
Single anastomosis (mini-) gastric bypass for the treatment of Asian type 2 diabetes mellitus
Introduction
Obesity is closely associated with type 2 diabetes mellitus (T2DM) (1). Both diseases are very difficult to control and became a major growing health problem in both developing and developed countries (2). Bariatric surgery proved to be the most effective therapy for maintaining a successful weight loss and improving obesity related co-morbidities (3). The success of bariatric surgery in the treatment of T2DM in morbidly obese patients made it a valid option in the treatment of not-well controlled T2DM in mildly obese patients (4). However, bariatric/metabolic operations have been evolving over the past 60 years and have witnessed a significant increase of the volume since the advent of laparoscopic surgery (5). Rutledge performed the first mini-gastric bypass (MGB) in 1997 and published his initial experience of 1,274 patients in 2001 (6). However, criticisms and controversies about this procedure were raised immediately from many surgeons who do not perform it (7,8). Their main concern was focused on the incidence of bile reflux associated with symptomatic gastritis and esophagitis as was mentioned in Mason’s original experience on loop gastric bypass. However, MGB gained worldwide acceptance after demonstrating its safety and efficacy which was proven by a randomized trial done by Lee et al. comparing it to the conventional Roux-en-Y gastric bypass (RYGB) (9). Tens of thousands of this procedure have reported throughout the world and the procedure was re-named to a laparoscopic single anastomosis (mini-) gastric bypass (LSAGB) in order to avoid the controversy (10). In addition to a sustained weight loss and remission of the obesity relation co-morbidities, this procedure provides other advantages such as technique simplicity, shorter learning curve and easy to conversion (10-12). Accordingly, this procedure was proposed as a metabolic operation for treating T2DM (13-15). In this article, we examine the current status, weight loss sustainability on the long-term and T2DM remission after this procedure.
Technical aspects
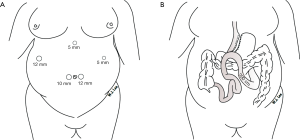
The operation consists of two components, the first component is performing a long-sleeved gastric tube along the lesser curvature of the stomach and the second component is a Billroth II loop gastrojejunostomy with a 200 cm afferent limb. The operation, LSAGB is routinely performed with a standard 5-port laparoscopic technique (Figure 1). Patients were placed in a gentle reverse Trendelenburg position. A total of five incisions placed at four sites of the abdomen including: (I) two incisions along the nature fold of the umbilicus (10 mm camera port and 12 mm port as a working channel); (II) a 5 mm port incision at the left lateral abdominal wall (working channel); (III) a 12 mm port incision at the right lateral abdominal wall (first assistant); (IV) a stab incision at subxyphoid level to provide retraction of the left lobe of the liver. Exposure of the E-G junction is achieved by dissecting the gastric fat pad. Starting from the antrum distal to the crow’s foot we begin the creation of a long sleeved gastric tube heading all the way to the E-G junction. Following this, we identify the jejunum at the ligament of Treitz and measure 150 to 250 cm distally according to the patient’s BMI. A whole length of the intestine is measured to make sure that the common channel is more than four meters. Antecolic Billroth type 2 side-side gastrojejunostomy was performed using a stapling technique. The gastro-enteric defect is then closed by hand-sewn technique over 18F nasogastric tube placed into the efferent loop to ensure the patency of the anastomosis. Anchoring the afferent limb with continuous suture to prevent bile reflux was performed. The efferent limb is then fixed to the antrum to avoid torsion of the loop (9,10).
Operative risk
SAGB has a lower operative risk in comparison to the standard RYGB. In comparing 40 patients with SAGB to 40 patients with RYGB, a randomized clinical trial showed that the SAGB arm had a shorter operative time, less post-operative analgesia use, shorter hospital stay and fewer post-operative complications (9), therefore reflecting the relative simplicity of the procedure as only one anastomosis is required leading to a better risk profile compared to RYGB. RYGB is technically more difficult as it requires two anastomoses, one higher in the abdomen to the gastric pouch and one jejuno-jejunal anastomosis in the lower abdomen. A RYGB requires a bivalve of the greater omentum in order to reduce the tension on the gastro-jejunostomy, in addition to closing the mesenteric defects which is not the case in SAGB, and this adds more time and complexity to the procedure. A bivalve of the greater omentum is not required in SAGB because the posterior aspect of the loop becomes adherent to the omentum. The learning curve of SAGB is estimated to be approximately 30 cases (11), much less than the 100 to 500 cases in RYGB (16,17). Currently this procedure represents around 1.5% of bariatric surgeries worldwide, which is a similar figure to biliopancreatic division (BPD)/duodenal switch (DS) (5). In the English literature, more than 11,000 cases of SAGB have been reported to date (18-26). A major complication rate of less than 3%, and a leak rate of approximately 1% is illustrated by the current published observational studies (Table 1). Average 30-day mortality rate was 0.11% (ranged from 0–0.9%).
Table 1
| Author | n | Starting year | Report year | Major C (%) | Mortality (%) | Initial BMI (kg/m2) | Final BMI (kg/m2) | %TWL | %EWL | DM Rem |
|---|---|---|---|---|---|---|---|---|---|---|
| Rutledge (18) | 2,410 | 1997 | 2005 | 5.9 | 0.08 | 46.0 | NA | NA | 80 | – |
| Lee (19) | 1,163 | 2001 | 2012 | 1.8 | 0.17 | 41.4 | 27.7 | NA | 72.9* | 93 |
| Carbajo (20) | 1,200 | 2002 | 2017 | 2.7 | 0.16 | 46.0 | 29.9 | 41 | 70.0* | 94 |
| Noun (21) | 1,000 | 2005 | 2012 | 3.4 | 0 | 42.5 | 28.4 | NA | 68.6 | – |
| Musella (22) | 974 | 2006 | 2014 | 2.0 | 0.2 | 48.0 | 28.0 | NA | 77.0 | 87 |
| Chevallier (23) | 1,000 | 2006 | 2015 | 5.5 | 0.2 | 46.7 | 31.5 | NA | 71.6 | 85.7 |
| Kular (24) | 1,054 | 2007 | 2014 | 1.3 | 0.18 | 43.2 | 25.9 | NA | 87.0 | 93.2 |
| Jammu (25) | 473 | 2007 | 2016 | 0.9 | 0 | 56.5 | NA | NA | 92.2 | 95.1 |
| Genser (26) | 2,321 | 2007 | 2016 | 1.5# | 0 | 48 | NA | NA | NA | – |
| Overall | 11,595 | – | – | 2.8 | 0.11 | 44.7 | 27.6 | 41 | 74.9 | – |
*, data of 10 years, others are data of 5 years; #, leakage only. NA, not available; BMI, body mass index; %TWL, percentage of total weight loss; %EWL, percentage excess weight loss; C, complication; Rem, remission.
Weight loss
The weight loss after SAGB was universally good and durable (Table 1). The reported mean %EWL at 5-year after SAGB was 76% (varied from 68.6% to 92.2%). The reported mean excess weight loss at 10 years in SAGB was 72.9%, slightly higher than the 60% plus in RYGB, as reported in a randomized clinical trial comparing both procedures (19). Two studies investigated the quality of life change after SAGB both showed a significantly increasing quality of life after surgery and non-inferior to RYGB (19,27). Using SAGB as a revision surgery for failed bariatric procedure also resulted a similar weight loss as primary SAGB with acceptable morbidities (11,28-31).
T2DM remission after SAGB
It is expected that a 90% remission rate of diabetes can be achieved after SAGB as reported by some large series (Table 1). However, these data are not adequate because those were for bariatric surgery not specific for T2DM treatment and using different criteria for T2DM resolution. Table 2 listed the outcome of T2DM treatment using SAGB as metabolic surgery for the treatment of T2DM from reports with adequate data (13,15,22,24,32-41). Lee was the first one to report the result of using SAGB for T2DM treatment (13). In this report, SAGB is an effective and durable treatment for T2DM patients but the remission rate was lower in low BMI patients. Several studies then from Asia and other parts of the world also confirmed the good results using SAGB for the treatment of Asian T2DM patients. A higher diabetes remission rate in SAGB might be a result of a better weight loss and a longer BP limb (10,12). In Asian, SAGB can provided a high T2DM complete remission rate up to 78.4% at 1-year and 56.8% at 5-year. However, the complete remission rate varied with BMI, 86% at BMI >35, 65–70% at BMI between 30 and 35 and dropped to 30% at BMI <30 kg/m2. Therefore, using SAGB to treat low BMI T2DM patients should be very carefully and the BP limb should be tailored short in low BMI patients to avoid the malnutrition problem after SAGB (42). A diabetes surgery scoring system ABCD score can also be applied for patient selection and choice of procedure (43,44).
Table 2
| Report | Patient No. | Mean pre-op BMI (kg/m2) | Mean pre-op A1c% | Complete remission (A1c <6.0%) (%) | Partial remission (A1c <6.5%) (%) | Improved or remission (A1c <7%) (%) |
|---|---|---|---|---|---|---|
| Lee (13)* | 201 | 40.1 | – | – | – | 87.1 (1 y) |
| Dixon (32)* | 88 | 39.0 | 9.1 | 85.5 (1 y) | – | 98 (1 y) |
| Lee (33,34)* | 30 | 30.3 | 10 | – | 93 (1 y) | – |
| 70.5 (5 y) | 85 (5 y) | |||||
| Dixon (35)* | 103 | 26.0 | 9.1 | 30.1 (1 y) | – | 67 |
| Kim (36)* | 107 | 25.3 | 9.0 | – | – | 53 (1 y) |
| García-Caballero (15) | 13 | 27 | 8.3 | – | 73 (1 y) | – |
| Kular (24)* | 674 | 43.2 | – | – | 93 (1 y) | 98 (1 y) |
| Kular (37)* | 128 | 33.4 | 10.7 | 64 (1 y) | 82 (1 y) | 100 (1 y) |
| 53 (5 y) | 70 (5 y) | 87 (5 y) | ||||
| Musella (38) | 229 | 48 | – | – | 87 (1 y) | 98 (1 y) |
| Musella (22) | 224 | 48.0 | – | – | – | 84.8 (5 y) |
| Milone (39) | 16 | 45.8 | – | 87.5(1 y) | – | |
| Lee (40)* | 33 | 31.0 | 9.1 | 46.9 (5 y) | 68.8% (5 y) | – |
| Lee (41)* | 249 | 39.9 | 8.6 | 85.7 (1 y) | – | 98.5 (1 y) |
| Overall (1 y) | 2,095 | 34.7 | 8.0 | 78.4 (1 y) | 87.3% (1 y) | 96.5 (1 y) |
| Overall (5 y) | 287 | 31.6 | 9.9 | 56.8 (5 y) | 69.4 (5 y) | 85.6 (5 y) |
*, Asian patients. pre-op, pre-operative; BMI, body mass index.
Comparison of SAGB and other procedures
Accumulating data is strongly indicating that SAGB is an effective and durable bariatric procedure. Long-term available database and randomized clinical trials indicate that SAGB can be considered as a simpler and safer alternative to the standard RYGB with a better weight loss and glycemic control (9,19). Two randomized controlled trial also demonstrated a better weight loss of SAGB than sleeve gastrectomy (SG) or gastric plication (34,45). Many comparative studies and meta-analysis also demonstrated a better weight loss of SAGB than RYGB or SG (29,46-49). In T2DM patients, SAGB had a higher and more durable T2DM remission rate than SG (29,34,38,40,41). However, SAGB had a higher incidence of micronutrient deficiencies than RYGB or SG because of a greater mal-absorptive component of longer BP limb (10,12). Revision surgery is rarely needed in SAGB but some patients may need a conversion surgery because of malnutrition (50-52).
Conclusions
After a journey of more than 15 years, SAGB is strongly becoming an accepted bariatric operation with a favorable risk profile and metabolic results. In term of weight loss, SAGB could achieve good %EWL of 80% at 5-year and 70% at 10-year follow-up. In obese Asian T2DM patients, SAGB can achieve a 5-year durable complete remission of more than 50% with a low complication rate but the remission rate varied according to the patient’s BMI. SAGB can be a valid treatment option for Asian obese T2DM patients.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Laparoscopic and Endoscopic Surgery for the series “Laparoscopic Metabolic Surgery for the Treatment of Type 2 Diabetes in Asia”. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2017.07.06). The series “Laparoscopic Metabolic Surgery for the Treatment of Type 2 Diabetes in Asia” was commissioned by the editorial office without any funding or sponsorship. WJL served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Annals of Laparoscopic and Endoscopic Surgery from Jun 2016 to May 2018. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chan JM, Rim EB, Colditz G, et al. Obesity, fat distributio, and weight gain as risk factors for clinical diabetes in men. Diabetes Care 1994;17:961-9. [Crossref] [PubMed]
- Kopelman PG. Obesity as a medical problem. Nature 2000;404:635-43. [PubMed]
- Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA 2004;292:1724-37. [Crossref] [PubMed]
- Dixon JB, Zimmet P, Alberti KG, et al. Bariatric surgery: an IDF statement for obese type 2 diabetes. Diabet Med 2011;28:628-42. [Crossref] [PubMed]
- Angrisani L, Santonicola A, Iovino P, et al. Bariatric surgery worldwide 2013. Obes Surg 2015;25:1822-32. [Crossref] [PubMed]
- Rutledge R. The mini-gastric bypass: experience with the first 1,274 cases. Obes Surg 2001;11:276-80. [Crossref] [PubMed]
- Fisher BL, Buchwald H, Clark W, et al. Mini-gastric bypass controversy. Obes Surg 2001;11:773-7. [Crossref] [PubMed]
- Johnson WH, Fernanadez AZ, Farrell TM, et al. Surgical revision of loop ("mini") gastric bypass procedure: multicenter review of complications and conversions to Roux-en-Y gastric bypass. Surg Obes Relat Dis 2007;3:37-41. [Crossref] [PubMed]
- Lee WJ, Yu PJ, Wang W, et al. Laparoscopic Roux-en-Y versus mini-gastric bypass for the treatment of morbid obesity: a prospective randomized controlled clinical trial. Ann Surg 2005;242:20-8. [Crossref] [PubMed]
- Lee WJ, Lin YH. Single-Anastomosis Gastric Bypass (SAGB): Appraisal of Clinical Evidence. Obes Surg 2014;24:1749-56. [Crossref] [PubMed]
- Wang W, Wei PL, Lee YC, et al. Short-term results of laparoscopic mini-gastric bypass. Obes Surg 2005;15:648-54. [Crossref] [PubMed]
- Mahawar KK, Jennings N, Brown J, et al. "Mini" gastric bypass: systematic review of a controversial procedure. Obes Surg 2013;23:1890-8. [Crossref] [PubMed]
- Lee WJ, Wang W, Lee YC, et al. Effect of laparoscopic mini-gastric bypass for type 2 diabetes mellitus: comparison of BMI>35 and <35 kg/m2. J Gastrointest Surg 2008;12:945-52. [Crossref] [PubMed]
- Kim Z, Hur KY. Laparoscopic mini-gastric bypass for type 2 diabetes: the preliminary report. World J Surg 2011;35:631-6. [Crossref] [PubMed]
- García-Caballero M, Valle M, Martinez-Moreno JM, et al. Resolution of diabetes mellitus and metabolic syndrome in normal weight 24-29 BMI patients with One Anastomosis Gastric Bypass. Nutr Hosp 2012;27:623-31. [PubMed]
- El-Kadre L, Tinoco AC, Tinoco RC, et al. Overcoming the learning curve of laparoscopic Roux-en-Y gastric bypass: a 12-year experience. Surg Obes Relat Dis 2013;9:867-72. [Crossref] [PubMed]
- Schauer P, Ikramuddin S, Hamad G, et al. The learning curve for laparoscopic Roux-en-Y gastric bypass is 100 cases. Surg Endosc 2003;17:212-5. [Crossref] [PubMed]
- Rutledge R, Walsh TR. Continued excellent results with the mini-gastric bypass: six-year study in 2,410 patients. Obes Surg 2005;15:1304-8. [Crossref] [PubMed]
- Lee WJ, Ser KH, Lee YC, et al. Laparoscopic Roux-en-Y vs. mini-gastric bypass for the treatment of morbid obesity: a 10-year experience. Obes Surg 2012;22:1827-34. [Crossref] [PubMed]
- Carbajo MA, Luque-de-leon E, Jimenez JM, et al. Laparoscopic one-anastomosis gastric bypass: Technique, results, and long-term follow-up in 1200 patients. Obes Surg 2017;27:1153-67. [Crossref] [PubMed]
- Noun R, Skaff J, Riachi E, et al. One thousand consecutive mini-gastric bypass: short- and long-term outcome. Obes Surg 2012;22:697-703. [Crossref] [PubMed]
- Musella M, Susa A, Greco F, et al. The laparoscopic mini-gastric bypass: the Italian experience: outcomes from 974 consecutive cases in a multicenter review. Surg Endosc 2014;28:156-63. [Crossref] [PubMed]
- Chevallier JM, Arman GA, Guenzi M, et al. One thousand single anastomosis (omega loop) gastric bypass to treat morbid obesity in a 7-year period: outcomes show few complications and good efficacy. Obes Surg 2015;25:951-8. [Crossref] [PubMed]
- Kular KS, Manchanda N, Rutledge R A. 6-Year Experience with 1,054 Mini-Gastric Bypasses-First Study from Indian Subcontinent. Obes Surg 2014;24:1430-5. [Crossref] [PubMed]
- Jammu GS, Sharma R. A 7-year clinical audit of 1107 cases comparing sleeve gastrectomy, Roux-en-Y gastric bypass and mini-gastric bypass, to determine an effective and safe bariatric and metabolic procedure. Obes Surg 2016;26:926-32. [Crossref] [PubMed]
- Genser L, Carandina S, Tabbara M, et al. Presentation and surgical management of leaks after mini-gastric bypass for morbid obesity. Surg Obes Relat Dis 2016;305-12. [Crossref] [PubMed]
- Bruzzi M, Rau C, Voron T, et al. Single anastomosis or mini-gastric bypass: long-term results and quality of life after a 5-year follow-up. Surg Obes Relat Dis 2015;11:321-26. [Crossref] [PubMed]
- Bruzzi M, Voron T, Zinzindohoue F, et al. Revisional single anastomosis gastric bypass for failed restrictive procedure: 5-year results. Surg Obes Relat Dis 2016;12:240-5. [Crossref] [PubMed]
- Chansaenroj P, Aung L, Lee WJ, et al. Revision procedures after failed adjustable gastric banding: comparison of efficacy and safety. Obes Surg 2017; [Epub ahead of print]. [Crossref] [PubMed]
- Piazza L, Di Stefano C, Ferrara F, et al. Revision of failed primary adjustable gastric banding to mini-gastric bypass: results in 48 consecutive patients. Updates Surg 2015;67:433-7. [Crossref] [PubMed]
- Meydan C, Raziel A, Sakran N, et al. Single anastomosis gastric bypass-comparative short-term outcome study of convertional and primary procedures. Obes Surg 2017;27:432-8. [Crossref] [PubMed]
- Dixon JB, Hur KY, Lee WJ, et al. Gastric bypass in Type 2 diabetes with BMI < 30: weight and weight loss have a major influence on outcomes. Diabet Med 2013;30:e127-34. [Crossref] [PubMed]
- Lee WJ, Chong K, Ser KH, et al. Gastric bypass vs sleeve gastrectomy for type 2 diabetes mellitus: a randomized controlled trial. Arch Surg 2011;146:143-8. [Crossref] [PubMed]
- Lee WJ, Chong K, Lin YH. Laparoscopic Sleeve Gastrectomy versus Single Anastomosis (Mini-) Gastric Bypass for the Treatment of Type 2 Diabetes Mellitus: 5-Year Results of a Randomized Trial and Study of Incretin Effect. Obes Surg 2014;24:1552-62. [Crossref] [PubMed]
- Dixon JB, Chuang LM, Chong K, et al. Predicting the glycemic response to gastric bypass surgery in patients with type 2 diabetes. Diabetes Care 2013;36:20-6. [Crossref] [PubMed]
- Kim MJ, Hur KY. Short-term outcomes of laparoscopic single anastomosis gastric bypass (LSAGB) for the treatment of type 2 diabetes in lower BMI (<30 Kg/m2) patients. Obes Surg 2014;24:1044-51. [Crossref] [PubMed]
- Kular KS, Manchanada N, Cheema G. Seven years of mini-gastric bypass in type II diabetes patients with a body mass index < 35 Kg/m2. Obes Surg 2016;26:1457-62. [Crossref] [PubMed]
- Musella M, Apers J, Rheinwalt K, et al. Efficacy of bariatric surgery in type 2 mellitus remission: the role of mini gastric bypass/one anastomosis gastric bypass and sleeve gastrectomy at 1 year of follow-up. A European survey. Obes Surg 2016;26:933-940. [Crossref] [PubMed]
- Milone M, Di Minno MN, Leongito M, et al. Bariatric surgery and diabetes remission: sleeve gastrectomy or mini-gastric bypass? World J Gastroenterol 2013;19:6590-7. [Crossref] [PubMed]
- Hsu CC, Almulaifi A, Chen JC, et al. Effect of bariatric surgery vs medical treatment on type 2 diabetes in patients with body mass index lower than 35:five-year outcomes. JAMA Surg 2015;150:1117-24. [Crossref] [PubMed]
- Lee WJ, Chong K, Aung L, et al. Metabolic surgery for diabetes treatment: sleeve gastrectomy or gastric bypass. World J Surg 2017;41:216-23. [Crossref] [PubMed]
- Lee WJ, Wang W, Lee YC, et al. Laparoscopic mini-gastric bypass: experience with tailored bypass limb according to body weight. Obes Surg 2008;18:294-9. [Crossref] [PubMed]
- Lee WJ, Almulaifi AM, Tsou JJ, et al. Laparoscopic Sleeve Gastrectomy for Type 2 Diabetes Mellitus: Predicting the Success by ABCD Score. Surg Obes Relat Dis 2015;11:991-6. [Crossref] [PubMed]
- Lee WJ, Almulaifi AM, Chong K, et al. The Effect and Predictive Score of Gastric Bypass and Sleeve Gastrectomy on Type 2 Diabetes Mellitus Patients with BMI < 30 kg/m2. Obes Surg 2015;25:1772-8. [Crossref] [PubMed]
- Darabi S, Talebpour M, Zeinoddini A, et al. Laparoscopic gastric plication versus mini-gastric bypass surgery in the treatment of morbid obesity: a randomized clinical trial. Surg Obes Relat Dis 2013;9:914-9. [Crossref] [PubMed]
- Disse E, Pasquer A, Espalieu P, et al. Greater weight loss with the omega loop bypass compared to the Roux-en-Y gastric bypass: a comparative study. Obes Surg 2014;24:841-6. [Crossref] [PubMed]
- Kruschitz R, Luger M, Kienbacher C, et al. The Effect of Roux-en-Y vs. Omega-Loop Gastric Bypass on Liver, Metabolic Parameters, and Weight Loss. Obes Surg 2016;26:2204-2212. [Crossref] [PubMed]
- Lee WJ, Pok EH, Almulaifi AM, et al. Medium-term Results of Laparoscopic Sleeve Gastrectomy: A Matched Comparison with Gastric Bypass. Obes Surg 2015;25:1431-8. [Crossref] [PubMed]
- Quan Y, Huang A, Ye M, et al. Efficacy of Laparoscopic Mini Gastric Bypass for Obesity and Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Gastroenterol Res Pract 2015;2015:152852.
- Lee WJ, Lee YC, Ser KH, et al. Revisional surgery for laparoscopic minigastric bypass. Surg Obes Relat Dis 2011;7:486-91. [Crossref] [PubMed]
- Facchiano E, Leuratti L, Lucchese M. Conversion of one anastomosis gastric bypass (OAGB) is rarely needed if standard operative techniques are performed. Obes Surg 2016;26:1594-5. [Crossref] [PubMed]
- Chen CY, Lee WJ, Lee HM, et al. Laparoscopic Conversion of Gastric Bypass Complicationto Sleeve Gastrectomy: Technique and Early Results. Obes Surg 2016;26:2014-21. [Crossref] [PubMed]
Cite this article as: Alkhalifah N, Lee WJ. Single anastomosis (mini-) gastric bypass for the treatment of Asian type 2 diabetes mellitus. Ann Laparosc Endosc Surg 2017;2:137.


