Update on robotic surgery for rectal cancer treatment
Introduction
While laparoscopic treatment for colon cancer has showed several undisputed short term advantages over the traditional open approach, with equivalent long term oncologic outcomes (1,2), two recently published trials have questioned this assumption for rectal cancer (3,4).
Total mesorectal excision (TME), since it was first described by Heald in 1982 (5), is considered the gold standard treatment for rectal cancer, due to the significant reduction in local recurrence rate (6). The standard and rigid laparoscopic instrumentation, with the camera hold by the assistant surgeon, add complexity to the TME. The pelvis is a confined location, that becomes even more unfavorable in males, and when dealing with bulky tumors or obese patients. Therefore, TME remains a challenging procedure, with high conversion rates (CRs) (7,8) and a steep learning curve (9). Robotic surgery, with its endowristed instruments with 7 degrees of freedom, 3D full HD vision with a stable optical platform, tremor filtering, and motion scaling could overcome some of the technical limitations of standard laparoscopy, reducing the degree of procedural complexity. This could potentially increase the diffusion of minimally-invasive TME, that still maintains a low penetration worldwide (10-12).
This paper aims to describe the surgical technique of robotic TME and to review the recent literature on robotic rectal surgery with a focus on short term results, functional and oncological outcomes, learning curve and costs.
Robotic TME: surgical technique
Herein we describe the technique for full-robotic TME with the DaVinci Xi surgical platform (Intuitive Surgical Inc., Sunnyvale, CA, USA). This system allows for an easier setup and multiquadrant access thanks to a faster docking, simpler OR setup (boom-mounted rotating arms) and longer instruments, when compared to previous robotic systems.
Patient positioning
The patient is placed in lithotomy position with arms alongside the body and is carefully secured with dedicated patient positioning system (Pink Pad, Xodus Medical Inc., PA, USA) to prevent sliding.
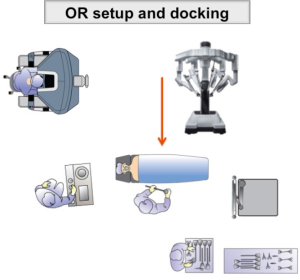
First assistant stands on the patient’s right side. The scrub nurse is at the lower right side of the table and the assistant’s monitor is located at the patient’s left shoulder. The cart is placed at the patient’s left side and is docked from the left lower quadrant (Figure 1).
Port placement
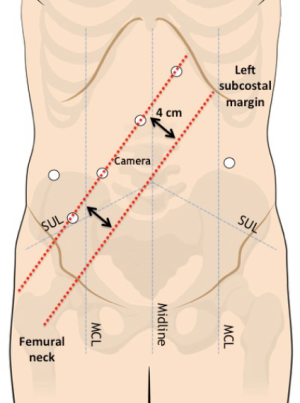
A Veress needle is inserted in the left hypochondrium (Palmer’s point) for the induction of a 12-mmHg pneumoperitoneum. A 12-mm optical port is inserted in the right flank. Four 8-mm robotic trocars are then inserted along a straight line that is parallel to and about 4 cm cranial to the costofemural line, maintaining a 6–8 cm distance between each port. Ports are also located at least 2–3 cm away from bony prominences. An additional 8-mm robotic port is placed in the left flank and will be used for the pelvic phase of the procedure (Figure 2).
Robot positioning and docking
A complete exploration of the abdominal cavity is performed and an intraoperative liver ultrasonography is carried out with a laparoscopic dedicated probe to complete the intraoperative staging of the disease. The patient is then placed in a 20–25° Trendelemburg position with a 20–25° right tilt: the greater omentum and the small bowel loops are retracted out of the pelvic area into the right upper quadrant laparoscopically.
The robotic cart is then approximated and deployed for docking from the patient’s left side. A green laser, emitted from the overhead boom, is used to define the correct cart position. The optical port is then docked and the endoscope is inserted to complete the automated targeting process towards the left iliac fossa. The other robotic arms are then docked and robotic instruments are inserted under visual control.
Step-by-step review of critical elements of the procedure
Full-robotic TME is essentially based on 3 steps:
- Splenic flexure mobilization;
- Vascular control;
- TME.
Splenic flexure mobilization
The trasverse mesocolon is lifted up with the grasper in R1 (Figure 3). The lesser sac is entered through the incision of transverse mesocolic root at the level of the anterior pancreatic border. Splenic flexure mobilization is carried out with a medial-to-lateral approach along the pancreatic body. A sponge is placed underneath the transverse mesocolon and the splenic flexure is retracted medially by the assistant. Splenic flexure mobilization is completed in a lateral-to-medial fashion along the white line of Toldt up to the inferior splenic pole and the plane previously developed is easily reached. Coloepiploic detachment is then carried out.
Vascular control
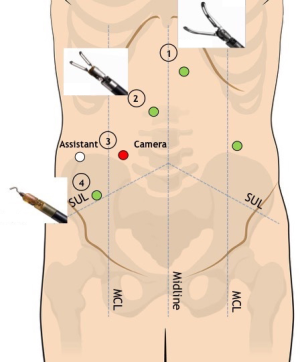
During this step of the procedure R2 and R4 are the operative arms, whereas R1 is used for stable retraction. A 30°-down robotic camera is mounted on robotic arm R3. The assistant trocar in the right flank is used for suction/irrigator, clip applier, swab introduction or additional retraction if needed.
The assistant grasper and the robotic grasper in R1 lift anteriorly and laterally the sigmoid colon and upper rectum to expose the root of the sigmoid mesocolon and the upper mesorectum. The peritoneum is then incised at the level of the sacral promontory to obtain the avascular presacral plane and the hypogastric nerves are identified. The robotic monopolar hook on R4 and bipolar grasper on R2 work synergically for the dissection of the IMA, which is freed by the surrounding lymphatic tissue, providing a wide locoregional lymphadenectomy and preserving the main trunks of the hypogastric plexus at the IMA origin.
Before IMA division, the Toldt’s fascia is identified underneath the inferior mesenteric vein (IMV) in a medial-to-lateral fashion and left gonadal vessels and left ureter are identified and preserved. The IMA is then dissected free and a high-tie is performed by the application of Hem-o-lok® (Teleflex, Weck, USA) clips. The dissection along the Toldt’s fascia, that has been previously identified cranially and caudally to the IMA origin, is completed in a medial-to-lateral fashion and the retroperitoneal structures are preserved. The IMV is isolated and dissected at its root between clips at the inferior border of the pancreas.
TME
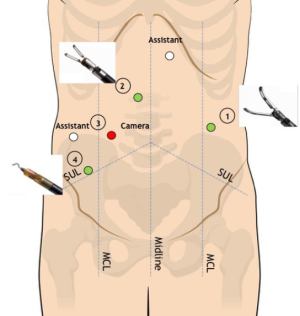
During this step, R1 is moved and connected to the left flank 8-mm trocar to achieve optimal access to the mesorectum, whereas R2 and R4 remain in their original position (Figure 4). An additional 8-mm epigastric trocar is now available in order to maximize the assistance with cranial retraction on the sigmoid colon and simultaneous suction/irrigation or additional gentle pelvic sidewall retraction.
Robotic R2 and R4 are the operative arms, whereas R1 is used to expose the pelvic area with lateral traction on pelvic sidewalls or anterior/upward traction on the Douglas peritoneal reflection, vaginal wall or seminal vesicles/Denonvilliers fascia. Frequent repositioning of R1 is fundamental to maintain the adequate countertraction that will allow the dissection plane to be continued up to the level of the pelvic floor.
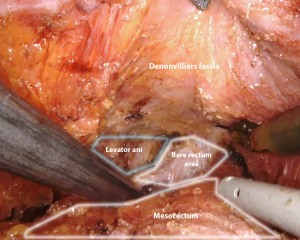
TME is carried out according to Heald’s principles through the so-called “holy plane”. This step is fundamental to avoid the transection of the hypogastric nerve plexus and sacral venous plexus located deep in the parietal layer within the presacral space. Dissection starts on the posterior aspect of the mesorectal envelope: the right lateral and the anterior plane are then subsequently dissected up to the seminal vesicles in a counterclockwise fashion. The left lateral pelvic fascia is then dissected up to its lower portion to allow for the identification of the pelvic nerve plexus and to gain access to the “bare rectum area” (Figure 5). Dissection is then completed anteriorly on the lower portion of the Denonvilliers fascia and circumferentially below the reflection of the mesorectal fascia to gain access to the levator ani plane. During the dissection of the lower mesorectum, it may be helpful to shift to a 0° camera in order to achieve better visualization.
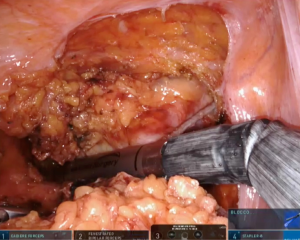
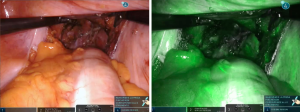
According to tumor distance from the anal verge, rectal transection is performed with 45-mm robotic staplers (Figure 6) after evaluation of rectal stump perfusion with the integrated fluorescence imaging system (Figure 7).
Short-term postoperative outcomes
To date, few studies with low level of evidence comparing robotic versus laparoscopic rectal resection are available. No multicentre randomized controlled trial has been published yet. Nevertheless, several meta-analysis have been published as the interest in robotic surgery is growing.
Several studies reported longer operative time for the robotic approach compared to laparoscopy (13-15), the available meta-analysis showed no significant differences between the two procedures (16-19). Only one study reported a shorter operative time for robotic rectal resection (20).
Laparoscopic rectal resection is a demanding procedure with still high CRs (21); the CLASICC trial reported a CR of 34% (7), while in the COLOR II trial CR to open surgery decreased to 17% (8). Recently, two randomized trials (3,4) found a CR of 9% and 11.3% respectively. To date, the majority of the studies showed lower CRs to open surgery for robotic rectal approach when compared to laparoscopy (22-24) and, probably, this is the most frequently reported finding in favour of robotic rectal surgery in the literature. Trastulli et al. (18) reported a CR of 2% for robotic rectal resections, and Xiong et al. (17) in a recent meta-analysis comparing robotic and laparoscopic TME, found a significantly lower CR for the robotic group.
This measure of surgical outcome has also been chosen as primary endpoint of the ROLARR Trial, whose results have been recently presented. In the study design (superiority trial), it was hypothesized that the CR could be reduced by 50% (from 25% to 12.5%) in the robotic group. Though the study failed to meet the criterion for statistical superiority, an a priori defined subgroup analysis showed a possible advantage in the most challenging cases, namely male patients, low anterior resections and obese patients.
Regarding blood loss, some reports demonstrated a lower mean operative blood loss in robotic procedures compared to either open or laparoscopic ones (23,25).
Two studies (26,27) found a significant reduction in length of hospital stay in the robotic group, probably due to the lower overall surgical trauma of the robotic technique and to lower CRs. To date, however, there is no high level of evidence that robotic surgery could reduce length of stay when compared to standard laparoscopy (17,18,28).
Post-operative complications rates are similar and particularly the anastomotic leak rate is not different between the two approaches (17,18,24,28,29). Only one recent meta-analysis by Sun and coworkers (19) demonstrated a lower overall post-operative complications rate for the robotic group compared to the laparoscopic one.
Functional outcomes
Whether or not laparoscopic surgery could have an advantage over open surgery in preserving urogenital function is still controversial, later studies suggest better outcomes with laparoscopy, but earlier reports stated the opposite (30). Undoubtedly, in this scenery the robotic system could find its place, combining the advantages of minimally invasive surgery with a more precise dissection.
Kim et al. (31), in their series of 69 patients, demonstrated that robotic TME is associated with earlier recovery of normal voiding (3 months versus 6 months) and erectile function (6 months versus 12 months) when compared to its laparoscopic counterpart. Recently, Panteleimonitis et al. (32) found that Robotic TME has better postoperative urogenital outcomes in men and urological outcomes in women. For female sexual function there was no difference between the two groups, but the sample was very small (9 laparoscopic, 4 robotic). Another series of 74 patients submitted to robotic TME, showed that robotic surgery allows for preservation of urinary and sexual function, with values at 1-year comparable to those measured before surgery (33). A similar trend for urogenital function has been observed in other studies (34,35).
Oncological outcomes
Studies comparing oncological outcomes of robotic and laparoscopic resections for rectal cancer have small number of cases, and for the most part are nonrandomized controlled trials with a short follow up (17,21).
A specimen with an adequate and complete mesorectum directly correlates with recurrence rates (36), but few studies on minimally invasive TME specify the macroscopic assessment of mesorectal excision according to the criteria initially described by Quirke (37). Baik et al. (38) reported statistical significance in the quality of the mesorectum in favor of the robotic group, ascribing this result to the technical advantages of the robotic platform. In a recent review that considered 11 studies assessing the quality of the mesorectum, complete excision ranged from 100% to 60% in the robotic series, and from 100% to 40% in the laparoscopic group (21).
Preliminary results of the ROLARR trial seam to show no statistical difference between laparoscopic and robotic resection in terms of CRM positivity, thus confirming the results of several others series (13,24,26,39,40).
Kang et al. (15) in their case matched study reported a significant decrease in CRM involvement in the robotic group compared to the open group in mid to low rectal cancer resections. 165 patients had robotic resection with a CRM positivity rate of 4.2%, while the open group (165 patients) presented with a CRM positivity rate of 10.3%. No significant difference was found between the robotic and the laparoscopic group (CRM+ 6.7%).
Ghezzi et al. (41) reported a significant higher number of retrieved lymph nodes in the robotic group, this finding was confirmed also by other authors (13,27,42), but the majority of the studies reports similar numbers of harvested nodes between laparoscopic and robotic rectal resection (17,21).
Survival data from the ROLARR trial are still unavailable and reports of long-term oncologic outcomes for robotic rectal surgery remain limited (Table 1). No significant difference has been reported in overall and disease free survival (DSF) or local recurrence rates (LR) (53). Park et al. (26) found no differences in the 5-year overall survival (OS), DSF and local recurrence rates. Similar results were reported by Cho et al in a case matched series of 278 patients (40), with a 5-year OS of 92.2% and a DSF of 81.8% in the robotic group. More recently, Kim et al. (45) showed that robotic surgery was a significant good prognostic factor for OS and cancer specific survival in multivariate analysis.
Table 1
| Ref | Year | Country | Technique | Pts. | Harvested nodes (mean) | CRM + (%) | Distal margin (mm, mean) | Median FU months | LR % | DSF % | OS % |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lim et al. (43) | 2017 | South Korea | ROB | 74 | 11.6 | 4 | 17 | 56.1 | 2.7 | 76.8 (5 y) | 90 (5 y) |
| LAP | 64 | 14.7 | 1.6 | 22 | 6.3 | 76 (5 y) | 93.3 (5 y) | ||||
| Tang et al. (44) | 2017 | China | ROB | 392 | 14.6 | 2.5 | 35 | 24* | 5.1 | 74.3 (3 y) | 86.4 (3 y) |
| Kim et al. (45) | 2017 | South Korea | ROB | 192 | 20.2 | 4 | 23 | 40.3 | − | 72.6 (5 y) | 90.5 (5 y) |
| LAP | 192 | 21 | 4.9 | 24 | − | 68 (5 y) | 78 (5 y) | ||||
| Law et al. (13) | 2016 | Hong Kong | ROB | 220 | 14*** | 4.1 | 30 | 31.4 | − | 81.9** (5 y) | 71.8 (5 y) |
| LAP | 171 | 12*** | 8.2 | 30 | − | 80** (5 y) | 74.3 (5 y) | ||||
| Sammour et al. (46) | 2016 | USA | ROB | 276 | 22*** | 2.5 | − | 23.8 | 2.4# (5 y) | 82# (5 y) | 87# (5 y) |
| Feroci et al. (27) | 2016 | Italy | ROB | 53 | 18 | 0 | 25 | 37.4 | 1.9 | 79.2 (3 y) | 90.2 (3 y) |
| LAP | 58 | 11 [3–27] | 1.7 | 15 | 5.2 | 83.4 (3 y) | 90 (3 y) | ||||
| Pai et al. (47) | 2016 | USA | ROB | 101 | 15 | 5 | 35 | 33 | 4 | 79.2 (3 y) | 90.1 (3 y) |
| Park et al. (26) | 2015 | South Korea | ROB | 133 | 16.34 | 6.8 | 27.5 | 58 | 2.3 | 81.9 (5 y) | 92.8 (5 y) |
| LAP | 84 | 16.63 | 7.1 | 28.5 | 1.2 | 78.7 (5 y) | 93.5 (5 y) | ||||
| Cho et al. (40) | 2015 | South Korea | ROB | 278 | 15 | 5 | 20 | 51.8* | 5.9 | 81.8 (5 y) | 92.2 (5 y) |
| LAP | 278 | 16.2 | 4.7 | 22 | 3.9 | 79.6 (5 y) | 93.1 (5 y) | ||||
| Ghezzi et al. (41) | 2014 | Brazil | ROB | 65 | 20.1 | 0 | 27 | 46.7* | 3.2 | 73.2 (5 y) | 85 (5 y) |
| OPEN | 109 | 14.1 | 1.8 | 22 | 55.1* | 16.1 | 69.5 (5 y) | 76 (5 y) | |||
| Yoo et al. (48) | 2014 | South Korea | ROB | 43 | 13.93 | 9.1 § | 13.3 | 33.9 | 12.8 | 76.7 (3 y) | 95.2 (3 y) |
| LAP | 26 | 21.42 | 19.2 § | 16.7 | 36.5 | 8.3 | 75 (3 y) | 88.5 (3 y) | |||
| Hara et al. (49) | 2014 | South Korea | ROB | 200 | 17*** | 2.5 | 18*** | 29.8 | 4.5 | 81.7 (5 y) | 92 (5 y) |
| Baik et al. (50) | 2013 | South Korea | ROB | 370 | 15.6 | 5.7 | 25 | 24 | 3.6 | 79.2 (3 y) | 93.1 (3 y) |
| Baek et al. (51) | 2010 | USA | ROB | 64 | 14.5 | 0 | 34 | 20.2 | 3.1 | 73.7 (3 y) | 96.2 (3 y) |
| Bianchi et al. (24) | 2010 | Italy | ROB | 25 | 18 | 0 | 20 | − | − | − | − |
| LAP | 25 | 17 | 4 | 20 | |||||||
| Pigazzi et al. (52) | 2010 | Multicentric (USA/Italy) | ROB | 143 | 14.1 | 0.7 | 29 | 17.4 | 1.5 | 77.6 (3 y) | 97 (3 y) |
| Patriti et al. (20) | 2009 | Italy | ROB | 29 | 10.3 | 0 | 21 | 29.2* | 0 | 100 | 96.6 |
| LAP | 37 | 11.2 | 0 | 45 | 18.7* | 5.4 | 83.7 | 97.2 |
ROB, robotic; LAP, laparoscopic; LR, local recurrence; DFS, disease free survival; OS, overall survival. *, mean; **, cancer specific survival; ***, median; #, 83 patients followed up for a minimum of 3 years; §, CRM ≤2 mm.
Learning curve
Rectal cancer surgery with TME is complex and demanding and the number of cases required to achieve the learning curve in laparoscopic rectal resection is estimated to be between 60 and 80 (9,54). The majority of the studies published so far suggest a shorter learning curve for robotic rectal cancer surgery, ranging from 15 to 30 cases (9,55,56). Yamaguchi et al. (57) found a learning curve of 25 cases before reaching the plateau. Robotic assistance could probably facilitate the adoption of minimally invasive rectal surgery (58), that still maintains a low penetration worldwide (10-12), by overcoming the technical constraints of standard laparoscopy and by shortening the learning curve.
The robotic technology represents a unique tool for training with the aid of the dual console and of the virtual simulator, but competence assessment is mandatory. Recently, a structured training protocol has been proposed (59), in an attempt to standardize and objectively evaluate the learning process.
The robotic system has the potential of shortening the learning curve for complex procedures not only for experienced surgeons (56), but also for novice surgeons that may learn a procedure approaching it directly with the robot. Foo et al. (60) evaluated the learning curve for robotic assisted rectal resection in a surgeon that had previously performed less than 5 open or laparoscopic rectal cancer resections. On a series of 39 consecutive TME they found the learning curve to be 25 cases. At our Institution, we recently introduced a structured training program in robotic colorectal surgery in order to assess its safety and efficacy for young surgeons without prior experience in both open and laparoscopic colorectal surgery. Robotic right colectomy with intracorporeal anastomosis has been chosen as a model and two junior attending surgeons were trained through sequential steps that included: virtual simulator, dry lab, wet lab hands-on courses, at least 20 procedures as table assistant and proctored clinical practice with the dual console. They were then privileged to perform right colectomy without mentoring. Preliminary results showed neither conversions nor intra- or postoperative complication. Mean operative time was 200 minutes and mean length of hospital stay was 6 days. The program will then involve novice surgeons in left-sided resections and, finally, in most complex procedures such as TME in a stepwise approach.
Costs
One of the biggest reasons for skepticism about robotic surgery is often related to high costs.
When focusing on robotic rectal cancer surgery, Kim et al. (61) and Park et al. (26) failed to prove the cost-effectiveness of robotic versus laparoscopic rectal resection based on short-term and long-term oncological outcomes, respectively. Baek et al. (62) in their case-matched study reported higher total hospitalization costs for the robotic group compared to the laparoscopic one, but without reaching a statistical significance. Besides, Byrn et al. (63) found that direct costs of robotic rectal resection decreased with time, showing that later procedures were actually less expensive. A recent Italian study (64) reported a similar finding: although overall mean costs of laparoscopic TME were significantly lower than those of robotic TME, costs gradually decreased in the robotic group as the surgeon advanced in his learning curve, thus suggesting a significant optimization of instruments and OR time use with experience.
Recently, Daskalaki et al. (65) reported that robotic and open liver resections were financially comparable. Though direct costs (purchase, maintainance and use of the system) were higher in the robotic group, they were balanced by shorter length of stay and better postoperative outcomes (lower rates of major complications and shorter ICU stay).
Conclusions
Robotic surgery is often considered to be associated with longer operative times because of the docking and system setup. However, these steps can be quickly performed with practice and are not among the main reasons for this finding. Robotic surgery represents a new concept of surgery, where precise dissection and careful visualization of even the smallest anatomical structure play a crucial role. This aspect could probably explain a trend towards better functional outcomes of robotic surgery versus the laparoscopic approach that should be further investigated.
The technological advantages provided by the robotic system translates into a shorter learning curve and lower CRs when compared to standard laparoscopy, and these aspects could probably facilitate the widespread adoption of minimally-invasive TME that still has a low penetration worldwide. Moreover, the educational capabilities of the platform, together with structured training programs, could allow novice surgeons to safely approach colorectal surgery directly with the robot, defining the “robotic surgeon” as a new paradigm.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Marco Milone) for the series “Minimally Invasive Treatment of Low Rectal Cancer” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: The series “Minimally Invasive Treatment of Low Rectal Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wilson MZ, Hollenbeak CS, Stewart DB. Laparoscopic colectomy is associated with a lower incidence of postoperative complications than open colectomy: a propensity score-matched cohort analysis. Colorectal Dis 2014;16:382-9. [Crossref] [PubMed]
- Wu Q, Wei M, Ye Z, et al. Laparoscopic Colectomy Versus Open Colectomy for Treatment of Transverse Colon Cancer: A Systematic Review and Meta-Analysis. J Laparoendosc Adv Surg Tech A 2017; [Epub ahead of print]. [Crossref] [PubMed]
- Fleshman J, Branda M, Sargent DJ, et al. Effect of Laparoscopic-Assisted Resection vs Open Resection of Stage II or III Rectal Cancer on Pathologic Outcomes: The ACOSOG Z6051 Randomized Clinical Trial. JAMA 2015;314:1346-55. [Crossref] [PubMed]
- Stevenson AR, Solomon MJ, Lumley JW, et al. Effect of Laparoscopic-Assisted Resection vs Open Resection on Pathological Outcomes in Rectal Cancer: The ALaCaRT Randomized Clinical Trial. JAMA 2015;314:1356-63. [Crossref] [PubMed]
- Heald RJ, Husband EM, Ryall RD. The mesorectum in rectal cancer surgery--the clue to pelvic recurrence? Br J Surg 1982;69:613-6. [Crossref] [PubMed]
- Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet 1986;1:1479-82. [Crossref] [PubMed]
- Guillou PJ, Quirke P, Thorpe H, et al. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet 2005;365:1718-26. [Crossref] [PubMed]
- Bonjer HJ, Deijen CL, Abis GA, et al. A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med 2015;372:1324-32. [Crossref] [PubMed]
- Barrie J, Jayne DG, Wright J, et al. Attaining surgical competency and its implications in surgical clinical trial design: a systematic review of the learning curve in laparoscopic and robot-assisted laparoscopic colorectal cancer surgery. Ann Surg Oncol 2014;21:829-40. [Crossref] [PubMed]
- Babaei M, Balavarca Y, Jansen L, et al. Minimally Invasive Colorectal Cancer Surgery in Europe: Implementation and Outcomes. Medicine (Baltimore) 2016;95:e3812 [Crossref] [PubMed]
- Taylor EF, Thomas JD, Whitehouse LE, et al. Population-based study of laparoscopic colorectal cancer surgery 2006-2008. Br J Surg 2013;100:553-60. [Crossref] [PubMed]
- Yeo H, Niland J, Milne D, et al. Incidence of minimally invasive colorectal cancer surgery at National Comprehensive Cancer Network centers. J Natl Cancer Inst 2014;107:362. [Crossref] [PubMed]
- Law WL, Foo DC. Comparison of short-term and oncologic outcomes of robotic and laparoscopic resection for mid- and distal rectal cancer. Surg Endosc 2017;31:2798-2807. [PubMed]
- Kwak JM, Kim SH, Kim J, et al. Robotic vs laparoscopic resection of rectal cancer: short-term outcomes of a case-control study. Dis Colon Rectum 2011;54:151-6. [Crossref] [PubMed]
- Kang J, Yoon KJ, Min BS, et al. The impact of robotic surgery for mid and low rectal cancer: a case-matched analysis of a 3-arm comparison--open, laparoscopic, and robotic surgery. Ann Surg 2013;257:95-101. [Crossref] [PubMed]
- Xiong B, Ma L, Huang W, et al. Robotic versus laparoscopic total mesorectal excision for rectal cancer: a meta-analysis of eight studies. J Gastrointest Surg 2015;19:516-26. [Crossref] [PubMed]
- Xiong B, Ma L, Zhang C, et al. Robotic versus laparoscopic total mesorectal excision for rectal cancer: a meta-analysis. J Surg Res 2014;188:404-14. [Crossref] [PubMed]
- Trastulli S, Farinella E, Cirocchi R, et al. Robotic resection compared with laparoscopic rectal resection for cancer: systematic review and meta-analysis of short-term outcome. Colorectal Dis 2012;14:e134-56. [Crossref] [PubMed]
- Sun Y, Xu H, Li Z, et al. Robotic versus laparoscopic low anterior resection for rectal cancer: a meta-analysis. World J Surg Oncol 2016;14:61. [Crossref] [PubMed]
- Patriti A, Ceccarelli G, Bartoli A, et al. Short- and medium-term outcome of robot-assisted and traditional laparoscopic rectal resection. JSLS 2009;13:176-83. [PubMed]
- Staderini F, Foppa C, Minuzzo A, et al. Robotic rectal surgery: State of the art. World J Gastrointest Oncol 2016;8:757-71. [Crossref] [PubMed]
- Tam MS, Kaoutzanis C, Mullard AJ, et al. A population-based study comparing laparoscopic and robotic outcomes in colorectal surgery. Surg Endosc 2016;30:455-63. [Crossref] [PubMed]
- Ramji KM, Cleghorn MC, Josse JM, et al. Comparison of clinical and economic outcomes between robotic, laparoscopic, and open rectal cancer surgery: early experience at a tertiary care center. Surg Endosc 2016;30:1337-43. [Crossref] [PubMed]
- Bianchi PP, Ceriani C, Locatelli A, et al. Robotic versus laparoscopic total mesorectal excision for rectal cancer: a comparative analysis of oncological safety and short-term outcomes. Surg Endosc 2010;24:2888-94. [Crossref] [PubMed]
- Shiomi A, Kinugasa Y, Yamaguchi T, et al. Robot-assisted versus laparoscopic surgery for lower rectal cancer: the impact of visceral obesity on surgical outcomes. Int J Colorectal Dis 2016;31:1701-10. [Crossref] [PubMed]
- Park EJ, Cho MS, Baek SJ, et al. Long-term oncologic outcomes of robotic low anterior resection for rectal cancer: a comparative study with laparoscopic surgery. Ann Surg 2015;261:129-37. [Crossref] [PubMed]
- Feroci F, Vannucchi A, Bianchi PP, et al. Total mesorectal excision for mid and low rectal cancer: Laparoscopic vs robotic surgery. World J Gastroenterol 2016;22:3602-10. [Crossref] [PubMed]
- Wang Y, Zhao GH, Yang H, et al. A Pooled Analysis of Robotic Versus Laparoscopic Surgery for Total Mesorectal Excision for Rectal Cancer. Surg Laparosc Endosc Percutan Tech 2016;26:259-64. [Crossref] [PubMed]
- Luo Z, Zeng D, Shi Y, et al. da Vinci robotic versus laparoscopic surgery in rectal cancer: a meta-analysis of postsurgery complications. Int J Colorectal Dis 2016;31:1405-6. [Crossref] [PubMed]
- Lim RS, Yang TX, Chua TC. Postoperative bladder and sexual function in patients undergoing surgery for rectal cancer: a systematic review and meta-analysis of laparoscopic versus open resection of rectal cancer. Tech Coloproctol 2014;18:993-1002. [Crossref] [PubMed]
- Kim JY, Kim NK, Lee KY, et al. A comparative study of voiding and sexual function after total mesorectal excision with autonomic nerve preservation for rectal cancer: laparoscopic versus robotic surgery. Ann Surg Oncol 2012;19:2485-93. [Crossref] [PubMed]
- Panteleimonitis S, Ahmed J, Ramachandra M, et al. Urogenital function in robotic vs laparoscopic rectal cancer surgery: a comparative study. Int J Colorectal Dis 2017;32:241-8. [Crossref] [PubMed]
- Luca F, Valvo M, Ghezzi TL, et al. Impact of robotic surgery on sexual and urinary functions after fully robotic nerve-sparing total mesorectal excision for rectal cancer. Ann Surg 2013;257:672-8. [Crossref] [PubMed]
- Panteleimonitis S, Ahmed J, Harper M, et al. Critical analysis of the literature investigating urogenital function preservation following robotic rectal cancer surgery. World J Gastrointest Surg 2016;8:744-54. [Crossref] [PubMed]
- D'Annibale A, Pernazza G, Monsellato I, et al. Total mesorectal excision: a comparison of oncological and functional outcomes between robotic and laparoscopic surgery for rectal cancer. Surg Endosc 2013;27:1887-95. [Crossref] [PubMed]
- Lino-Silva LS, Garcia-Gomez MA, Aguilar-Romero JM, et al. Mesorectal pathologic assessment in two grades predicts accurately recurrence, positive circumferential margin, and correlates with survival. J Surg Oncol 2015;112:900-6. [Crossref] [PubMed]
- Quirke P, Durdey P, Dixon MF, et al. Local recurrence of rectal adenocarcinoma due to inadequate surgical resection. Histopathological study of lateral tumour spread and surgical excision. Lancet 1986;2:996-9. [Crossref] [PubMed]
- Baik SH, Kwon HY, Kim JS, et al. Robotic versus laparoscopic low anterior resection of rectal cancer: short-term outcome of a prospective comparative study. Ann Surg Oncol 2009;16:1480-7. [Crossref] [PubMed]
- Speicher PJ, Englum BR, Ganapathi AM, et al. Robotic Low Anterior Resection for Rectal Cancer: A National Perspective on Short-term Oncologic Outcomes. Ann Surg 2015;262:1040-5. [Crossref] [PubMed]
- Cho MS, Baek SJ, Hur H, et al. Short and long-term outcomes of robotic versus laparoscopic total mesorectal excision for rectal cancer: a case-matched retrospective study. Medicine (Baltimore) 2015;94:e522 [Crossref] [PubMed]
- Ghezzi TL, Luca F, Valvo M, et al. Robotic versus open total mesorectal excision for rectal cancer: comparative study of short and long-term outcomes. Eur J Surg Oncol 2014;40:1072-9. [Crossref] [PubMed]
- Levic K, Donatsky AM, Bulut O, et al. A Comparative Study of Single-Port Laparoscopic Surgery Versus Robotic-Assisted Laparoscopic Surgery for Rectal Cancer. Surg Innov 2015;22:368-75. [Crossref] [PubMed]
- Lim DR, Bae SU, Hur H, et al. Long-term oncological outcomes of robotic versus laparoscopic total mesorectal excision of mid-low rectal cancer following neoadjuvant chemoradiation therapy. Surg Endosc 2017;31:1728-37. [Crossref] [PubMed]
- Tang B, Zhang C, Li C, et al. Robotic Total Mesorectal Excision for Rectal Cancer: A Series of 392 Cases and Mid-Term Outcomes from A Single Center in China. J Gastrointest Surg 2017;21:569-76. [Crossref] [PubMed]
- Kim J, Baek SJ, Kang DW, et al. Robotic Resection is a Good Prognostic Factor in Rectal Cancer Compared with Laparoscopic Resection: Long-term Survival Analysis Using Propensity Score Matching. Dis Colon Rectum 2017;60:266-73. [PubMed]
- Sammour T, Malakorn S, Bednarski BK, et al. Oncological Outcomes After Robotic Proctectomy for Rectal Cancer: Analysis of a Prospective Database. Ann Surg 2016; [Epub ahead of print]. [Crossref] [PubMed]
- Pai A, Marecik SJ, Park JJ, et al. Oncologic and Clinicopathologic Outcomes of Robot-Assisted Total Mesorectal Excision for Rectal Cancer. Dis Colon Rectum 2015;58:659-67. [Crossref] [PubMed]
- Yoo BE, Cho JS, Shin JW, et al. Robotic versus laparoscopic intersphincteric resection for low rectal cancer: comparison of the operative, oncological, and functional outcomes. Ann Surg Oncol 2015;22:1219-25. [Crossref] [PubMed]
- Hara M, Sng K, Yoo BE, et al. Robotic-assisted surgery for rectal adenocarcinoma: short-term and midterm outcomes from 200 consecutive cases at a single institution. Dis Colon Rectum 2014;57:570-7. [Crossref] [PubMed]
- Baik SH, Kim NK, Lim DR, et al. Oncologic outcomes and perioperative clinicopathologic results after robot-assisted tumor-specific mesorectal excision for rectal cancer. Ann Surg Oncol 2013;20:2625-32. [Crossref] [PubMed]
- Baek JH, McKenzie S, Garcia-Aguilar J, et al. Oncologic outcomes of robotic-assisted total mesorectal excision for the treatment of rectal cancer. Ann Surg 2010;251:882-6. [Crossref] [PubMed]
- Pigazzi A, Luca F, Patriti A, et al. Multicentric study on robotic tumor-specific mesorectal excision for the treatment of rectal cancer. Ann Surg Oncol 2010;17:1614-20. [Crossref] [PubMed]
- Ishihara S, Otani K, Yasuda K, et al. Recent advances in robotic surgery for rectal cancer. Int J Clin Oncol 2015;20:633-40. [Crossref] [PubMed]
- Son GM, Kim JG, Lee JC, et al. Multidimensional analysis of the learning curve for laparoscopic rectal cancer surgery. J Laparoendosc Adv Surg Tech A 2010;20:609-17. [Crossref] [PubMed]
- Jiménez-Rodríguez RM, Rubio-Dorado-Manzanares M, Díaz-Pavón JM, et al. Learning curve in robotic rectal cancer surgery: current state of affairs. Int J Colorectal Dis 2016;31:1807-15. [Crossref] [PubMed]
- Bianchi PP, Luca F, Petz W, et al. The role of the robotic technique in minimally invasive surgery in rectal cancer. Ecancermedicalscience 2013;7:357. [PubMed]
- Yamaguchi T, Kinugasa Y, Shiomi A, et al. Learning curve for robotic-assisted surgery for rectal cancer: use of the cumulative sum method. Surg Endosc 2015;29:1679-85. [Crossref] [PubMed]
- Bianchi PP, Petz W, Luca F, et al. Laparoscopic and robotic total mesorectal excision in the treatment of rectal cancer. Brief review and personal remarks. Front Oncol 2014;4:98. [Crossref] [PubMed]
- Petz W, Spinoglio G, Choi GS, et al. Structured training and competence assessment in colorectal robotic surgery. Results of a consensus experts round table. Int J Med Robot 2016;12:634-41. [Crossref] [PubMed]
- Foo CC, Law WL. The Learning Curve of Robotic-Assisted Low Rectal Resection of a Novice Rectal Surgeon. World J Surg 2016;40:456-62. [Crossref] [PubMed]
- Kim CW, Baik SH, Roh YH, et al. Cost-effectiveness of robotic surgery for rectal cancer focusing on short-term outcomes: a propensity score-matching analysis. Medicine (Baltimore) 2015;94:e823 [Crossref] [PubMed]
- Baek JH, Pastor C, Pigazzi A. Robotic and laparoscopic total mesorectal excision for rectal cancer: a case-matched study. Surg Endosc 2011;25:521-5. [Crossref] [PubMed]
- Byrn JC, Hrabe JE, Charlton ME. An initial experience with 85 consecutive robotic-assisted rectal dissections: improved operating times and lower costs with experience. Surg Endosc 2014;28:3101-7. [Crossref] [PubMed]
- Morelli L, Guadagni S, Lorenzoni V, et al. Robot-assisted versus laparoscopic rectal resection for cancer in a single surgeon's experience: a cost analysis covering the initial 50 robotic cases with the da Vinci Si. Int J Colorectal Dis 2016;31:1639-48. [Crossref] [PubMed]
- Daskalaki D, Gonzalez-Heredia R, Brown M, et al. Financial Impact of the Robotic Approach in Liver Surgery: A Comparative Study of Clinical Outcomes and Costs Between the Robotic and Open Technique in a Single Institution. J Laparoendosc Adv Surg Tech A 2017;27:375-82. [Crossref] [PubMed]
Cite this article as: Esposito S, Formisano G, Giuliani G, Misitano P, Krizzuk D, Salvischiani L, Bianchi PP. Update on robotic surgery for rectal cancer treatment. Ann Laparosc Endosc Surg 2017;2:132.