignancies. Appropriate selection of candidates for endoscopic resection is crucial. Estimation of eligibility relies entirely in an adequate pre-procedural evaluation of depth of invasion, which correlates with the risk of lymph node metastasis. Widespread use of some of these techniques has been limited due to its technical complexity, its flat learning curves, and its long procedure times.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: Dr. A Bhatt is a consultant for Aries Pharma. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [Crossref] [PubMed]
- Luketich JD, Pennathur A, Awais O, et al. Outcomes after minimally invasive esophagectomy: review of over 1000 patients. Ann Surg 2012;256:95-103. [Crossref] [PubMed]
- Birkmeyer JD, Stukel TA, Siewers AE, et al. Surgeon volume and operative mortality in the United States. N Engl J Med 2003;349:2117-27. [Crossref] [PubMed]
- Orringer MB, Marshall B, Chang AC, et al. Two thousand transhiatal esophagectomies: changing trends, lessons learned. Ann Surg 2007;246:363,72; discussion 372-4.
- Ngamruengphong S, Wolfsen HC, Wallace MB. Survival of patients with superficial esophageal adenocarcinoma after endoscopic treatment vs surgery. Clin Gastroenterol Hepatol 2013;11:1424-1429.e2; quiz e81.
- McCulloch P, Ward J, Tekkis PP, et al. Mortality and morbidity in gastro-oesophageal cancer surgery: initial results of ASCOT multicentre prospective cohort study. BMJ 2003;327:1192-7. [Crossref] [PubMed]
- Suzuki H, Oda I, Abe S, et al. High rate of 5-year survival among patients with early gastric cancer undergoing curative endoscopic submucosal dissection. Gastric Cancer 2016;19:198-205. [Crossref] [PubMed]
- Rogers BH. The safety of carbon dioxide insufflation during colonoscopic electrosurgical polypectomy. Gastrointest Endosc 1974;20:115-7. [Crossref] [PubMed]
- Rosenberg N. Submucosal saline wheal as safety factor in fulguration or rectal and sigmoidal polypi. AMA Arch Surg 1955;70:120-2. [Crossref] [PubMed]
- ASGE Technology Committee. Endoscopic mucosal resection and endoscopic submucosal dissection. Gastrointest Endosc 2008;68:11-8. [Crossref] [PubMed]
- Ono H, Kondo H, Gotoda T, et al. Endoscopic mucosal resection for treatment of early gastric cancer. Gut 2001;48:225-9. [Crossref] [PubMed]
- Ono S, Fujishiro M, Niimi K, et al. Long-term outcomes of endoscopic submucosal dissection for superficial esophageal squamous cell neoplasms. Gastrointest Endosc 2009;70:860-6. [Crossref] [PubMed]
- Oyama T, Tomori A, Hotta K, et al. Endoscopic submucosal dissection of early esophageal cancer. Clin Gastroenterol Hepatol 2005;3:S67-70. [Crossref] [PubMed]
- Tsujitani S, Oka S, Saito H, et al. Less invasive surgery for early gastric cancer based on the low probability of lymph node metastasis. Surgery 1999;125:148-54. [Crossref] [PubMed]
- Rösch T, Sarbia M, Schumacher B, et al. Attempted endoscopic en bloc resection of mucosal and submucosal tumors using insulated-tip knives: a pilot series. Endoscopy 2004;36:788-801. [Crossref] [PubMed]
- Cao Y, Liao C, Tan A, et al. Meta-analysis of endoscopic submucosal dissection versus endoscopic mucosal resection for tumors of the gastrointestinal tract. Endoscopy 2009;41:751-7. [Crossref] [PubMed]
- Xu MD, Cai MY, Zhou PH, et al. Submucosal tunneling endoscopic resection: a new technique for treating upper GI submucosal tumors originating from the muscularis propria layer (with videos). Gastrointest Endosc 2012;75:195-9. [Crossref] [PubMed]
- Yahagi N, Fujishiro M, Kakushima N, et al. Endoscopic submucosal dissection for early gastric cancer using the tip of an electrosurgical snare (thin type). Digestive Endoscopy 2004;16:34-8. [Crossref]
- Watanabe T, Itabashi M, Shimada Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) Guidelines 2014 for treatment of colorectal cancer. Int J Clin Oncol 2015;20:207-39. [Crossref] [PubMed]
- Higuchi K, Tanabe S, Azuma M, et al. A phase II study of endoscopic submucosal dissection for superficial esophageal neoplasms (KDOG 0901). Gastrointest Endosc 2013;78:704-10. [Crossref] [PubMed]
- Kothari S, Kaul V. Endoscopic Mucosal Resection and Endoscopic Submucosal Dissection for Endoscopic Therapy of Barrett's Esophagus-related Neoplasia. Gastroenterol Clin North Am 2015;44:317-35. [Crossref] [PubMed]
- Matsui N, Akahoshi K, Nakamura K, et al. Endoscopic submucosal dissection for removal of superficial gastrointestinal neoplasms: A technical review. World J Gastrointest Endosc 2012;4:123-36. [Crossref] [PubMed]
- Uraoka T, Saito Y, Yamamoto K, et al. Submucosal injection solution for gastrointestinal tract endoscopic mucosal resection and endoscopic submucosal dissection. Drug Des Devel Ther 2009;2:131-8. [PubMed]
- Yamamoto H, Yahagi N, Oyama T, et al. Usefulness and safety of 0.4% sodium hyaluronate solution as a submucosal fluid “cushion” in endoscopic resection for gastric neoplasms: a prospective multicenter trial. Gastrointest Endosc 2008;67:830-9. [Crossref] [PubMed]
- Draganov PV, Gotoda T, Chavalitdhamrong D, et al. Techniques of endoscopic submucosal dissection: application for the Western endoscopist? Gastrointest Endosc 2013;78:677-88. [Crossref] [PubMed]
- Arantes V, Albuquerque W, Benfica E, et al. Submucosal injection of 0.4% hydroxypropyl methylcellulose facilitates endoscopic mucosal resection of early gastrointestinal tumors. J Clin Gastroenterol 2010;44:615-9. [Crossref] [PubMed]
- ASGE Technology Committee. Endoscopic submucosal dissection. Gastrointest Endosc 2015;81:1311-25. [Crossref] [PubMed]
- Mehta N, Strong AT, Franco M, et al. Optimal injection solution for endoscopic submucosal dissection: A randomized controlled trial of Western solutions in a porcine model. Dig Endosc 2017; [Epub ahead of print]. [Crossref] [PubMed]
- Kawahara Y, Hori K, Takenaka R, et al. Endoscopic submucosal dissection of esophageal cancer using the Mucosectom2 device: a feasibility study. Endoscopy 2013;45:869-75. [Crossref] [PubMed]
- Bhatt A, Abe S, Kumaravel A, et al. Indications and Techniques for Endoscopic Submucosal Dissection. Am J Gastroenterol 2015;110:784-91. [Crossref] [PubMed]
- Oyama T. Counter traction makes endoscopic submucosal dissection easier. Clin Endosc 2012;45:375-8. [Crossref] [PubMed]
- He Y, Fu K, Leung J, et al. Traction with dental floss and endoscopic clip improves trainee success in performing gastric endoscopic submucosal dissection (ESD): a live porcine study (with video). Surg Endosc 2016;30:3138-44. [Crossref] [PubMed]
- Koike Y, Hirasawa D, Fujita N, et al. Usefulness of the thread-traction method in esophageal endoscopic submucosal dissection: randomized controlled trial. Dig Endosc 2015;27:303-9. [Crossref] [PubMed]
- Wang XY, Xu MD, Yao LQ, et al. Submucosal tunneling endoscopic resection for submucosal tumors of the esophagogastric junction originating from the muscularis propria layer: a feasibility study (with videos). Surg Endosc 2014;28:1971-7. [Crossref] [PubMed]
- Liu BR, Song JT, Kong LJ, et al. Tunneling endoscopic muscularis dissection for subepithelial tumors originating from the muscularis propria of the esophagus and gastric cardia. Surg Endosc 2013;27:4354-9. [Crossref] [PubMed]
- Tan Y, Lv L, Duan T, et al. Comparison between submucosal tunneling endoscopic resection and video-assisted thoracoscopic surgery for large esophageal leiomyoma originating from the muscularis propria layer. Surg Endosc 2016;30:3121-7. [Crossref] [PubMed]
- von Delius S, Gillen S, Doundoulakis E, Schneider A, et al. Comparison of transgastric access techniques for natural orifice transluminal endoscopic surgery. Gastrointest Endosc 2008;68:940-7. [Crossref] [PubMed]
- Ye LP, Zhang Y, Mao XL, et al. Submucosal tunneling endoscopic resection for small upper gastrointestinal subepithelial tumors originating from the muscularis propria layer. Surg Endosc 2014;28:524-30. [Crossref] [PubMed]
- Zhou DJ, Dai ZB, Wells MM, et al. Submucosal tunneling and endoscopic resection of submucosal tumors at the esophagogastric junction. World J Gastroenterol 2015;21:578-83. [Crossref] [PubMed]
- Zhang Y, Ye LP, Mao XL. Endoscopic treatments for small gastric subepithelial tumors originating from muscularis propria layer. World J Gastroenterol 2015;21:9503-11. [Crossref] [PubMed]
- Lee IL, Lin PY, Tung SY, et al. Endoscopic submucosal dissection for the treatment of intraluminal gastric subepithelial tumors originating from the muscularis propria layer. Endoscopy 2006;38:1024-8. [Crossref] [PubMed]
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [Crossref] [PubMed]
- DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin 2014;64:252-71. [Crossref] [PubMed]
- Rustgi AK, El-Serag HB. Esophageal carcinoma. N Engl J Med 2014;371:2499-509. [Crossref] [PubMed]
- Rice TW, Gress DM, Patil DT, et al. Cancer of the esophagus and esophagogastric junction-Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin 2017;67:304-17.
- Wang H, Shen Y, Feng M, et al. Outcomes, quality of life, and survival after esophagectomy for squamous cell carcinoma: A propensity score-matched comparison of operative approaches. J Thorac Cardiovasc Surg 2015;149:1006,14; discussion 1014-5.e4.
- Raymond DP, Seder CW, Wright CD, et al. Predictors of Major Morbidity or Mortality After Resection for Esophageal Cancer: A Society of Thoracic Surgeons General Thoracic Surgery Database Risk Adjustment Model. Ann Thorac Surg 2016;102:207-14. [Crossref] [PubMed]
- Arnold M, Soerjomataram I, Ferlay J, et al. Global incidence of oesophageal cancer by histological subtype in 2012. Gut 2015;64:381-7. [Crossref] [PubMed]
- Devesa SS, Blot WJ, Fraumeni JF Jr. Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer 1998;83:2049-53. [Crossref] [PubMed]
- Kubo A, Corley DA. Marked multi-ethnic variation of esophageal and gastric cardia carcinomas within the United States. Am J Gastroenterol 2004;99:582-8. [Crossref] [PubMed]
- Simard EP, Ward EM, Siegel R, et al. Cancers with increasing incidence trends in the United States: 1999 through 2008. CA Cancer J Clin 2012;62:118-28. [Crossref] [PubMed]
- Dubecz A, Solymosi N, Stadlhuber RJ, et al. Does the Incidence of Adenocarcinoma of the Esophagus and Gastric Cardia Continue to Rise in the Twenty-First Century?-a SEER Database Analysis. J Gastrointest Surg 2014;18:124-9. [Crossref]
- Pohl H, Sirovich B, Welch HG. Esophageal adenocarcinoma incidence: are we reaching the peak? Cancer Epidemiol Biomarkers Prev 2010;19:1468-70. [Crossref] [PubMed]
- Leers JM, DeMeester SR, Oezcelik A, et al. The prevalence of lymph node metastases in patients with T1 esophageal adenocarcinoma a retrospective review of esophagectomy specimens. Ann Surg 2011;253:271-8. [Crossref] [PubMed]
- Nafteux PR, Lerut TE, Villeneuve PJ, et al. Signet ring cells in esophageal and gastroesophageal junction carcinomas have a more aggressive biological behavior. Ann Surg 2014;260:1023-9. [Crossref] [PubMed]
- Desai M, Saligram S, Gupta N, et al. Efficacy and safety outcomes of multimodal endoscopic eradication therapy in Barrett's esophagus-related neoplasia: a systematic review and pooled analysis. Gastrointest Endosc 2017;85:482-495.e4. [Crossref] [PubMed]
- Bahin FF, Jayanna M, Hourigan LF, et al. Long-term outcomes of a primary complete endoscopic resection strategy for short-segment Barrett's esophagus with high-grade dysplasia and/or early esophageal adenocarcinoma. Gastrointest Endosc 2016;83:68-77. [Crossref] [PubMed]
- Moss A, Bourke MJ, Hourigan LF, et al. Endoscopic resection for Barrett's high-grade dysplasia and early esophageal adenocarcinoma: an essential staging procedure with long-term therapeutic benefit. Am J Gastroenterol 2010;105:1276-83. [Crossref] [PubMed]
- Yang D, Coman RM, Kahaleh M, et al. Endoscopic submucosal dissection for Barrett's early neoplasia: a multicenter study in the United States. Gastrointest Endosc 2017;86:600-7. [Crossref] [PubMed]
- Pech O, Behrens A, May A, et al. Long-term results and risk factor analysis for recurrence after curative endoscopic therapy in 349 patients with high-grade intraepithelial neoplasia and mucosal adenocarcinoma in Barrett's oesophagus. Gut 2008;57:1200-6. [Crossref] [PubMed]
- Tanabe S, Koizumi W, Higuchi K, et al. Clinical outcomes of endoscopic oblique aspiration mucosectomy for superficial esophageal cancer. Gastrointest Endosc 2008;67:814-20. [Crossref] [PubMed]
- Katada C, Muto M, Manabe T, et al. Local recurrence of squamous-cell carcinoma of the esophagus after EMR. Gastrointest Endosc 2005;61:219-25. [Crossref] [PubMed]
- Pech O, May A, Manner H, et al. Long-term efficacy and safety of endoscopic resection for patients with mucosal adenocarcinoma of the esophagus. Gastroenterology 2014;146:652-660.e1. [Crossref] [PubMed]
- Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2015;47:829-54. [Crossref] [PubMed]
- ASGE Standards of Practice Committee. The role of endoscopy in the assessment and treatment of esophageal cancer. Gastrointest Endosc. 2013;77:328-34. [Crossref] [PubMed]
- Raja S, Rice TW, Goldblum JR, et al. Esophageal submucosa: the watershed for esophageal cancer. J Thorac Cardiovasc Surg 2011;142:1403-11.e1. [Crossref] [PubMed]
- Chevaux JB, Piessevaux H, Jouret-Mourin A, et al. Clinical outcome in patients treated with endoscopic submucosal dissection for superficial Barrett's neoplasia. Endoscopy 2015;47:103-12. [PubMed]
- Neuhaus H, Terheggen G, Rutz EM, et al. Endoscopic submucosal dissection plus radiofrequency ablation of neoplastic Barrett's esophagus. Endoscopy 2012;44:1105-13. [Crossref] [PubMed]
- Kagemoto K, Oka S, Tanaka S, et al. Clinical outcomes of endoscopic submucosal dissection for superficial Barrett's adenocarcinoma. Gastrointest Endosc 2014;80:239-45. [Crossref] [PubMed]
- Peters FP, Brakenhoff KP, Curvers WL, et al. Endoscopic cap resection for treatment of early Barrett's neoplasia is safe: a prospective analysis of acute and early complications in 216 procedures. Dis Esophagus 2007;20:510-5. [Crossref] [PubMed]
- Terheggen G, Horn EM, Vieth M, et al. A randomised trial of endoscopic submucosal dissection versus endoscopic mucosal resection for early Barrett's neoplasia. Gut 2017;66:783-93. [Crossref] [PubMed]
- Kim MP, Brown KN, Schwartz MR, et al. Advanced esophageal cancer in patients who underwent radiofrequency ablation for barrett esophagus with high-grade dysplasia. Innovations (Phila) 2013;8:17-22. [Crossref] [PubMed]
- Yang D, Zou F, Xiong S, et al. Endoscopic submucosal dissection for early Barrett's neoplasia: a meta-analysis. Gastrointest Endosc 2017; [Epub ahead of print]. [PubMed]
- Subramaniam S, Chedgy F, Longcroft-Wheaton G, et al. Complex early Barrett’s neoplasia at 3 Western centers: European Barrett’s Endoscopic Submucosal Dissection Trial (E-BEST). Gastrointest Endosc 2017;86:608-18. [Crossref] [PubMed]
- Kuwano H, Nishimura Y, Oyama T, et al. Guidelines for Diagnosis and Treatment of Carcinoma of the Esophagus April 2012 edited by the Japan Esophageal Society. Esophagus 2015;12:1-30.
- Guo HM, Zhang XQ, Chen M, et al. Endoscopic submucosal dissection vs endoscopic mucosal resection for superficial esophageal cancer. World J Gastroenterol 2014;20:5540-7. [Crossref] [PubMed]
- Ishihara R, Iishi H, Takeuchi Y, et al. Local recurrence of large squamous-cell carcinoma of the esophagus after endoscopic resection. Gastrointest Endosc 2008;67:799-804. [Crossref] [PubMed]
- Takahashi H, Arimura Y, Masao H, et al. Endoscopic submucosal dissection is superior to conventional endoscopic resection as a curative treatment for early squamous cell carcinoma of the esophagus (with video). Gastrointest Endosc 2010;72:255-64, 264.e1-2.
- Repici A, Hassan C, Carlino A, et al. Endoscopic submucosal dissection in patients with early esophageal squamous cell carcinoma: results from a prospective Western series. Gastrointest Endosc 2010;71:715-21. [Crossref] [PubMed]
- Fujishiro M, Yahagi N, Kakushima N, et al. Endoscopic submucosal dissection of esophageal squamous cell neoplasms. Clin Gastroenterol Hepatol 2006;4:688-94. [Crossref] [PubMed]
- Sgourakis G, Gockel I, Lang H. Endoscopic and surgical resection of T1a/T1b esophageal neoplasms: a systematic review. World J Gastroenterol 2013;19:1424-37. [Crossref] [PubMed]
- Fujinami H, Hosokawa A, Ogawa K, et al. Endoscopic submucosal dissection for superficial esophageal neoplasms using the stag beetle knife. Dis Esophagus 2014;27:50-4. [Crossref] [PubMed]
- Hirasawa K, Kokawa A, Oka H, et al. Superficial adenocarcinoma of the esophagogastric junction: long-term results of endoscopic submucosal dissection. Gastrointest Endosc 2010;72:960-6. [Crossref] [PubMed]
- Ishihara R, Iishi H, Uedo N, et al. Comparison of EMR and endoscopic submucosal dissection for en bloc resection of early esophageal cancers in Japan. Gastrointest Endosc 2008;68:1066-72. [Crossref] [PubMed]
- Probst A, Aust D, Markl B, et al. Early esophageal cancer in Europe: endoscopic treatment by endoscopic submucosal dissection. Endoscopy 2015;47:113-21. [PubMed]
- Kanzaki H, Ishihara R, Ohta T, et al. Randomized study of two endo-knives for endoscopic submucosal dissection of esophageal cancer. Am J Gastroenterol 2013;108:1293-8. [Crossref] [PubMed]
- Toyonaga T, Man-i M, East JE, et al. 1,635 Endoscopic submucosal dissection cases in the esophagus, stomach, and colorectum: complication rates and long-term outcomes. Surg Endosc 2013;27:1000-8. [Crossref] [PubMed]
- Yamashita T, Zeniya A, Ishii H, et al. Endoscopic mucosal resection using a cap-fitted panendoscope and endoscopic submucosal dissection as optimal endoscopic procedures for superficial esophageal carcinoma. Surg Endosc 2011;25:2541-6. [Crossref] [PubMed]
- Mochizuki Y, Saito Y, Tsujikawa T, et al. Combination of endoscopic submucosal dissection and chemoradiation therapy for superficial esophageal squamous cell carcinoma with submucosal invasion. Exp Ther Med 2011;2:1065-8. [Crossref] [PubMed]
- Kim JS, Kim BW, Shin IS. Efficacy and safety of endoscopic submucosal dissection for superficial squamous esophageal neoplasia: a meta-analysis. Dig Dis Sci 2014;59:1862-9. [Crossref] [PubMed]
- Ono S, Fujishiro M, Niimi K, et al. Predictors of postoperative stricture after esophageal endoscopic submucosal dissection for superficial squamous cell neoplasms. Endoscopy 2009;41:661-5. [Crossref] [PubMed]
- Abe S, Iyer PG, Oda I, et al. Approaches for stricture prevention after esophageal endoscopic resection. Gastrointest Endosc 2017;86:779-91. [Crossref] [PubMed]
- Yamaguchi N, Isomoto H, Nakayama T, et al. Usefulness of oral prednisolone in the treatment of esophageal stricture after endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma. Gastrointest Endosc 2011;73:1115-21. [Crossref] [PubMed]
- Hashimoto S, Kobayashi M, Takeuchi M, et al. The efficacy of endoscopic triamcinolone injection for the prevention of esophageal stricture after endoscopic submucosal dissection. Gastrointest Endosc 2011;74:1389-93. [Crossref] [PubMed]
- Deprez PH. Esophageal strictures after extensive endoscopic resection: hope for a better outcome? Gastrointest Endosc 2013;78:258-9. [Crossref] [PubMed]
- Bahin FF, Jayanna M, Williams SJ, et al. Efficacy of viscous budesonide slurry for prevention of esophageal stricture formation after complete endoscopic mucosal resection of short-segment Barrett's neoplasia. Endoscopy 2016;48:71-4. [PubMed]
- Mori H, Rafiq K, Kobara H, et al. Steroid permeation into the artificial ulcer by combined steroid gel application and balloon dilatation: prevention of esophageal stricture. J Gastroenterol Hepatol 2013;28:999-1003. [Crossref] [PubMed]
- Wen J, Lu Z, Linghu E, et al. Prevention of esophageal strictures after endoscopic submucosal dissection with the injection of botulinum toxin type A. Gastrointest Endosc 2016;84:606-13. [Crossref] [PubMed]
- Ezoe Y, Muto M, Horimatsu T, et al. Efficacy of preventive endoscopic balloon dilation for esophageal stricture after endoscopic resection. J Clin Gastroenterol 2011;45:222-7. [Crossref] [PubMed]
- Iizuka T, Kikuchi D, Yamada A, et al. Polyglycolic acid sheet application to prevent esophageal stricture after endoscopic submucosal dissection for esophageal squamous cell carcinoma. Endoscopy 2015;47:341-4. [PubMed]
- Kim YJ, Park JC, Chung H, et al. Polyglycolic acid sheet application to prevent esophageal stricture after endoscopic submucosal dissection for recurrent esophageal cancer. Endoscopy 2016;48:E319-20. [Crossref] [PubMed]
- Sakaguchi Y, Tsuji Y, Ono S, et al. Polyglycolic acid sheets with fibrin glue can prevent esophageal stricture after endoscopic submucosal dissection. Endoscopy 2015;47:336-40. [PubMed]
- Lua GW, Tang J, Liu F, et al. Prevention of Esophageal Strictures After Endoscopic Submucosal Dissection: A Promising Therapy Using Carboxymethyl Cellulose Sheets. Dig Dis Sci 2016;61:1763-9. [Crossref] [PubMed]
- Takagi R, Murakami D, Kondo M, et al. Fabrication of human oral mucosal epithelial cell sheets for treatment of esophageal ulceration by endoscopic submucosal dissection. Gastrointest Endosc 2010;72:1253-9. [Crossref] [PubMed]
- Ohki T, Yamato M, Ota M, et al. Application of regenerative medical technology using tissue-engineered cell sheets for endoscopic submucosal dissection of esophageal neoplasms. Dig Endosc 2015;27:182-8. [Crossref] [PubMed]
- Ponsaing LG, Kiss K, Hansen MB. Classification of submucosal tumors in the gastrointestinal tract. World J Gastroenterol 2007;13:3311-5. [Crossref] [PubMed]
- Melstrom LG, Phillips JD, Bentrem DJ, et al. Laparoscopic versus open resection of gastric gastrointestinal stromal tumors. Am J Clin Oncol 2012;35:451-4. [Crossref] [PubMed]
- Ha CY, Shah R, Chen J, et al. Diagnosis and management of GI stromal tumors by EUS-FNA: a survey of opinions and practices of endosonographers. Gastrointest Endosc 2009;69:1039-44.e1. [Crossref] [PubMed]
- Grotz TE, Donohue JH. Surveillance strategies for gastrointestinal stromal tumors. J Surg Oncol 2011;104:921-7. [Crossref] [PubMed]
- Lu J, Jiao T, Zheng M, et al. Endoscopic resection of submucosal tumors in muscularis propria: the choice between direct excavation and tunneling resection. Surg Endosc 2014;28:3401-7. [Crossref] [PubMed]
- Chen H, Xu Z, Huo J, et al. Submucosal tunneling endoscopic resection for simultaneous esophageal and cardia submucosal tumors originating from the muscularis propria layer (with video). Dig Endosc 2015;27:155-8. [Crossref] [PubMed]
- Tan Y, Huo J, Liu D. Current status of submucosal tunneling endoscopic resection for gastrointestinal submucosal tumors originating from the muscularis propria layer. Oncol Lett 2017;14:5085-90. [PubMed]
- Chen T, Zhou PH, Chu Y, et al. Long-term Outcomes of Submucosal Tunneling Endoscopic Resection for Upper Gastrointestinal Submucosal Tumors. Ann Surg 2017;265:363-9. [Crossref] [PubMed]
- Wang H, Tan Y, Zhou Y, et al. Submucosal tunneling endoscopic resection for upper gastrointestinal submucosal tumors originating from the muscularis propria layer. Eur J Gastroenterol Hepatol 2015;27:776-80. [Crossref] [PubMed]
- Jain D, Desai A, Mahmood E, et al. Submucosal tunneling endoscopic resection of upper gastrointestinal tract tumors arising from muscularis propria. Ann Gastroenterol 2017;30:262-72. [PubMed]
- Ge N, Sun S, Wang S, et al. Endoscopic Ultrasound-Assisted Tunnel-Type Endoscopic Submucosal Dissection for the Treatment of Esophageal Tumors Arising in the Muscularis Propria (with video). Endosc Ultrasound 2013;2:11-5. [Crossref] [PubMed]
- Gong W, Xiong Y, Zhi F, et al. Preliminary experience of endoscopic submucosal tunnel dissection for upper gastrointestinal submucosal tumors. Endoscopy 2012;44:231-5. [Crossref] [PubMed]
- Li QL, Chen WF, Zhang C, et al. Clinical impact of submucosal tunneling endoscopic resection for the treatment of gastric submucosal tumors originating from the muscularis propria layer (with video). Surg Endosc 2015;29:3640-6. [Crossref] [PubMed]
- Inoue H, Ikeda H, Hosoya T, et al. Submucosal endoscopic tumor resection for subepithelial tumors in the esophagus and cardia. Endoscopy 2012;44:225-30. [Crossref] [PubMed]
- Du C, Ma L, Chai N, et al. Factors affecting the effectiveness and safety of submucosal tunneling endoscopic resection for esophageal submucosal tumors originating from the muscularis propria layer. Surg Endosc 2018;32:1255-64. [Crossref] [PubMed]
- Lv XH, Wang CH, Xie Y. Efficacy and safety of submucosal tunneling endoscopic resection for upper gastrointestinal submucosal tumors: a systematic review and meta-analysis. Surg Endosc 2017;31:49-63. [Crossref] [PubMed]
- Du C, Linghu E. Submucosal Tunneling Endoscopic Resection for the Treatment of Gastrointestinal Submucosal Tumors Originating from the Muscularis Propria Layer. J Gastrointest Surg 2017;21:2100-9. [Crossref] [PubMed]
- Du C, Linghu E. Treatment of esophageal submucosal tumors: endoscopic submucosal tunneling dissection versus thoracoscopic enucleation. Gastrointest Endosc 2017;86:925. [Crossref] [PubMed]
- Jeong ES, Hong SJ, Han JP, et al. Submucosal Tunneling Endoscopic Resection of a Leiomyoma Originating from the Muscularis Propria of the Gastric Cardia (with Video). Korean J Gastroenterol 2015;66:340-4. [Crossref] [PubMed]
- Maeda Y, Hirasawa D, Fujita N, et al. A pilot study to assess mediastinal emphysema after esophageal endoscopic submucosal dissection with carbon dioxide insufflation. Endoscopy 2012;44:565-71. [Crossref] [PubMed]
- Higashiyama M, Oka S, Tanaka S, et al. Risk factors for bleeding after endoscopic submucosal dissection of gastric epithelial neoplasm. Dig Endosc 2011;23:290-5. [Crossref] [PubMed]
- Oda I, Saito D, Tada M, et al. A multicenter retrospective study of endoscopic resection for early gastric cancer. Gastric Cancer 2006;9:262-70. [Crossref] [PubMed]
- Hamashima C, Shibuya D, Yamazaki H, et al. The Japanese guidelines for gastric cancer screening. Jpn J Clin Oncol 2008;38:259-67. [Crossref] [PubMed]
- Park HA, Nam SY, Lee SK, et al. The Korean guideline for gastric cancer screening. J Korean Med Assoc 2015;58:373-84. [Crossref]
- Saragoni L. Upgrading the definition of early gastric cancer: better staging means more appropriate treatment. Cancer Biol Med 2015;12:355-61. [PubMed]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 2011;14:113-23. [Crossref] [PubMed]
- Shim CN, Lee SK. Endoscopic submucosal dissection for undifferentiated-type early gastric cancer: do we have enough data to support this? World J Gastroenterol 2014;20:3938-49. [Crossref] [PubMed]
- Fukase K, Kato M, Kikuchi S, et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet 2008;372:392-7. [Crossref] [PubMed]
- Chung IK, Lee JH, Lee SH, et al. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc 2009;69:1228-35. [Crossref] [PubMed]
- Ahn JY, Jung HY, Choi KD, et al. Endoscopic and oncologic outcomes after endoscopic resection for early gastric cancer: 1370 cases of absolute and extended indications. Gastrointest Endosc 2011;74:485-93. [Crossref] [PubMed]
- Choi MK, Kim GH, Park DY, et al. Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: a single-center experience. Surg Endosc 2013;27:4250-8. [Crossref] [PubMed]
- Choi IJ, Lee NR, Kim SG, et al. Short-Term Outcomes of Endoscopic Submucosal Dissection in Patients with Early Gastric Cancer: A Prospective Multicenter Cohort Study. Gut Liver 2016;10:739-48. [Crossref] [PubMed]
- Matsuda T, Ajiki W, Marugame T, et al. Population-based survival of cancer patients diagnosed between 1993 and 1999 in Japan: a chronological and international comparative study. Jpn J Clin Oncol 2011;41:40-51. [Crossref] [PubMed]
- Peng LJ, Tian SN, Lu L, et al. Outcome of endoscopic submucosal dissection for early gastric cancer of conventional and expanded indications: systematic review and meta-analysis. J Dig Dis 2015;16:67-74. [Crossref] [PubMed]
- Cho JH, Cha SW, Kim HG, et al. Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: a comparison study to surgery using propensity score-matched analysis. Surg Endosc 2016;30:3762-73. [Crossref] [PubMed]
- Chiu PW, Teoh AY, To KF, et al. Endoscopic submucosal dissection (ESD) compared with gastrectomy for treatment of early gastric neoplasia: a retrospective cohort study. Surg Endosc 2012;26:3584-91. [Crossref] [PubMed]
- Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell 1990;61:759-67. [Crossref] [PubMed]
- Zauber AG, Winawer SJ, O'Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med 2012;366:687-96. [Crossref] [PubMed]
- Hotta K, Saito Y, Matsuda T, et al. Local recurrence and surveillance after endoscopic resection of large colorectal tumors. Dig Endosc 2010;22:S63-8. [Crossref] [PubMed]
- Sakamoto T, Matsuda T, Otake Y, et al. Predictive factors of local recurrence after endoscopic piecemeal mucosal resection. J Gastroenterol 2012;47:635-40. [Crossref] [PubMed]
- Tanaka S, Kashida H, Saito Y, et al. JGES guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig Endosc 2015;27:417-34. [Crossref] [PubMed]
- Tanaka S, Oka S, Chayama K. Colorectal endoscopic submucosal dissection: present status and future perspective, including its differentiation from endoscopic mucosal resection. J Gastroenterol 2008;43:641-51. [Crossref] [PubMed]
- Fujishiro M, Yahagi N, Kakushima N, et al. Outcomes of endoscopic submucosal dissection for colorectal epithelial neoplasms in 200 consecutive cases. Clin Gastroenterol Hepatol 2007;5:678-83; quiz 645. [Crossref] [PubMed]
- Isomoto H, Nishiyama H, Yamaguchi N, et al. Clinicopathological factors associated with clinical outcomes of endoscopic submucosal dissection for colorectal epithelial neoplasms. Endoscopy 2009;41:679-83. [Crossref] [PubMed]
- Niimi K, Fujishiro M, Kodashima S, et al. Long-term outcomes of endoscopic submucosal dissection for colorectal epithelial neoplasms. Endoscopy 2010;42:723-9. [Crossref] [PubMed]
- Kotake K, Honjo S, Sugihara K, et al. Changes in colorectal cancer during a 20-year period: an extended report from the multi-institutional registry of large bowel cancer, Japan. Dis Colon Rectum 2003;46:S32-43. [PubMed]
- Yoshii S, Nojima M, Nosho K, et al. Factors associated with risk for colorectal cancer recurrence after endoscopic resection of T1 tumors. Clin Gastroenterol Hepatol 2014;12:292-302.e3. [Crossref] [PubMed]
- Ikematsu H, Yoda Y, Matsuda T, et al. Long-term outcomes after resection for submucosal invasive colorectal cancers. Gastroenterology 2013;144:551-9; quiz e14.
- Barendse RM, van den Broek FJ, van Schooten J, et al. Endoscopic mucosal resection vs transanal endoscopic microsurgery for the treatment of large rectal adenomas. Colorectal Dis 2012;14:e191-6. [Crossref] [PubMed]
- Arezzo A, Passera R, Saito Y, et al. Systematic review and meta-analysis of endoscopic submucosal dissection versus transanal endoscopic microsurgery for large noninvasive rectal lesions. Surg Endosc 2014;28:427-38. [Crossref] [PubMed]
- Kudo S, Hirota S, Nakajima T, et al. Colorectal tumours and pit pattern. J Clin Pathol 1994;47:880-5. [Crossref] [PubMed]
- Matsuda T, Fujii T, Saito Y, et al. Efficacy of the invasive/non-invasive pattern by magnifying chromoendoscopy to estimate the depth of invasion of early colorectal neoplasms. Am J Gastroenterol 2008;103:2700-6. [Crossref] [PubMed]
- Tanaka S, Tamegai Y, Tsuda S, et al. Multicenter questionnaire survey on the current situation of colorectal endoscopic submucosal dissection in Japan. Dig Endosc 2010;22:S2-8. [Crossref] [PubMed]
- Tanaka S, Terasaki M, Hayashi N, et al. Warning for unprincipled colorectal endoscopic submucosal dissection: accurate diagnosis and reasonable treatment strategy. Dig Endosc 2013;25:107-16. [Crossref] [PubMed]
- Sakamoto T, Saito Y, Nakajima T, et al. Comparison of magnifying chromoendoscopy and narrow-band imaging in estimation of early colorectal cancer invasion depth: a pilot study. Dig Endosc 2011;23:118-23. [Crossref] [PubMed]
- Yoshida N, Hisabe T, Inada Y, et al. The ability of a novel blue laser imaging system for the diagnosis of invasion depth of colorectal neoplasms. J Gastroenterol 2014;49:73-80. [Crossref] [PubMed]
- Santoro GA, Gizzi G, Pellegrini L, et al. The value of high-resolution three-dimensional endorectal ultrasonography in the management of submucosal invasive rectal tumors. Dis Colon Rectum 2009;52:1837-43. [Crossref] [PubMed]
- Saitoh Y, Obara T, Einami K, et al. Efficacy of high-frequency ultrasound probes for the preoperative staging of invasion depth in flat and depressed colorectal tumors. Gastrointest Endosc 1996;44:34-9. [Crossref] [PubMed]
- Yamada M, Saito Y, Takamaru H, et al. Long-term clinical outcomes of endoscopic submucosal dissection for colorectal neoplasms in 423 cases: a retrospective study. Endoscopy 2017;49:233-42. [Crossref] [PubMed]
- Yamada M, Saito Y, Sakamoto T, et al. Endoscopic predictors of deep submucosal invasion in colorectal laterally spreading tumors. Endoscopy 2016;48:456-64. [Crossref] [PubMed]
- Oka S, Tanaka S, Saito Y, et al. Local recurrence after endoscopic resection for large colorectal neoplasia: a multicenter prospective study in Japan. Am J Gastroenterol 2015;110:697-707. [Crossref] [PubMed]
- Puli SR, Kakugawa Y, Saito Y, et al. Successful complete cure en-bloc resection of large nonpedunculated colonic polyps by endoscopic submucosal dissection: a meta-analysis and systematic review. Ann Surg Oncol 2009;16:2147-51. [Crossref] [PubMed]
- Fujiya M, Tanaka K, Dokoshi T, et al. Efficacy and adverse events of EMR and endoscopic submucosal dissection for the treatment of colon neoplasms: a meta-analysis of studies comparing EMR and endoscopic submucosal dissection. Gastrointest Endosc 2015;81:583-95. [Crossref] [PubMed]
- Saito Y, Uraoka T, Yamaguchi Y, et al. A prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video). Gastrointest Endosc 2010;72:1217-25. [Crossref] [PubMed]
- Fuccio L, Hassan C, Ponchon T, et al. Clinical outcomes after endoscopic submucosal dissection for colorectal neoplasia: a systematic review and meta-analysis. Gastrointest Endosc 2017;86:74-86.e17. [Crossref] [PubMed]
- Kiriyama S, Saito Y, Yamamoto S, et al. Comparison of endoscopic submucosal dissection with laparoscopic-assisted colorectal surgery for early-stage colorectal cancer: a retrospective analysis. Endoscopy 2012;44:1024-30. [Crossref] [PubMed]
- Nakamura F, Saito Y, Sakamoto T, et al. Potential perioperative advantage of colorectal endoscopic submucosal dissection versus laparoscopy-assisted colectomy. Surg Endosc 2015;29:596-606. [Crossref] [PubMed]
- Nakamura F, Saito Y, Haruyama S, et al. Short-term Prospective Questionnaire Study of Early Postoperative Quality of Life After Colorectal Endoscopic Submucosal Dissection. Dig Dis Sci 2017;62:3325-35. [Crossref] [PubMed]
- Kiriyama S, Saito Y, Matsuda T, et al. Comparing endoscopic submucosal dissection with transanal resection for non-invasive rectal tumor: a retrospective study. J Gastroenterol Hepatol 2011;26:1028-33. [Crossref] [PubMed]
- Buchner AM, Guarner-Argente C, Ginsberg GG. Outcomes of EMR of defiant colorectal lesions directed to an endoscopy referral center. Gastrointest Endosc. 2012;76:255-63. [Crossref] [PubMed]
- Terasaki M, Tanaka S, Oka S, et al. Clinical outcomes of endoscopic submucosal dissection and endoscopic mucosal resection for laterally spreading tumors larger than 20 mm. J Gastroenterol Hepatol 2012;27:734-40. [Crossref] [PubMed]
- Moss A, Bourke MJ, Williams SJ, et al. Endoscopic mucosal resection outcomes and prediction of submucosal cancer from advanced colonic mucosal neoplasia. Gastroenterology 2011;140:1909-18. [Crossref] [PubMed]
- Tate DJ, Desomer L, Klein A, et al. Adenoma recurrence after piecemeal colonic EMR is predictable: the Sydney EMR recurrence tool. Gastrointest Endosc 2017;85:647-56.e6. [Crossref] [PubMed]
- Kobayashi N, Yoshitake N, Hirahara Y, et al. Matched case-control study comparing endoscopic submucosal dissection and endoscopic mucosal resection for colorectal tumors. J Gastroenterol Hepatol 2012;27:728-33. [Crossref] [PubMed]
- Nakajima T, Saito Y, Tanaka S, et al. Current status of endoscopic resection strategy for large, early colorectal neoplasia in Japan. Surg Endosc 2013;27:3262-70. [Crossref] [PubMed]
- Kikuchi T, Fu KI, Saito Y, et al. Transcutaneous monitoring of partial pressure of carbon dioxide during endoscopic submucosal dissection of early colorectal neoplasia with carbon dioxide insufflation: a prospective study. Surg Endosc 2010;24:2231-5. [Crossref] [PubMed]
- Repici A, Pellicano R, Strangio G, et al. Endoscopic mucosal resection for early colorectal neoplasia: pathologic basis, procedures, and outcomes. Dis Colon Rectum 2009;52:1502-15. [Crossref] [PubMed]
- Ballas KD, Rafailidis SF, Triantaphyllou A, et al. Retroperitoneal, mediastinal, and subcutaneous emphysema, complicating colonoscopy and rectal polypectomy. J Laparoendosc Adv Surg Tech A 2008;18:717-20. [Crossref] [PubMed]
- Liaquat H, Rohn E, Rex DK. Prophylactic clip closure reduced the risk of delayed postpolypectomy hemorrhage: experience in 277 clipped large sessile or flat colorectal lesions and 247 control lesions. Gastrointest Endosc 2013;77:401-7. [Crossref] [PubMed]
- Shioji K, Suzuki Y, Kobayashi M, et al. Prophylactic clip application does not decrease delayed bleeding after colonoscopic polypectomy. Gastrointest Endosc 2003;57:691-4. [Crossref] [PubMed]
- Imagawa A, Okada H, Kawahara Y, et al. Endoscopic submucosal dissection for early gastric cancer: results and degrees of technical difficulty as well as success. Endoscopy 2006;38:987-90. [Crossref] [PubMed]
- Yoshida M, Takizawa K, Suzuki S, et al. Conventional versus traction-assisted endoscopic submucosal dissection for gastric neoplasms: a multicenter, randomized controlled trial (with video). Gastrointest Endosc 2017; [Epub ahead of print]. [Crossref] [PubMed]
Cite this article as: Mejía Pérez LK, Abe S, McNamara M, Sohal D, Erim T, Sanaka MR, Raja S, Murthy S, Vargo JJ, Saito Y, Bhatt A. Minimally invasive treatment of early gastrointestinal cancers. Ann Laparosc Endosc Surg 2018;3:23.


