
The role of endoscopic submucosal dissection for Tis/T1 rectal cancer
Introduction
Colorectal endoscopic submucosal dissection (ESD) is widely used as a minimally invasive treatment for neoplasms of the colon and rectum. Colorectal ESD plays an important role because surgical treatment sometimes impairs patient’s quality of life (QOL) especially for rectal lesions. Transanal resection (TAR) and transanal endoscopic microsurgery (TEM) are options for rectal neoplasms, however, several studies reported relatively high local recurrence rates with these techniques (8% to 33%) (1-4). On the other hand, colorectal ESD is associated with low recurrence rates. ESD is technically difficult, time-consuming and the procedure requires longer time for training for manipulation of endoscope and management of adverse effects such as bleeding and intra-procedural perforation. Intra-procedural perforation seems to be decreasing according to advancement of ESD technique and improvement in equipment. Recent studies also have reported that patients with colon perforation could be managed conservatively after endoscopic clipping of the defect (5). For rectal neoplasms, surgery is associated with decreased patient’s QOL and rectal perforation rarely causes peritonitis due to anatomical reasons. Compared with the proximal colon, endoscope manipulation is easy in the rectum, therefore even less-expert endoscopists can perform ESD relatively with ease for rectal lesion (6).
Here we investigated the short-term outcome of ESD for lesions in the upper rectum (Ra), lower rectum (Rb) and anal canal (P).
Our experience
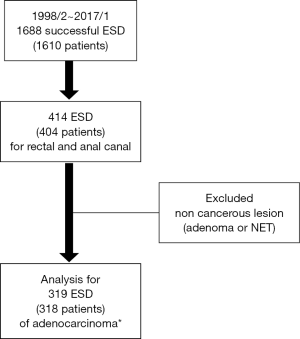
From February 1998 to January 2017, 1,610 consecutive patients with 1,688 colorectal lesions underwent ESD at the National Cancer Center Hospital, Tokyo, Japan. Among these patients, 404 patients with 414 lesions located at upper rectum, lower rectum and anal canal are analyzed (Figure 1). Data from these procedures were entered into a prospective database [NEXUS endoscopic database from Sep 2007 and Japan Endoscopy Database (JED) from Jan 2015]. Age, gender, macroscopic feature, lesion size, location of the lesion, procedure time, histological diagnosis, depth of invasion and complication (intra-procedural perforation/delayed perforation/delayed bleeding) were all entered into the database prospectively. If delayed perforation or delayed bleeding occurred, these were also prospectively added to the database. Then, univariate analysis of the lesions with depth of Tis/T1a and T1b was performed. These lesions were histologically evaluated as adenocarcinoma. All histological evaluation was based on guideline by the Japanese Society for Cancer of the Colon and Rectum (JSCCR) (7). All ESD procedures were performed by one of 11 expert staff endoscopists or 29 trainee endoscopists under the direct supervision of an expert. All 11 endoscopists are certified endoscopists by Japan Gastroenterological Endoscopy Society (JGES) and considered experts in ESD.
R0 resection was defined as one-piece resection of an entire lesion with carcinoma-free lateral and vertical resection margins. Curative resection was defined when the resection was R0 or Rx without deep submucosal (SM) invasion (>1,000 µm) or lymphovascular invasion or poorly differentiated adenocarcinoma component. We defined colonic perforation as a full thickness defect of the muscular layer with recognition of connective tissue, the abdominal cavity, or serosa. Delayed perforation was defined as perforation occurring after completion of the ESD procedure without any intra-procedural perforation (8).
Results were expressed as mean ± SD or median and range. Continuous variables were compared using a Student’s t-test in two groups. Categorical variables were compared between the two groups using the chi-squared test or Fisher’s exact test, as appropriate. A two-tailed P value <0.05 was considered to be statistically significant. Statistical analysis was performed using the JMP SAS version 12.0.1 (SAS Institute, Cary, NC, USA).
This study was approved by the internal review board in our institution, and informed written consent was obtained from all patients for each specific endoscopic treatment.
Table 1 shows clinical features of the patients and lesions. The mean age of patients was 63.6 years (SD ±11.5), 235 of 404 (58.1%) patients were male. The mean size of the lesions was 37.3 mm (SD ±23.4) and number of the lesions located at upper rectum (Ra), lower rectum (Rb) and anal canal (P) were 104, 292 and 12, respectively. Most of the lesions (260 lesions, 63%) showed protruded features (Is and Is + IIa). Table 2 shows the short-term outcome of rectal ESDs. Mean procedure time was 109.2 minutes (SD ±77.5) and en bloc resection was performed in 95.2% of lesions. Sixty-five (15.7%) lesions were evaluated as T1b or T2 in depth diagnosis. These lesions were diagnosed as non-curative resection. Overall ratio of curative resection was 80.9%. The ratio of intra-procedural perforation, delayed perforation and delayed bleeding were 1.0%, 0% and 3.9%, respectively.
Table 1
| Characteristics | N=414 |
|---|---|
| Gender (M/F) (n=404) | 231/173 |
| Age (mean ± SD) | 63.6±11.5 |
| Lesion size (mean ± SD) (mm) | 37.3±23.4 |
| Macroscopic feature | |
| Protruded (Ip/Is/Is + IIa) | 260 |
| Flat/elevated (Is + IIc/IIa/IIa + IIc/IIb/IIc) | 106 |
| Recurrence | 39 |
| Others (SMT etc.) | 9 |
| Location of the lesion | |
| Ra (upper rectum) | 104 |
| Rb (lower rectum) | 298 |
| P (anal canal) | 12 |
M, man; F, female; SD, standard deviation; SMT, submucosal tumor.
Table 2
| Variables | N=414 |
|---|---|
| Histological diagnosis | |
| Adenocarcinoma | 319 |
| Adenoma | 74 |
| Others | 21 |
| Procedure time (mean ± SD) (min) | 109.2±77.5 |
| En bloc resection† [n (%)] | 394 (95.2) |
| Depth of invasion | |
| Tis | 225 |
| T1a | 29 |
| T1b | 61 |
| T2 | 4 |
| Others (including adenoma) | 95 |
| Curative resection‡ [n (%)] | 335 (80.9) |
| Intraoperative perforation [n (%)] | 4 (1.0) |
| Delayed perforation [n (%)] | 0 (0) |
| Delayed bleeding [n (%)] | 15 (3.9) |
†, en bloc resection is defined as 1-piece resection of an entire lesion; ‡, curative resection is defined as R0 or Rx without deep submucosal (SM) invasion (>1,000 ìm)/lymphovascular invasion/poorly differentiated adenocarcinoma component. SD, standard deviation.
Next, we compared the short-term outcome of the T1b lesions to Tis/T1a lesions (Table 3). There was no difference in size of the lesions, procedure time, intra-procedural or delayed perforation, and delayed bleeding between Tis/T1a and T1b groups. In the T1b group, R1 resection was higher than Tis/T1a group (21.0% vs. 5.5%).
Table 3
| Variables | Tis/T1a (n=254) | T1b (n=61) | P value |
|---|---|---|---|
| Lesion size (mean ± SD) (mm) | 40.9±24.0 | 39.0±23.7 | 0.584 |
| Procedure time (mean ± SD) (min) | 111.9±78.4 | 127.5±84.8 | 0.180 |
| Resection† | <0.001 | ||
| R0 (complete) [n (%)] | 219 (86.2) | 44 (71.0) | |
| R1 [n (%)] | 14 (5.5) | 13 (21.0) | |
| Rx [n (%)] | 21 (8.3) | 5 (8.1) | |
| Curative resection‡ [n (%)] | 228 (89.7) | 0 (0) | <0.001 |
| Intraoperative perforation [n (%)] | 3 (1.3) | 0 (0) | 1.000 |
| Delayed perforation ratio [n (%)] | 0 (0) | 0 (0) | – |
| Delayed bleeding [n (%)] | 8 (3.8) | 3 (5.5) | 0.432 |
†, R0 resection is defined as 1-piece resection of an entire lesion with carcinoma-free lateral and vertical resection margins; ‡, curative resection is defined as R0 or Rx without deep submucosal (SM) invasion (>1,000 ìm)/lymphovascular invasion/poorly differentiated adenocarcinoma component; SD, standard deviation.
State of Art
The technique of colorectal ESD has been previously described in detail (9,10). Briefly, we used an endoscope (PCF-Q260JI or GIF-Q260J; Olympus Medical Systems Co., Tokyo, Japan) with a water-jet pump system (OFP; Olympus Medical Systems Co.). A carbon dioxide (CO2) gas supply system (Olympus Medical Systems Co.) (11) was introduced in 2004. Glycerol® (10% glycerin and 5% fructose) with a small amount of indigo carmine dye and epinephrine (1 mL of 0.1%) solution was injected into the submucosal (SM) layer first to confirm appropriate SM elevation. Next, hyaluronic acid solution MucoUp® (Seikagaku Co., Tokyo, Japan) was injected into the properly elevated SM layer (12). Washing the lesion with the water jet was very important process of colorectal ESD.
Additionally, the patient was positioned appropriately for gravity to provide downward traction on the lesion, which both facilitates SM dissection. Basically, an initial incision and marginal resection of the oral side was made with the bipolar knife (Jet B knife®, XEMEX Co., Tokyo, Japan) using the retroflex view, except for the patients with lesions that were close to the dentate line. After the lesion was partially dissected, a monopolar ESD knife (IT knife nano®, Olympus Medical Systems Co., Tokyo, Japan) was used to complete the dissection of the SM layer quickly and safely (10).
West vs. East
In the West, piecemeal endoscopic mucosal resection (EMR) is performed even if the lesions are more than 20 mm. The ESGE guideline states that the majority of colonic and rectal superficial lesions can be removed by standard EMR (13). ESD can be considered for colonic and rectal lesions with high suspicion of limited SM invasion. This is based endoscopic visualization of depressed morphology and irregular or nongranular surface pattern, particularly if the lesions are larger than 20 mm. As preoperative examinations, endoscopic ultrasound (EUS) is mainly used for assessment of depth of invasion and presence of lymph node metastasis. Early invasive superficial neoplasia such as T1a and T1b are indication for surgical resection. If the lesions are located near to the dentate line, TEM may be considered, especially in Europe. Resected lesions that showed evidence of deep SM invasion (T1b) or lymphovascular invasion are better served by additional surgery or chemo-radio therapy (CRT).
In Japan, on the other hand, ESD is recommended for lesions more than 20 mm or T1a invasive lesions when en bloc EMR is considered difficult (14). While magnified chromo-endoscopy (MCE) is not yet available in the West, MCE is generally used as a detailed preoperative examination to assess depth of invasion in Japan (15). Surgery is highly recommended for T1b lesions (14). Currently in Japan, patients with histologically high-risk lesions such as deep SM invasion (T1b) or lymphovascular invasion, only additional surgery is the standard option while CRT is now chosen as a clinical trial.
In the West, most large benign rectal lesions are removed by piecemeal EMR due to not widely available ESD. Data have shown that piecemeal EMR is associated with increased recurrence risk (odds ratio, 4.14) (16). These differences of practical approach to rectal lesions between the West and the East are partially due to the difference of histological definition of cancer. Tis (intra-mucosal) lesions are considered as cancer in Japan, whereas it is recognized as a non-cancer but high-grade dysplasia in most Western countries. However, local recurrences with invasive cancer after piecemeal EMR have been reported, although the rate of such recurrences are quite low. From this standpoint, we believe en bloc resection should be recommended when the intra-mucosal lesions are detected as far as cost-effectiveness permits it.
Fu et al. reported that MCE is at least accurate as EUS for preoperative staging of early colorectal cancers (17). As the examination tools of detailed depth of invasion, MCE has been developed by repeated conference and discussion between endoscopists and pathologists, especially in Japan. Generally, Japanese pathologists make histological section of endoscopically resected specimen every 2 mm apart in order to evaluate precisely for high risk features such as lymphovascular invasion. Furthermore, this contributes to the detailed discussion of comparison between MCE and histological findings.
Another difference between the West and the East is the reimbursement provided by the insurance companies for ESD procedures. In the US, for example, currently ESD doesn’t have a current procedural terminology (CPT) code and medical insurance doesn’t cover colorectal ESD.
Colorectal ESD is a minimally invasive therapy compared with open or laparoscopic assisted surgery for superficial SM invasive rectal lesions and thus provide a better QOL for patients (15,18). Due to the technical difficulty and longer learning curve for ESD as well as limitation of reimbursement, ESD is not widely available in the West. Several studies have reported that rectal ESD is an effective training for Western endoscopists who want to learn about ESD (19). Currently, a few Western endoscopists are now performing colorectal ESD with high en bloc resection and low complication rate. Therefore, rectal ESD may be feasible and safe if appropriate training is taken and in the right selected patients. Therefore, we believe optimized indication for rectal ESD is very important.
Discussion
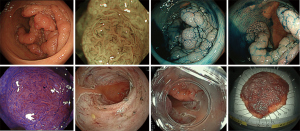
To our knowledge, this study is the largest retrospective study investigating the short-term outcomes of rectal ESD. Ikematsu et al. (20) had reported that the local recurrence rate for patients with SM invasive rectal cancer were worse than patients with SM colon cancer when treated by endoscopic resection alone and no additional surgery. According to Japanese guidelines (7), SM invasive colorectal cancer with negative vertical margins, well-differentiated or moderately differentiated adenocarcinomas, no evidence of vascular or lymphatic invasions, and invasion depths of less than 1,000 µm are classified as low risk for lymph node metastasis and local recurrence. SM invasive colorectal cancer that is positive for any of these risk factors are classified as high-risk lesions. Therefore, it is thought that additional surgical treatment with lymph node dissection is needed when the lesion is histologically evaluated as a high-risk lesion. On the other hand, endoscopic resection is sufficient treatment for low-risk SM invasive cancers. Therefore, ESD has an important role because it allows precise histological evaluation by en bloc resection. Figure 2 showed that we could achieve en bloc resection for semi-circumferential rectal lesions by ESD, so it is thought that there is no limitation in size of the lesion. The prevalence of cancer in rectal lesions was 79.0%, no statistically significant differences compared with the prevalence of cancer in colon lesions (77.3%) in our institution (Table S1). This indicates that rectal and colon lesions should be treated according to endoscopic findings.
In this study, R0 resection and curative resection for rectal cancers with depth of Tis or T1a was 86.2% and 89.7%, respectively. It is notable that non-curative resections due to lymphovascular infiltration was high (24.1%, 7/29 lesions) among these T1a cancers. This illustrates the importance of en bloc resection by ESD for accurate histopathological diagnosis. Among rectal cancers histologically diagnosed as T1b, 47 lesions (78.3%) were difficult to distinguish T1a from T1b by pre-operative endoscopic examination, while 13 lesions (21.7%) were diagnosed correctly but resected considering patients’ high mortality. The R1 resection rate of the lesions of histologically T1b was significantly higher than Tis or T1a. To consider additional surgery, precise histological evaluation is required. The lesions should be resected en bloc with R0 when endoscopic resection was chosen.
Next, we compared the group of Tis/T1a lesions and group of T1b lesions. There is no significant difference in procedure time and adverse events between two groups. However, the ratio of positive vertical margin is significantly higher in T1b group than Tis/T1a group (13.1% vs. 0.8%, P <0.001). It is important to improve the R0 resection ratio, especially negative vertical margin for T1b lesions in the future.
In summary, endoscopic resection is feasible for Tis and T1a rectal cancers. Additionally, en bloc resection by ESD is desirable for lesions that are suspected T1a or T1b in endoscopic depth diagnosis for precise histological evaluation. Concerning rectal T1b cancer, the problem of positive vertical margin, lymph node metastasis and local recurrence is still remaining. Collaboration with surgical local resection should be considered and endoscopic resection is an appropriate treatment options for lesions spreading to anal canal. Abdominoperineal resection (APR) is sometime chosen as surgical method for cancer located in the lower rectum. In such cases, CRT with en bloc ESD may be also one of the options for patients who are not suitable for surgery due to patient risk factors, or patients who refuse a radical rectal excision with APR. We are now preparing a clinical trial for ESD following CRT for rectal cancer.
This study has several limitations. First, it is a retrospective analysis from clinical records. The second limitation is that this study has a possible selection bias.
In conclusion, ESD for Tis or T1a rectal cancers is thought to be feasible from the aspect of good short-term outcomes and less adverse events, while higher R1 resection ratio was still problem of ESD for T1b lesions. Collaboration between ESD and surgery, and ESD following CRT are potential future treatment options.
Table S1
| Variables | Colon | Rectum | P value |
|---|---|---|---|
| Number of the patients | 1,206 | 404 | – |
| Gender (M/F) | 675/531 | 231/173 | 0.693 |
| Age (mean ± SD) | 66.9±10.1 | 63.6 ±11.5 | <0.001 |
| Number of ESD | 1,274 | 414 | – |
| Lesion size (mean ± SD) (mm) | 34.8±17.1 | 37.3 ±23.4 | 0.003 |
| Histological diagnosis | 0.116 | ||
| Adenocarcinoma [n (%)] | 932 (77.3) | 319 (79.0) | |
| Adenoma/others [n (%)] | 342 (28.3) | 95 (21.0) |
Almost all lesions showed relationship between size of the lesion and procedure time. Several lesions were recognized as outlier of this relationship (within the ellipse). M, man; F, female; SD, standard deviation; ESD, endoscopic submucosal resection.
Acknowledgments
Funding: This study was financially supported in part by the National Cancer Center Research and Development Fund (25-A-12) to Dr. Saito.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Marco Milone) for the series “Minimally Invasive Treatment of Low Rectal Cancer” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2017.07.01). The series “Minimally Invasive Treatment of Low Rectal Cancer” was commissioned by the editorial office without any funding or sponsorship. YS serves as an unpaid editorial board member of Annals of Laparoscopic and Endoscopic Surgery from Dec 2016 to Nov 2018. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by our institutional ethics committee (approval number 2016-447). Informed written consent was obtained from all patients for each specific endoscopic treatment.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Junginger T, Goenner U, Hitzler M, et al. Long-term Oncologic Outcome After Transanal Endoscopic Microsurgery for Rectal Carcinoma. Dis Colon Rectum 2016;59:8-15. [Crossref] [PubMed]
- Allaix ME, Arezzo A, Morino M. Transanal endoscopic microsurgery for rectal cancer: T1 and beyond? An evidence-based review. Surg Endosc 2016;30:4841-52. [Crossref] [PubMed]
- Doornebosch PG, Ferenschild FT, de Wilt JH, et al. Treatment of recurrence after transanal endoscopic microsurgery (TEM) for T1 rectal cancer. Dis Colon Rectum 2010;53:1234-9. [Crossref] [PubMed]
- Lee W, Lee D, Choi S, et al. Transanal endoscopic microsurgery and radical surgery for T1 and T2 rectal cancer. Surg Endosc 2003;17:1283-7. [Crossref] [PubMed]
- Takamaru H, Saito Y, Yamada M, et al. Clinical impact of endoscopic clip closure of perforations during endoscopic submucosal dissection for colorectal tumors. Gastrointest Endosc 2016;84:494-502.e1. [Crossref] [PubMed]
- Imai K, Hotta K, Yamaguchi Y, et al. Preoperative indicators of failure of en bloc resection or perforation in colorectal endoscopic submucosal dissection: implications for lesion stratification by technical difficulties during stepwise training. Gastrointest Endosc 2016;83:954-62. [Crossref] [PubMed]
- Watanabe T, Muro K, Ajioka Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int J Clin Oncol 2017; [Epub ahead of print]. [Crossref] [PubMed]
- Saito Y, Kawano H, Takeuchi Y, et al. Current status of colorectal endoscopic submucosal dissection in Japan and other Asian countries: progressing towards technical standardization. Dig Endosc 2012;24:67-72. [Crossref] [PubMed]
- Saito Y, Uraoka T, Yamaguchi Y, et al. A prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video). Gastrointest Endosc 2010;72:1217-25. [Crossref] [PubMed]
- Saito Y, Otake Y, Sakamoto T, et al. Indications for and technical aspects of colorectal endoscopic submucosal dissection. Gut Liver 2013;7:263-9. [Crossref] [PubMed]
- Saito Y, Uraoka T, Matsuda T, et al. A pilot study to assess the safety and efficacy of carbon dioxide insufflation during colorectal endoscopic submucosal dissection with the patient under conscious sedation. Gastrointest Endosc 2007;65:537-42. [Crossref] [PubMed]
- Yamamoto H, Kawata H, Sunada K, et al. Success rate of curative endoscopic mucosal resection with circumferential mucosal incision assisted by submucosal injection of sodium hyaluronate. Gastrointest Endosc 2002;56:507-12. [Crossref] [PubMed]
- Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2015;47:829-54. [Crossref] [PubMed]
- Tanaka S, Kashida H, Saito Y, et al. JGES guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig Endosc 2015;27:417-34. [Crossref] [PubMed]
- Saito Y, Bharr A, Nakajima T, et al. Colorectal endoscopic submucosal dissection (ESD) could reduce the need for surgery of colonic polyps in the West. Ann Laparosc Endosc Surg 2016;1:16. [Crossref]
- Sakamoto T, Matsuda T, Otake Y, et al. Predictive factors of local recurrence after endoscopic piecemeal mucosal resection. J Gastroenterol 2012;47:635-40. [Crossref] [PubMed]
- Fu KI, Kato S, Sano Y, et al. Staging of early colorectal cancers: magnifying colonoscopy versus endoscopic ultrasonography for estimation of depth of invasion. Dig Dis Sci 2008;53:1886-92. [Crossref] [PubMed]
- Nakamura F, Saito Y, Sakamoto T, et al. Potential perioperative advantage of colorectal endoscopic submucosal dissection versus laparoscopy-assisted colectomy. Surg Endosc 2015;29:596-606. [Crossref] [PubMed]
- Iacopini F, Bella A, Costamagna G, et al. Stepwise training in rectal and colonic endoscopic submucosal dissection with differentiated learning curves. Gastrointest Endosc 2012;76:1188-96. [Crossref] [PubMed]
- Ikematsu H, Yoda Y, Matsuda T, et al. Long-term outcomes after resection for submucosal invasive colorectal cancers. Gastroenterology 2013;144:551-9; quiz e14.
Cite this article as: Takamaru H, Saito Y, Aadam AA, Hammoud GM, Sekiguchi M, Yamada M, Abe S, Sakamoto T, Nakajima T, Matsuda T. The role of endoscopic submucosal dissection for Tis/T1 rectal cancer. Ann Laparosc Endosc Surg 2017;2:129.


