
Laparoscopic rectal resection—the road to safety surgery
Introduction
Total mesorectal excision (TME) indicates a surgical technique for the removal of the rectum and mesorectum during an anterior resection for rectal cancer. The concept of complete TME was first described by Heald in 1979 (1). The mesorectum is the fat tissue that surrounds the rectum, it contains blood vessels, lymph vessels and lymph nodes and autonomic nerves. Rectal cancer, in most cases, is confined to the mesorectum, therefore, to obtain definitive cure of the disease the complete removal of this package is necessary. In fact, being associated with low local recurrence rates, this technique became the gold standard for rectal cancer (2). To fully understand the surgical technique described by Heald, it is important to define what in literature is called the ‘holy plane’. In the pelvis, the visceral compartment is enveloped in the visceral fascia, which posteriorly covers the mesorectum. This fascia is connected with a layer of loose tissue which can be divided, opening the space of the Retzius anteriorly and the retrorectal space posteriorly. This dissection plane is called ‘holy plane’ or TME plane, described by Heald in 1988 (3).
In the last decade laparoscopic total mesorectal excision (LTME) has been introduced as an alternative to open TME (OTME) and proved to be equivalent by many multicentric trials (4-6). Anyhow, the laparoscopic approach is technically more challenging than the open one, and its learning curve is often longer. Moreover, TME is proven to be the hardest task to learn to perform, among the various phases of the anterior resection (7).
To facilitate the learning of this technique and, therefore, to improve its safety, many Authors all over the world have tried to standardize it. For an example Miskovic et al. in their recent work tried to establish a structured international consensus on a detailed technical description of laparoscopic TME. At the moment most of the phases of the operation have been internationally standardized, but a few controversies are still open. In fact, a debate is still going on about the height of section of the inferior mesenteric artery (IMA) and the routine mobilization of the splenic flexure.
To better understand the implications of the ligation of the IMA it is necessary to understand its anatomical features. The IMA, originates from the abdominal aorta and supplies the splenic flexure, the descending colon, the sigmoid colon and the upper part of the rectum. IMA’s branches are the left colic artery, the sigmoid branches and the superior rectal artery. Proximally, its territory of distribution overlaps with the middle colic artery, a branch of the superior mesenteric artery (SMA). In fact, the SMA and IMA anastomose at two levels: the artery of Drummond and the Riolan’s arcade. These anastomotic sites are essential to the correct vascularization of the colon. It is worth mentioning the existence of a vascular site particularly susceptible to ischemia in case of insufficiency or deficiency of these anastomotic branches, resulting particularly interesting in rectal surgery: Griffith’s critical point. It is defined as the site of communication of the ascending left colic artery with the marginal artery of Drummond and anastomotic bridging between the right and left terminal branches of the ascending left colic artery at the splenic flexure of the colon (8). Therefore, splenic flexure vascularization results dependent from the collateral circulation between the SMA and the marginal artery branch of the IMA, supplying the descending colon.
This critical point described become even more critical when associated to vascular arteriopathy, in particular in the elderly.
Considering the anterior resection of the rectum, the ligation of the IMA at its origin is preferred by many surgeons (9). Anyway, the high-tie section of the IMA leads to the sacrifice of the left colic artery, leaving the Drummond artery supplying blood for the splenic flexure.
Splenic flexure mobilization is usually performed to prevent tension on the anastomosis. When the proximal colon stump can reach the pubic symphysis, the anastomosis will be tension-free.
It is worth mentioning that, as demonstrated by Buunen et al., only in 20% of cases the descending branch of the left colic artery represents a limitation factor for the creation of a tension-free anastomosis, so in 80% of cases low-tie ligation can be performed.
It is important to underline how IMA ligation and splenic flexure mobilization are interconnected and if badly managed can lead to complications, such as the anastomotic leakage, which is due to the combination of low perfusion and tension on the anastomosis.
In our opinion, if the operator decides to go for high-tie ligation of the IMA, the splenic flexure should be always mobilized to prevent ischaemic complications at Griffith’s critical point. While if the operator decides to perform a low-tie ligation, the splenic flexure can be spared or mobilized, in case of short rectal stump, to prevent anastomotic tension.
All considered, laparoscopic rectal resection represents a challenging operation characterized by a long learning curve that must be performed by expert operators.
The main purpose of this study is to suggest a standardization of the procedure. As Strasberg (10,11) did for colecistectomia procedure, we suggested a critical view of safety for laparoscopic rectal resection, advising some tips and tricks which could benefit its learning and its performance.
Operative technique
Equipe positioning
Operator (surgeon) on the right side of the patient; 1st Assistant on the right side of the patient, at left of the operator; 2nd Assistant between the legs of the patient; nurse on the left side of the patient.
Port placement
T1: 10 mm port placed 2 cm above the umbilicus along the midline.
T2: 10 mm port placed in the right flank, 1 cm above the transverse umbilical line, along the longitudinal emiclavear line.
T3: 5 mm port in the left flank, 1 cm above the transverse umbilical line, along the longitudinal emiclavear line.
T4: 12 mm port placed in right iliac region, at least 2 cm far from the hipbone.
T5: 12 mm port placed in the sovrapubic region, along the midline, on the Pfannenstiel incision line.
Splenic flexure mobilization
T1 camera (1st assistant) T2 operator’s left hand T3 operator’s right hand T4 2nd assistant’s left hand
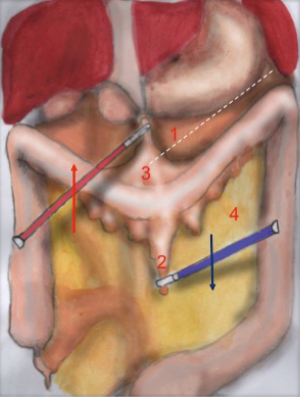
The operator with his left hand in T2 grabs the omentum, pulling it up and keeping it in traction. The colon is pulled downwards by the 2nd assistant in T4, by grasping epiploic appendix. The dissection line is highlighted by the operator’s traction and assistant’s counter traction (Figure 1). Surgeon’s right hand performs the coloepiploic detachment, opening the omental bursa. Dissection proceeds from medial to lateral, up to reach the splenocolic ligament, which gets resected to complete the mobilization of the splenic flexure (Figure 2).
Critical view of safety
The main landmarks of the dissection line are: superiorly the limit of the omentum flipped upwards and inferiorly the colon profile counter-tractioned downwards.
The identification of the posterior gastric wall confirms the right opening of the lasser sack. The detachment of splenic flexure is considered complete when the colon can be moved completely (‘like the page of a book’) and if it results completely detached from the omentum. It must be reminded that, sometimes, the omentum can lay on the antimesenteric side of the colon, strongly adhering to it. In these cases it is important to perform a careful detachment of the two.
Tricks: the instruments of both the operator and the assistant must proceed gradually in the direction of the dissection, as it moves on. The continuous traction and counter traction of gastrocolic and splenocolic ligaments allow an easier dissection, additionally facilitated by the intra-bdominal pressure created by the pneumoperitoneum.
Vascular dissection
T2 camera (1st assistant) T1 operator’s left hand T4 operator’s right hand T3 1st assistant’s left hand
The peritoneum is opened from the Treitz to the pelvic cavity, following the descending path of the aorta.
Vascular dissection is based on the identification of the embryological plane which divides Toldt’s fascia from Gerota’s fascia.
The plane is dissected at first under the inferior mesenteric vein. Thereafter the dissection proceeds under the IMA at its origin, 2 cm from the aorta (high-tie vascular dissection). Eventually, the two planes are joined and the colon detachment is complete.
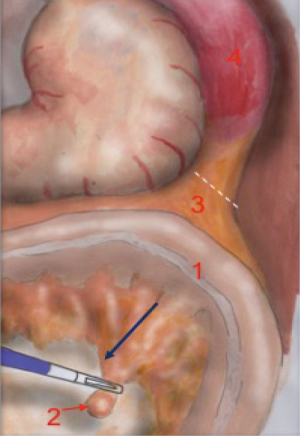
The operator pulls the mesenteric vein up to create an arch-shaped space underneath which he can divide the Toldt’s fascia from the Gerota’s fascia. The dissection begins right next to the Treitz ligament, where the identification of the vein is easier (Figure 3).
The dissection moves towards the plane already dissected during splenic mobilization. The pancreatic margin can be clearly identified on the left. The dissection of the vein ends with the detachment of the mesocolon from the pancreatic margin, after which the mobilization of the colon is completed.
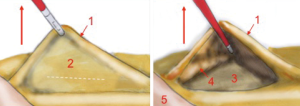
Thereafter, the operator, with his left hand grabs the IMA with a clamp and keeps it suspended. By doing so, all the retroperitoneal structures, like gonadal vessels, ureter, sympathetic nervous plexus are left behind the meso of the colon. This is made possible also by the assistant who pulls up and left the sigmoid and, consequently, the vascular axes (Figure 4).
The surgeon opens the visceral peritoneum and proceeds with the identification of the hypogastric nervous plexus, the abdominal aorta, the iliac vessels and the left ureter. This phase of identification of the anatomic landmarks is done by a dissection that goes from medial to lateral and upwards, inside the peritoneal widow just created. The dissection moves upwards, until the origin of the IMA, and laterally until the posterior muscular plane. After identifying the left ureter, always pulling up the mesosigmoid, the inferior mesenteric axis is put in tension, isolated and dissected at its origin.
The operation goes on by pulling up the vascular axis and the meso of the sigmoid and of the descending colon, and sticking the laparoscope and the laparoscopic tools under these structures. By doing so, the dissection can proceed from medially to laterally and upwards. Laparoscopic magnification and pressure caused by pneumoperitoneum allow the precise identification of the posterior side of the mesocolon, always kept in tension.
Critical view of safety
The anatomical landmarks that must be identified during mesenteric vein dissection are: the mesenteric vein tractioned upwards, creating a vascular arch superiorly and the Treitz ligament inferiorly. The floor of the plane is represented by the retroperitoneal space. The pancreatic margin must be identified on the left and is slightly elevated compared to the retroperitoneal plane created by the surgeon.
The anatomical landmarks that must be identified during mesenteric artery dissection are: the vascular axis perpendicular to the plane, the sigmoid superiorly and the aorta inferiorly. The floor of the plane is represented by the plane of the ureter that should be identified before artery dissection.
Tricks: to facilitate the dissection it is essential to create the right amount of tension on the tissues. This is made possible by a continuous gentle traction of the mesenteric vein before and of the sigmoid and vascular axis thereafter.
Sharp dissection must not be performed. The division line between the Toldt’s fascia and Gerota’s fascia has to be found with gentle dissection movements. The operator’s tools move gently up and down between the two fascial tissues, in order to gently separate them (‘just like opening a plastic grocery bag’). The identification of the correct plane is guaranteed by the avascularity of the dissection. If you meet any bleeding during dissection, you may got lost.
Paracolic gut detachment
The parietal peritoneum is dissected alongside the lateral margin of the colon, beginning from the proximal sigma up to the splenic flexure. The first assistant grabs the colon from T1, keeping a few cm from the grab of the operator, and suspends it promoting the separation from the parietal peritoneum (Figure 5).
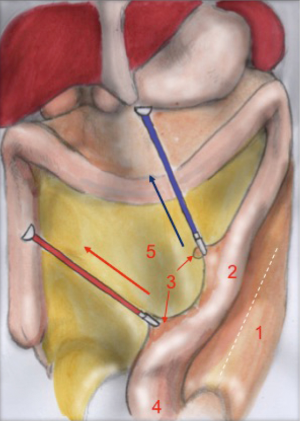
Mesorectal dissection
T2 camera (1st assistant) T1 operator’s left hand T4 operator’s right hand T3 1st assistant’s left hand T5 2nd assistant
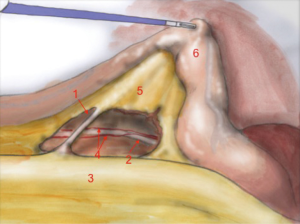
The first assistant (T3) holds rectosigmoid and retracts it cranially, so that the operator can start circular dissection of pelvic peritoneum. The assistant moves the rectosigmoid laterally, anteriorly or posteriorly synchronously with the operator’s position, in order to facilitate surgeon’s circular dissection (Figure 6).
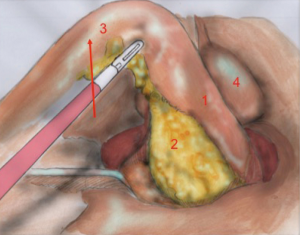
Also in laparoscopy, times of operation are the same described by Heald, starting from the posterior dissection moving down the pelvis, while the rectum is held high by the assistant (a laparoscopic retractor can be inserted is inserted in T5). Posterior dissection gets deeper and deeper, keeping on the sacral plane, paying attention to keep the mesorectal fascia intact. The posterior plane proceeds vertically down until the tip of the coccyx where, after the dissection of rectal-sacral ligament, it turns horizontal at the level of the elevator of the anus. It is advisable to go down as far as possible with the posterior dissection before starting the lateral and the anterior dissection. Once the posterior part is completely dissected, the dissection moves laterally (left and right). The operator is helped by the assistant, who performs countertraction. The retractor holds up the uterus in women and the bladder in both sexes. Laterally the ‘rectal wings’ are dissected. In this plane the vascularization is minimum but attention must be paid to the nervous system. Anteriorly the peritoneum is incised at 1 cm from the Douglas reflection so that the Douglas pouch can be entirely removed. The dissection moves in front of the Denonvilliers’ fascia where, in men, seminal vesicles can be identified while in women the vaginal wall.
A laparoscopic retractor in inserted in T5 during the dissection of the anterior side of the rectum. Its main function is to place a retractor to lift uterus and vagina in women and prostate and seminal vesicles in men.
Critical view of safety
The anatomical landmarks are: superiorly the rectum retracted upwards, inferiorly the sacrum, laterally pelvic walls and nervous plexus and anteriorly the vaginal wall, in women, and the seminal vesicles, in men.
Tricks: mesorectal dissection occurs in a total avascular plane that, once identified, can be easily dissected, exposing ‘the white side of the yellow’ as Heald first described in 1979. As the posterior dissection continues downward, the mesorectum looks more and more bilobate (indicating a good quality of the dissection) until it thins out and disappears.
Once again traction and counter traction are essential in lateral and anterior resection to identify the dissection plane. Working in the right dissection plane means to protect the nervous structures laterally and the seminal vesicles/vagina anteriorly. Moreover, the identification of the nervous structures at this level is facilitated by laparoscopic magnification.
Rectal dissection
Upwards continuous traction (left and right), counter traction obtained by the laparoscopic retractor and, sometimes, pushing the pelvic floor up for a few centimeters, associated with laparoscopic vision, allow an extremely precise dissection. Distal rectum gets dissected using a laparoscopic linear stapler, inserted from T5.
Acknowledgments
Thanks to Dr. Bianco Paolo for the medical illustrations.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Laparoscopic and Endoscopic Surgery for the series “Minimally Invasive Treatment of Low Rectal Cancer”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2017.08.09). The series “Minimally Invasive Treatment of Low Rectal Cancer” was commissioned by the editorial office without any funding or sponsorship. Marco Milone served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Annals of Laparoscopic and Endoscopic Surgery from Aug 2016 to Jul 2018. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Informed Consent: Written informed consent was obtained from the patients for publication of this article and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Heald RJ. A new approach to rectal cancer. Br J Hosp Med 1979;22:277-81. [PubMed]
- Dahlberg M, Påhlman L, Bergström R, et al. Improved survival in patients with rectal cancer: a population-based register study. Br J Surg 1998;85:515-20. [Crossref] [PubMed]
- Heald RJ. The 'Holy Plane' of rectal surgery. J R Soc Med 1988;81:503-8. [PubMed]
- Bonjer HJ, Hop WC, Nelson H, et al. Laparoscopically assisted vs open colectomy for colon cancer: a meta-analysis. Arch Surg 2007;142:298-303. [Crossref] [PubMed]
- van der Pas MH, Haglind E, Cuesta MA, et al. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol 2013;14:210-8. [Crossref] [PubMed]
- Kang SB, Park JW, Jeong SY, et al. Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): short-term outcomes of an open-label randomised controlled trial. Lancet Oncol 2010;11:637-45. [Crossref] [PubMed]
- Hartley JE, Mehigan BJ, Qureshi AE, et al. Total mesorectal excision: assessment of the laparoscopic approach. Dis Colon Rectum 2001;44:315-21. [Crossref] [PubMed]
- Meyers MA. Griffiths' point: critical anastomosis at the splenic flexure. Significance in ischemia of the colon. AJR Am J Roentgenol 1976;126:77-94. [Crossref] [PubMed]
- Buunen M, Lange MM, Ditzel M, et al. Level of arterial ligation in total mesorectal excision (TME): an anatomical study. Int J Colorectal Dis 2009;24:1317-20. [Crossref] [PubMed]
- Strasberg SM, Brunt LM. The Critical View of Safety: Why It Is Not the Only Method of Ductal Identification Within the Standard of Care in Laparoscopic Cholecystectomy. Ann Surg 2017;265:464-5. [Crossref] [PubMed]
- Strasberg SM. A perspective on the critical view of safety in laparoscopic cholecystectomy. Ann Laparosc Endosc Surg 2017;2:91. [Crossref]
Cite this article as: Milone M, Manigrasso M, Burati M. Laparoscopic rectal resection—the road to safety surgery. Ann Laparosc Endosc Surg 2017;2:141.


