
Laparoscopic distal gastrectomy with nodal dissection for clinical stage I gastric cancer
Introduction
Since the first report of laparoscopic gastrectomy in 1991 in Japan, an increasing number of patients are undergoing this procedure. In 2013, 52% of distal gastrectomies and 43% of total and partial gastrectomies were performed using laparoscopy (1). In the Japanese gastric cancer treatment guidelines published in 2014, a laparoscopic procedure became an option for routine medical practice in clinical stage I gastric cancer for which distal gastrectomy is indicated. Therefore, it is likely that more patients will undergo this procedure in the future. Alarmingly, however, there are complications during and after laparoscopic distal gastrectomy with nodal dissection in 1.1% and 5% of the cases, respectively (2), even though laparoscopy should theoretically be a minimally invasive technique that lowers the incidence of complications. Therefore, a continued effort is required to prevent such complications. In our hospital, we use technology with a focus on safety by simplifying and standardizing the technique. In this article, we describe the techniques that we use in laparoscopic distal gastrectomy with nodal dissection for clinical stage I gastric cancer, and we discuss issues that require attention during the operation.
Surgical techniques
Positions of the patient, monitors, and ports
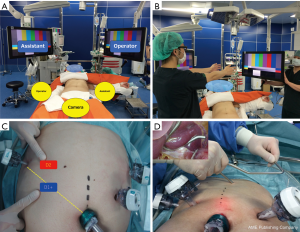
The patient lies supine with legs open. Two monitors are placed near the patient’s head. The monitor for the surgeon is facing straight at the surgeon. Likewise, the monitor for the surgical assistant is facing straight at the assistant (Figure 1A,B). The surgeon stands on the right side of the patient, while the assistant stands on the left side. The camera assistant stands between the patient’s legs. Only during dissection of station 6, duodenal dissection, and dissection of the right lymph nodes on the superior margin of the pancreas, the surgeon and the surgical assistant switch their positions so that the surgeon stands on the left side of the patient. The ports are positioned in a reverse trapezoid shape, as commonly practiced. In performing D2 dissection, the port to the right hand of the surgeon is moved toward the head along the outer edge of the rectus abdominis muscle for dissection of station 11p (Figure 1C). The umbilical port is 12 mm in size. The port near the left hand of the surgeon is 5 mm in size, and the other three ports are 12 mm. A liver retractor is used to expand the surgical field in the left hepatic lobe (Figure 1D). In dissecting the lymph nodes along the lesser curvature, it is crucial that there is a stable and clear view of the surgical field. To achieve this, we prefer using a liver retractor. Postoperative elevation of liver enzymes is occasionally observed, but this is rarely a clinically significant problem. After placing all the ports, the patient’s head is elevated at about ten degrees.
Energy devices
An electric scalpel, ultrasonic coagulating shears (UCS), and vessel sealing system (VSS) are used differentially, depending on the site and purpose. We use an electric scalpel in incision of the membrane, UCS in lymphadenectomy of sites that have capillaries and adipose tissues, and a VSS at sites that require cluster ligations. However, in principle, the surgeon should choose an appropriate device that matches his/her skills.
Key points in expanding the surgical field
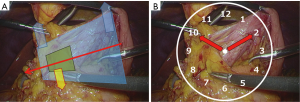
In attending to the axis of the device held by the surgeon at all times, the surgical field is expanded in the shape of a triangle with depth. Care should be taken so that the assistant can control the large surgical field, while the surgeon controls the small field (Figure 2A). Describing the directions using the twelve clock positions on the screen facilitates communication in orienting the expansion (Figure 2B).
Dissection techniques and reconstruction
Dissection of the left gastroepiploic artery (LGEA) and vein (dissection of station 4sb)
The operation is performed from the right side of the patient. While the patient’s head is elevated, the operating table is rotated ten degrees to the right of the patient. The omentum, colon, and spleen move medially due to gravity in this position, which facilitates the operation and expansion of the view of the organs. The surgical assistant uses the right hand to grasp the adipose tissue containing the LGEA and the left hand to grasp the omentum. Then, the surgeon uses the right hand to grasp the adipose tissue containing the LGEA and creates the surgical field in the shape of a triangle with depth. The key here is to pay attention to the surgeon’s right hand at all times in shaping the field.
In entering the omental bursa cavity, the incision from the site left of the round ligament of the liver facilitates the operation. In removing the omentum toward the inferior pole of the spleen, care must be taken as the LGEA may be present near the transverse colon. By examining the omental bursa cavity from the dorsal side, it is easy to recognize the incision line on the boundaries of the omentum and transverse colon (Figure 3A).
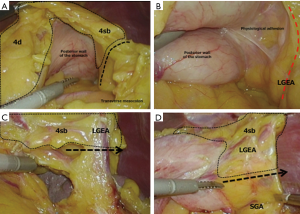
Because an epiploic branch may be found during dissection of the omentum in some cases, one must be careful not to misidentify it as the main trunk of the LGEA. Physiological adhesion is always present near the main trunk of the LGEA. In removing this adhesion, the LGEA can be seen lifting itself from the tail of the pancreas (Figure 3B). The dissection area of station 4sb is determined from the site in which the first branch from the LGEA enters the stomach wall, and the blood vessels are removed (Figure 3C). Furthermore, it is advised that the boundaries of stations 4sb and 4sa from the posterior wall of the stomach are determined (Figure 3D). Finally, the anterior wall of the stomach is expanded and station 4sb is resected from the stomach wall to complete dissection of station 4sb. While the patient’s head is kept elevated, only the operating table is rotated to make it level. At this point, the surgeon moves to the left side of the patient.
Infrapyloric lymphadenectomy (dissection of station 6: mesenterization and the lateral approach)
The surgeon operates from the left side of the patient. The key points are to perform mesenterization (3) and to identify the excised layer in front of the pancreas head.
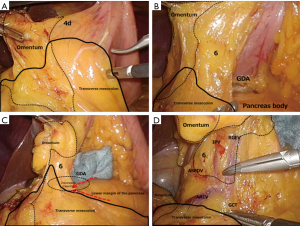
Mesenterization should be performed in attending to the adipose tissue in the right gastroepiploic arterial field (stations 4d–6), transverse mesocolon, and omentum. Again, expanding the surgical field in the shape of a triangle with depth is effective. Viewing and removing the omentum from the dorsal side are also effective. The assistant uses both hands to move the adipose tissue containing the right gastroepiploic artery (RGEA) to the ventral side in creating a large surgical field. Then, the surgeon uses both hands to begin dissecting the omentum at a distance of about 3 cm from the RGEA. Inspection of the physiological adhesion of the posterior wall of the stomach will reveal covering adhesion of the transverse mesocolon to the front of the body of the pancreas and to the posterior wall of the stomach. In separating these adhesions, the adipose tissues surrounding stations 4d–6, the transverse mesocolon, and the omentum can be distinguished (Figure 4A). In further separation, the gastroduodenal artery (GDA) is identified upon exposure of the front of the body of the pancreas near the posterior wall of the duodenum. Based on our experience, the height of the GDA is often the same as that of the anterior superior pancreaticoduodenal vein (ASPDV). This observation is useful in determining the lower margin of the dissection line of station 6 (Figure 4B). The assistant uses the left hand to hold the adipose tissue containing the RGEA and expands the surgical field by pulling them out to the lateroventral side and left side of the patient, like pulling out a duodenum. Then, the assistant uses the right hand to expand the field so that the RGEA pedicle becomes perpendicular to the screen (expansion with a pedicle). In removing the part, in which the line of the GDA and the lower margin of the pancreas intersect, to the dorsal side, the transverse mesocolon with the middle colic artery can be easily removed from the pancreas (Figure 4C). In maintaining the layer, the transverse mesocolon is removed up to the pancreas head and the front of the duodenum. This completes mesenterization (Figure 4D). Because the right gastroepiploic vein (RGEV) becomes exposed during this process, dissection of station 6 will begin from this point. We perform dissection of station 6 using a technique called the lateral approach. This is a method that sets the RGEA in the middle of the dissection area of station 6, which is then removed from the periphery (outside) to the middle. There are two advantages to using this method. When performing the dissection from the left side of the patient, the device held by the surgeon’s right hand is aligned parallel to the pancreatic parenchyma. This facilitates identification of the excised layer between the pancreatic parenchyma and the dissection tissue, and surgical injuries to the pancreas can be avoided. Another advantage is that the area of station 6 including the infrapyloric artery (IPA) can be dissected completely.
The following describes the actual technique. The cranial margin of the area of station 6 is the lower margin of the duodenum. The caudal margin is the ASPDV, and the medial margin is the GDA. First, the ASPDV is identified in front of the pancreas head. Then, the lower margin of the dissection line of station 6 is determined and the RGEV dissected. Once the RGEV is dissected, the incision layer between the adipose tissue surrounding station 6 and the front of the pancreas head can be identified. This part should be removed along the ASPDV up to the duodenum (Figure 5A). Then, in changing the point of view, the incision to the capsule is made along the lower margin of the duodenum.
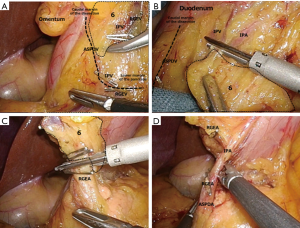
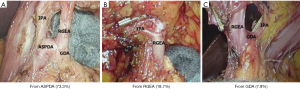
As the layer in front of the infrapyloric vein (IPV) is removed, it will reach the foregoing layer of the ASPDV in front of the pancreas head (the lower margin of the dissection line of station 6) (Figure 5B). Further dissection toward the middle RGEA is performed while maintaining the anterior pancreatic fascia in front of the pancreas head. Once the IPA or ASPDA is exposed, dissection is discontinued. With the procedure up to this point, dissection of the IPA area is complete. The area of station 6 has been dissected from the ASPDV and the lower margin of the duodenum in both sides, whereas the RGEA and IPA have been retained. In restarting the expansion of the surgical field with the pedicle, only the RGEA and IPA remain. When these are removed, the dissection is complete (Figure 5C,D). Attention must be paid to identify the branches of the IPA so that the ASPDA or GDA is not dissected by mistake. We have observed ASPDA branching in 73% of patients at our hospital. RGEA branching is observed in about 19% of patients, and GDA branching is found in 8%. The frequency and patterns of branching are nearly the same as those reported previously (4). It is good to remember that many patients have the IPA on the dorsal side of the RGEA (Figure 6).
Duodenal dissection
The surgeon operates from the left side of the patient. A small perforation for duodenal dissection is created from the posterior wall of the pylorus. This will facilitate the inspection of the right gastric artery’s course to avoid surgical damage. Irrespective of the method used in the reconstruction, all duodenal dissections are performed in the direction of the posterior wall to the anterior wall using the port inserted in the left lower side of the patient.
Dissection of the right lymph nodes on the superior margin of the pancreas (dissection of stations 8a–12a using the crossover method)
For lymph node dissection around suprapancreatic area, we applied the “cross over method”. In this procedure, lymph node dissection around station 8a is performed from the left side of the patient, while that around 11p is performed from the right side of the patient. We named this procedure “cross over method” because the axes of forceps are crossed at the central point during lymph node dissection. This procedure facilitates safe and easy lymph node dissection around station 8a and 11p. The details of the procedure are described as follows.
The surgeon operates from the left side of the patient. In attending to the outermost layer of the nerve (5), the lymph nodes on the superior margin of the pancreas are dissected. To do so, it is desirable for the assistant to use both hands to expand the surgical field such that the surgeon can use both hands for the procedure. Thus, our hospital has proposed a method in which the lymph nodes near the hepatic artery (station 8a) are dissected from the left side of the patient, whereas those near the splenic artery (station 11p) are dissected from the right side of the patient. We call this method the crossover method and we have standardized the procedure.
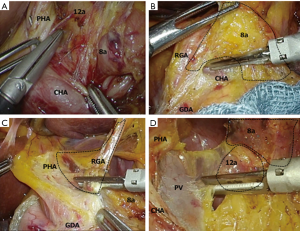
This method allows us to secure proper distances from the ports. Furthermore, since the device held by the surgeon's right hand becomes aligned parallel to the blood vessels, such as the hepatic artery and portal vein, it facilitates the procedures of expansion and removal. In D2 dissections, the port inserted into the right lower side of the patient is moved toward the head. Because the forceps must enter the surgical field in a perpendicular angle, this puts a strain on the surgeon, as he/she has to lift the right elbow to perform dissection of stations 5–8a (Figure 7A). In the crossover method, however, the port inserted into the left side of the patient does not change its position. Therefore, the method is easy to standardize, which is another advantage. The method does not require any special skills. After duodenal dissection, the surgeon remains in the same position and performs dissection of the right lymph nodes on the superior margin of the pancreas (station 9R). After turning the right side of the stomach to the left upper abdomen, the assistant expands the pedicles of the left and right gastric arteries to the ventral side. The surgeon uses the left hand to gently roll the pancreas and the right hand to make an incision in the pancreatic capsule. This way, it is easy to gain access to the outermost layer of the nerve between the dorsal side of station 8a and the common hepatic artery (CHA) (Figure 7B). While maintaining this layer, as the incision proceeds towards the left side of the proper hepatic artery (PHA), the origin of the right gastric artery can be identified. Care must be taken as the right and left PHA may branch early, and this can be misidentified as the right gastric artery. At our hospital, we have observed the right gastric artery branching from the main trunk of the PHA in 42% of patients, from the point past the bifurcation of the right and left PHA in 31%, from the GDA in 15%, from the CHA in 6%, and from the confluence of the three arteries in 5%. It should be noted that, unexpectedly, there are many cases of branching from a point past the bifurcation of the right and left PHA. After removing the left side of the PHA up to the cranial side to prepare the field, attention is returned to the right margin of the PHA, and the right gastric artery is identified and dissected (Figure 7C). From this point, as the dissection proceeds on the dorsal side along the nerve surrounding the hepatic artery, the portal vein becomes exposed. In D1+ dissections, the base of dissection is determined to be at a site between stations 8a and 12a. In D2 dissections, the base of dissection is defined at a site between station 12a and the left wall of the portal vein (Figure 7D). If the left gastric vein enters the portal vein, it is dissected during the foregoing procedure. At this point, the surgeon returns to the right side of the patient and prepares for dissection of the left lymph nodes on the superior margin of the pancreas (around the splenic artery).
Dissection of the left lymph nodes on the superior margin of the pancreas (dissection of station 11p using the cross-over method)
The operation is performed from the right side of the patient. The first step is incision of the lesser omentum. The assistant uses the right hand to hold the left gastric artery (LGA) around the ascending branch and the left hand to grasp the adipose tissue of the lesser omentum, expanding the surgical field along the surgeon’s axis. If a left accessory hepatic artery is found during the operation, the surgeon should make a decision whether to dissect or preserve the artery based on its size, and treat it accordingly. After the incision of the lesser omentum, the superior margin of station 1 is decided in the layer that exposes the anterior wall of the stomach. Subsequently, the adipose tissue containing station 9 is removed from the right crus of the diaphragm. This operation determines the cranial margin of the dissection. Then, adipose tissue containing stations 1 and 9 is dissected in the cranial to caudal direction, creating a large space in front of the posterior wall of the stomach and crura of the diaphragm. A piece of gauze is inserted into this space (Figure 8A). This gauze will provide an important clue later when the dissection is performed in the caudal to cranial direction. The stump of the stomach and duodenum are rolled to the cranial side. The assistant further expands the surgical field using the right hand to roll the LGA pedicle to the ventral side and the left hand to roll the pancreas.
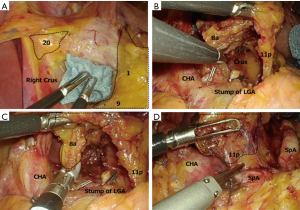
At our hospital, we perform dissection of the lymph nodes on the superior margin of the pancreas using the medial approach as a fundamental approach. First, an incision is made at the pancreatic capsule along the splenic artery to enter the outermost layer of the nerve. Subsequently, a loose layer between both sides of the LGA is identified and removed in the cranial direction along the celiac artery. The incision eventually runs into the gauze, which has previously been inserted. In expanding the space in both the ventral and dorsal directions, a space extending from the LGA to the crura of the diaphragm is created. In removing the LGA at this point, a large space is formed, and the dissection tissue containing stations 8a, 12a, 9, and 11p lifts like a wall or screen (Figure 8B). The surgery will then switch to dissection of the right lymph nodes on the superior margin of the pancreas (station 9R). First, incision proceeds along the nerve on the right wall of the celiac artery to isolate the tissue toward the dorsal side. This then reveals the inner side of station 9R. Since the dissection has already been completed on the portal vein side, the surgeon determines the base of station 8a and dissects the lymph nodes from both sides (Figure 8C). Finally, dissection of the left lymph nodes on the superior margin of the pancreas (station 9L) is performed. First, the excised layer between the Gerota’s fascia and Toldt’s fusion fascia is removed to the height of the dorsal pancreas or splenic vein. While maintaining the outermost layer of the nerve from the ventral side of the splenic artery, station 11p is dissected from both sides to complete D2 dissection (Figure 8D). In D1+ dissections, the procedure can be performed efficiently if the tissues on both sides of the LGA are dissected first and then station 9L, followed by the LGA and station 9R at the end. Finally, stations 1 and 3 are dissected from the posterior wall of the stomach and the surgery proceeds to resection of the stomach.
Resection of the stomach
The surgeon operates from the right side of the patient. Resection of the stomach is performed in the direction of the lesser curvature to the greater curvature. This method allows for easy handling of a linear stapler, even in a narrow abdominal cavity of a thin female patient, and enables stable gastrectomy. Because the dissected stump of the gastric remnant at the greater curvature does not dwindle, we consider the method to also be useful from the standpoint of tissue blood flow in intracorporeal anastomosis. The incision line is determined after injecting a dye into the submucosa and visualizing the lesion using intraoperative endoscopy (6). However, any method can be used here as long as it is reliable.
Reconstruction (Delta anastomosis)
The assistant performs the operation from the port inserted in the left lower side of the patient. We use Delta anastomosis for the Billroth I method and functional end-to-end anastomosis for the Roux-en-Y method. Delta anastomosis is a type of Billroth I anastomosis in which intracorporeal anastomosis is performed. This technique was first reported by Kanaya et al. (7). It is a simple technique characterized by functional end-to-end anastomosis of the posterior wall of the gastric remnant and the posterior wall of the duodenum. The main complications after Delta anastomosis are anastomotic leakage, anastomotic bleeding, stenosis, and stasis. We compared the short-term postoperative results of the patients who underwent Delta anastomosis (n=266) and those who underwent Billroth-I anastomosis using a circular stapler (n=147) between November 2004 and March 2017 in our institute. The frequency of anastomotic leakage, anastomotic bleeding, stenosis, and stasis (Clavien-Dindo grade II or greater) after Delta anastomosis were 1.12%, 0.37%, 0%, and 0.37%, respectively. On the other hand, those after Billroth-I anastomosis using a circular stapler were 1.36%, 1.36%, 0.68%, and 2.72%, respectively. Complication rates tended to be lower in patients after Delta anastomosis, and that of stasis was significantly lower in Delta anastomosis group (P=0.036). Possible explanation for this is less tension at anastomosis site in Delta anastomosis, compared to anastomosis with a circular stapler, because in Billroth-I anastomosis using a circular stapler, the remnant stomach is taken out from small incision and anastomosis is performed extracorporeally while anastomosis is performed intracorporeally in Delta anastomosis.
Other reconstruction methods after distal gastrectomy include Roux-en-Y and Billroth-II anastomosis. There is a report that Roux-en-Y reconstruction is safer and better in postoperative functional aspect compared to Billroth-I reconstruction (8). However, Delta anastomosis has some advantages such as simple procedure which takes shorter time and natural passage of diet after surgery.
We encourage readers to refer to the original publication for details. Here, we describe tips for performing Delta anastomosis. There are four key points: (I) securing the mobility of the duodenum; (II) the Delta check; (III) rotation before anastomosis; (IV) a V-shaped closure.
In duodenal dissection, the key at the beginning is to improve the mobility of the duodenum. The duodenum is an organ fixed to the retroperitoneum. In removing 2 to 3 supraduodenal vessels to mobilize the dorsal side of the duodenum and dissecting the duodenum from the posterior wall to the anterior wall while leaving a long neck of the duodenum, anastomosis can be carried out in a relaxed, unstrained manner (Figure 9A).
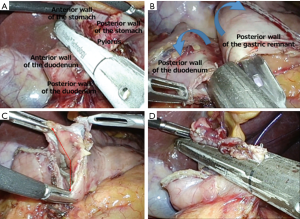
The Delta check is a step in which we confirm whether anastomosis of the gastric remnant and the duodenum can be carried out in a relaxed, unstrained manner. Because a 45-mm suture instrument is used in anastomosis, it is sufficient if there is room for a single cartilage to be overlaid on the gastric remnant and the duodenum.
In anastomosis of the gastric remnant and duodenum, insertion openings for the suture instrument are created in the posterior wall of the duodenum and the greater curvature of the gastric remnant stump. The opening in the duodenum is made in the one third of the posterior wall of the stump. The opening in the gastric remnant stump is made in a manner similar to resecting a horn. The assistant then uses the left hand to insert the cartilage of the suture instrument into the stomach. At this point, the surgeon rotates the gastric remnant stump outward.
As this keeps the distance between the dissected stump and the proposed anastomotic line, a temporary closure is made with the suture instrument. While the assistant is holding the gastric remnant stump with the right hand, the suture instrument and gastric remnant are moved closer to the duodenal stump, and the suture instrument is released. The surgeon then covers the fork of the suture instrument with the duodenum. Both the surgeon and assistant rotate (supinate) the tissues around the instrument outward and perform suture. Because the distance between the dissected stump and the anastomotic site are maintained in this operation, it is believed to prevent ischemia (Figure 9B).
In operating from the port inserted at the left lower side of the patient, the common opening can be closed in a V-shape. At this step, a temporary closure made using a suture facilitates the process of aligning the suture instrument and the direction of the closure (Figure 9C,D). A 60-mm suture instrument is used once for the closure. Before closure, the operation must be simulated. After closure, full-thickness excision of the stump must be verified extracorporeally.
In Delta anastomosis, it is crucial that both the surgeon and assistant have an understanding of the concept, procedure, and techniques of surgery. If a practice simulation is performed before carrying out anastomosis, the surgery is more likely to go smoothly. If there is excessive strain on the closure during the simulation, it is important to be ready to switch decisively to the Roux-en-Y method. Therefore, it is also necessary to have understanding and skills regarding the latter method.
Conclusions
We have described laparoscopic distal gastrectomy with nodal dissection. The procedure has been standardized at our hospital. However, since surgery techniques change over time, one should not adhere to a single technique. We hope that our techniques are utilized as a reference for providing safe and reliable surgery.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2017.08.02). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- The Education Committee of Japan Society for Endoscopic Surgery. 10th nationwide survey of endoscopic surgery in Japan. J Jpn Soc Endoscopic Surg 2010;15:567-77.
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 2011;14:113-23. [Crossref] [PubMed]
- Hisashi S, Shusuke H. Mesenterization: a theoretical method for overcoming a limitation. Rinsho Geka 2013;68:576-85.
- Haruta S, Shinohara H, Ueno M, et al. Anatomical considerations of the infrapyloric artery and its associated lymph nodes during laparoscopic gastric cancer surgery. Gastric Cancer 2015;18:876-80. [Crossref] [PubMed]
- Kanaya S, Ohara K, Kawata H, et al. Novel technique for dissection of the lymph nodes along the celiac artery using laparoscopy. Jpn J Gastroenterol Surg 2007;30:1683-92.
- Nakagawa M, Ehara K, Ueno M, et al. Accurate, safe, and rapid method of intraoperative tumor identification for totally laparoscopic distal gastrectomy: injection of mixed fluid of sodium hyaluronate and patent blue. Surg Endosc 2014;28:1371-5. [Crossref] [PubMed]
- Kanaya S, Gomi T, Momoi H, et al. Delta-shaped anastomosis in totally laparoscopic Billroth I gastrectomy: new technique of intraabdominal gastroduodenostomy. J Am Coll Surg 2002;195:284-7. [Crossref] [PubMed]
- Kojima K. A comparison of Roux-en-Y and Billroth-I reconstruction after laparoscopy-assisted distal gastrectomy. Ann Surg 2008;247:962-7. [Crossref] [PubMed]
Cite this article as: Ehara K, Nakamura S, Yamada T, Mori Y, Arai S, Kageyama Y, Kawashima Y, Sakamoto H, Tanaka Y. Laparoscopic distal gastrectomy with nodal dissection for clinical stage I gastric cancer. Ann Laparosc Endosc Surg 2017;2:134.


