Laparoscopic approach for retrorectal tumors—results of a series of 11 cases
Introduction
Retrorectal tumor (RRT) is infrequent entity in the daily surgical practice. Its incidence is not well known (1), although some authors report an incidence between 2 and 6 cases per year in reference centers (2,3).
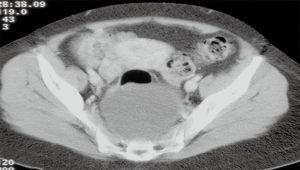
They are located in the retrorectal space (Figure 1), between the presacral fascia, posteriorly, and the rectum, anteriorly, with the Waldeyer fascia and the peritoneal reflection as well as the inferior and superior verge, respectively. This space contains tissues from different embryological origins (vessels, nerves and mesenchymal tissue). This justifies the heterogeneity of observed tumors, originated from different etiological strains, with different possibilities of malignancy (1). Its anatomical location justifies the possible difficulties in the surgical approach.
Different RRT classifications are reported in literature (Table 1), the Uhlig and Johnson modified by Dozois (1) and Pappalardo et al. (4) classifications are both most used.
Table 1
| Uhlig and Johnson, modified by Dozois | Pappalardo et al. | Lev. Chelouche et al. |
|---|---|---|
| Congenital (60–70%) | Retrorectal space: tail gut, teratoma, dermoid, epidermoid, rhabdomyosarcoma, liposarcoma, unclassifiable | Benign congenital |
| Neurogenic (10%) | Sacral spinal cord: meningocele, Ewing’s tumor, neurofibroma, neurofibrosarcoma, neurilemoma, chondrosarcoma, osteogenic sarcoma | Malignant congenital |
| Miscellanea (10%) | Rectum: rectal duplication, leiomyosarcoma, lymphoma | Benign acquired |
| Malignant acquired |
The RRTs are usually asymptomatic, and are often diagnosed routinely in gynaecological controls. If they are not early detected they can achieve a considerable volume, which will determine a series of typical symptoms based on their location: constipation, chronic abdominal pain, perianal abscess, faecal/urinary incontinence or sexual dysfunctions (1,5). Most commonly these tumors are diagnosed in female patients between 40 and 60 years (6).
The diagnosis of RRTs is usually achieved by imaging test such as ultrasound (US), abdominal computed tomography (CT) scan (Figure 1) or magnetic resonance imaging (MRI), and the radiological characteristics can guide on the possible origin of the lesion (5-7). Histologic diagnosis is usually postoperative. Diagnostic biopsy should be avoided due to the difficult access and possible complications, such as the infection (8) or potential neoplastic spreading (1,2).
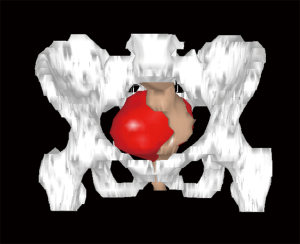
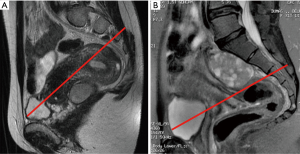
Usually, the perineal or abdominal surgical approach is chosen based on the level of the lesion. The line connecting the pubis with the sacral vertebrae S3–S4, is employed as anatomical landmark, according to the so-called Woodfield’s algorithm (9) (Figure 2). The abdominal or perineal approach are performed based on the relationship between the RRT and the S3 vertebra, based on involvement of different organs and on the lesion size (Figure 3).
The introduction of laparoscopy, and its application in low rectal surgery, allowed to extend the indication of the approach for the treatment of RRT, and questioning about the Woodfield’s algorithm validity (9).
The aim of the present study is to report the authors’ experience with the laparoscopic approach for the treatment of RRT, technically difficult to access.
Methods
This study is a retrospective analysis of prospectively collected data of the Service of Surgery of the Hospital de Sant Pau, Barcelona, Spain, of patients underwent surgery for RRT from 1998 to 2016. Institutional review board (IRB) approval was obtained. Since 1998, 11 patients were treated by laparoscopy for RRT. This study is an update, of a previously published analysis regarding the first 4 patients (10).
Patients’ preoperative clinical and demographic data (age, sex, previous surgery RRT lesion, exploration performed, diagnostic orientation), intraoperative data (operative time, conversion rate, blood loss) and postoperative data (complications, re-intervention) were extracted. Abdominal CT scan was performed periodically to early diagnose potential recurrences.
Surgical technique
Resection of RRT was performed as previously described (10). Preoperative mechanical bowel preparation, the use of elastic stockings and heparin for thrombosis prophylaxis were recommended before surgery.
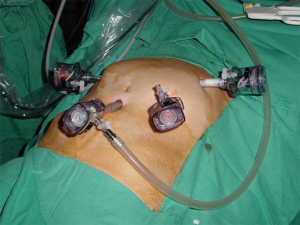
Surgery was performed with patient supine and in extreme Trendelenburg position, as well as in case of laparoscopic approach of the rectum. The first 10 mm optical trocar is inserted at the umbilicus site, one 5 mm and one 12 mm operating trocars are located in the right flank and iliac fossa, respectively, and the last 12 mm trocar is placed in left flank for the assistant (Figure 4). Sometimes a 5 or 11 mm trocar in hypogastrium may be useful to use the retractor or the aspirator (10).
The lesion located at the level of the lower pelvis usually protrudes through the peritoneum that is divided to access in the retrorectal space. This maneuver allows to achieve a wide dissection plane. The blunt dissection is performed with the aim to enucleate the lesion. Sometimes, specially in case of large cystic tumors, it is possible the incidental open the lesion, or to perform a deliberate aspiration, to reduce the tumor size and to facilitate the dissection. In case of cystic lesions, puncture-aspiration of the contents and the tumor capsule extraction by bag, through one of the trocar site, or through a small suprapubic incision such as Pfannenstiel, is performed (10).
A 30° optic is also a useful tool for the surgeon, allowing a better visualization of the small operative field, specially in case of male patients’ narrow pelvis. An essential maneuver, during surgery, is the control of the rectal wall integrity. In fact rectal perforation is considered the most important complication. To avoid this one, a careful technique, adequate traction and exposure of the rectum and digital intra-rectal control during dissection, are required (10).
Results
From 1998 to 2016, 11 patients (4 males, 7 females, mean age 54 years, range 32–86 years) with RRT (Table 2) underwent surgery in authors’ Hospital.
Table 2
| Age (years) | Gender | Symptoms | Approach | Operative time (minutes) | Size (cm) | Complications | Recurrences | Definitive histology | LOS (days) |
|---|---|---|---|---|---|---|---|---|---|
| 33 | Female | Gynecological examination | Laparoscopic | 90 | 7.5-5-4.4 | – | – | Fibrous tumor | 6 |
| 59 | Female | Sciatica | Laparoscopic | 75 | 6-5-3 | Abscess | – | Neurofibroma | 10 |
| 38 | Female | Gynecological examination | Laparoscopic | 145 | 11-5.5-3.5 | – | – | Hamartoma | 5 |
| 84 | Male | Abdominal pain | Laparoscopic | 180 | 7-6-4 | – | – | Teratoma | 6 |
| 86 | Female | Obstructed defecation | Laparoscopic | 190 | 7-7-3.6 | – | – | Hamartoma | 8 |
| 63 | Male | Lumbar pain | Laparoscopic | 200 | 5-6-4 | Abscess | – | Schwannoma | 11 |
| 35 | Male | Abdominal pain | Laparoscopic | 250 | 10-6-1.5 | Wound infection | – | Schwannoma | 7 |
| 32 | Female | Perianal abscess | Combined laparoscopic/perineal | 190 | 7-4-2 | – | – | Tail gut: cystic hamartoma | 5 |
| 43 | Female | Incidentally by CT scan | Laparoscopic | 120 | – | – | – | Salivary gland | 6 |
| 69 | Male | Incidentally by MRI | Laparoscopic | – | – | – | – | Glomus tumor | 7 |
| 38 | Female | Radiological exam | Laparoscopic/converted to open surgery | 210 | 3-5-6 | – | – | Neurofibroma | 11 |
LOS, length of hospital stay; CT, computed tomography; MRI, magnetic resonance imaging.
Preoperative diagnosis was performed radiologically in all cases. MRI was the most useful diagnostic test, providing information regarding the diagnostic orientation and the anatomical lesions relationships (1). Puncture biopsy is not considered mandatory in any case, based on the debate that exists about it clinical use. Moreover in any case, determines a change about surgical indication or approach. In all cases, the preoperative diagnostic orientation was benign lesion, without specifying the origin. All treated RRT were primary diagnoses.
One patient affected by a recurrent hamartoma and previously operated perineally, was treated satisfactorily by the abdominal approach.
Laparoscopic approach was employed in all cases, with mean operative time of 160 minutes (range 75–250 minutes). In one patient, with a presacral neurinoma, previously treated by a total proctocolectomy and ileoanal reservoir by laparotomy, the procedure was converted in open surgery. No intraoperative complications were observed. In all cases, a radical tumor excision was achieved. Blood loss was negligible.
Postoperative complications were observed in two cases (17%): 1 wound infection and 1 pelvic abscess treated by intravenous antibiotic therapy (grade I and II according to Dindo Classification, respectively). In both cases, for easier management during surgery, due to the lesions diameter, an evacuation of lesions contents was performed. Mean postoperative hospital stay was 7.5 days (range 5–11 days).
Mean size of excised lesion was 6 cm (range 5–11 cm). Definitive histology was benign in all cases. Table 2 shows the histological characteristics of the present series. At mean follow up of 4 years (range 1–10 years), recurrences were not observed.
Discussion
The most common and the best treatment established, was surgery by open abdominal or perineal approach or combined. However, these options result in a significant morbidity. A logical development is the laparoscopic approach, after achieving good results with rectal cancer, prolapse and prostate surgery. The laparoscopic option has confuted the traditional Woodfield’s algorithm that recommended in case of upper lesions, above S3, the transabdominal approach, while in case of lower lesion, the perineal route. The laparoscopic approach for these lesions was described for the first time by Sharpe et al. in 1995, for the treatment of a benign RRT (dermoid cyst) (11).
We report our experience, with a selected series of 11 patients who underwent surgery in our Service of Digestive Surgery in the last 19 years, treated by laparoscopic approach. This series only included lesions considered benign after radiological tests, therefore without affectation of bone and nerve structures, and of size beyond which caused difficulties for their extraction, unless in case of cystic lesions. During this long period no changes regarding surgical technique or approach have been adopted (10).
The most important controversy, in the treatment of RRTs, begins with diagnosis, which is basically radiological. The majority of the authors consider that a biopsy should be performed only in case of well-founded suspicion of malignancy or in cases in which, due to the patients’ comorbidity or advanced age, surgery is not suggested and confirmation of benign lesion is required. In our series, all patients underwent surgery after preoperative diagnosis of benign lesions, without preoperative biopsy, and in all cases, definitive histology confirmed benign tumors.
The decision, regarding the most adequate open surgical approach (abdominal versus perineal), is not only determined by the lesion location (Woodfield’s algorithm), but also by the potential malignancy, size, histology or adjacent structures invasion (9,12) (Figures 2,3). The present series confirms the feasibility and the safety of the laparoscopic approach and provides good outcomes as well as also in other series similar to the present for the sample size (8–12 cases) (Table 3) (4,13,14).
Table 3
| Authors | Mean age (years) | Gender | Lesions size, range (cm) | Mean operative time (minutes) | Conversion, n (%) | Complications | LOS (days) | Definitive histology | Mean follow up (months) | Recurrences |
|---|---|---|---|---|---|---|---|---|---|---|
| Nedelcu et al. (4) | 35 | 7 females, 2 males | 3–11.5 | – | 1 (11.1) | Neurological, hematoma | 4.7 | All benign lesions | – | – |
| Duclos et al. (13) | 55 | 12 females | 3–12 | 145 | 2 (16.6) (bleeding, technical difficulty) | Bleeding, urinary infection, rectal injury | 8 | 10 benign and 2 malignant lesions (misdiagnosis) | 34 | – |
| Zhou et al. (14) | 36 | 6 females, 2 males | 6.6–11.8 | 122±36 | – | – | 5 | All benign lesions | 11 | – |
| Present series | 54 | 7 females, 4 males | 5–11 | 160 | 1 (9.1) | 2 | 7.5 | Benign lesions | 48 | – |
LOS, length of hospital stay; RRT, retrorectal tumor.
An extensive experience in laparoscopic rectal surgery, for surgeon who decides to approach these lesions by laparoscopy, is mandatory, such as the surgeon’s familiarity with the laparoscopic dissection of this region, that allows to treat lesions located below S3–S4, for which, following the Woodfield’s algorithm, the open abdominal approach is not recommended. In case of large cystic lesions, the opening and aspiration of their contents significantly facilitate the surgery.
In the present series, conversion to open surgery rate was low (1/11, 9%), and was observed in a young female patients with retrorectal neurofibroma and history of previous proctocolectomy and ileal reservoir for ulcerative colitis, due to severe adhesions that did not allow the laparoscopic approach. In contrast, we had the example of a patient with a first perineal approach for the treatment of a hamartoma that was possible to resect in a second attempt laparoscopically, without recurrence so far.
The lesion size (up to 11 cm) in the present study did not complicate the laparoscopic approach, but cystic tumors have required puncture and aspiration to be resected and extracted by a trocar site, protected with an extractor bag. Although, in some cases, suprapubic mini-laparotomy to extract the specimen, was required. In case of leak of benign lesions contents, there is no risk of neoplastic spreading, in fact aspiration and washing can be performed with saline solution and diluted by povidone iodine at 5%.
The advantages of the laparoscopic approach such as less pain, shorter hospital stay and better recovery in working life, are widely known and reported in several studies about rectal or prostatic surgery. It is important to note, that in literature, three articles, about laparoscopic approach, are reported, and due to the rarity of this pathology these series are very small, with 8, 9 and 12 cases (4,13,14), respectively. In a fourth article (15), 4 out of 15 patients underwent laparoscopic surgery, while the other patients underwent surgery by different combinations of open abdominal and perineal approaches. Conversion rate reported in literature is low (11–16%), and it is comparable with the present series (9%). Mean operative time, in this study, was 146 minutes, similar to this reported in literature (122 and 145 minutes) (13,14) such as blood loss and hospital stay.
Other surgical techniques, less employed for their invasiveness, are reported, as well as the Kraske and York Mason transrectal technique, that results in significant morbidity, especially regarding wound infection (2,15). Transanal endoscopic microsurgery (TEM) is also proposed in literature, mostly in case of small lesions (4).
At mean follow up of 48 months, the recurrence rate, in our series, was 0%. Recurrences of these lesions are observed both in case of malignant tumor, due to their nature, or in case of benign tumor due to an incomplete resection. This means that, in the present series, the R0 has been achieved.
In the present series, the complication rate observed was low (18%), without major complications (grade I and II according to Dindo Classification), if compared to those observed in other series (4,13,14), that include major complications such as neurological lesions and the accidental rectal wall opening, that results in a rectal leak late postoperatively. Therefore, adequate training in rectal oncologic surgery is necessary, with the aim to preserve the hypogastric plexus and the integrity of the rectum.
Definitive histology observed is similar to other series (Table 3), but, as mentioned above, only patients with suspicion of benign lesions, confirmed at postoperative histology, underwent surgery. The great histological variability, that it is possible to achieve, is reported by Baek et al., in a review published in 2016 (16), in which are reported data from 241 studies for a total of 1,708 patients.
The weaknesses of the present study are its retrospective nature, the small sample of patients and the lack of long-term results.
In conclusion, RRTs are infrequent, despite the poor experience accumulated, and studies with a small sample of patients are reported, laparoscopic approach is feasible and safe (10). An adequate experience with laparoscopic surgery, although in other areas such as rectal cancer, greatly facilitates the decision to approach these lesions. It is important to select adequately the patients who can benefit of the laparoscopic approach, based on the suspicion of benignity/malignancy, locoregional dissemination, type and size of the lesion, and incompatibilities for the laparoscopic approach, such as in case of previous abdominal surgery.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2017.07.10). EMT serves as an unpaid editorial board member of Annals of Laparoscopic and Endoscopic Surgery from Oct 2016 to Sep 2018. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethnical committee. Written informed consent was obtained from the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Neale JA. Retrorectal tumors. Clin Colon Rectal Surg 2011;24:149-60. [Crossref] [PubMed]
- Hobson KG, Ghaemmaghami V, Roe JP, et al. Tumors of the retrorectal space. Dis Colon Rectum 2005;48:1964-74. [Crossref] [PubMed]
- Johnson WR. Postrectal neoplasms and cysts. Aust N Z J Surg 1980;50:163-6. [Crossref] [PubMed]
- Nedelcu M, Andreica A, Skalli M, et al. Laparoscopic approach for retrorectal tumors. Surg Endosc 2013;27:4177-83. [Crossref] [PubMed]
- Macafee DA, Sagar PM, El-Khoury T, et al. Retrorectal tumours: optimization of surgical approach and outcome. Colorectal Dis 2012;14:1411-7. [Crossref] [PubMed]
- Glasgow SC, Birnbaum EH, Lowney JK, et al. Retrorectal tumors: a diagnostic and therapeutic challenge. Dis Colon Rectum 2005;48:1581-7. [Crossref] [PubMed]
- Messick CA, Hull T, Rosselli G, et al. Lesions originating within the retrorectal space: a diverse group requiring individualized evaluation and surgery. J Gastrointest Surg 2013;17:2143-52. [Crossref] [PubMed]
- Verazin G, Rosen L, Khubchandani IT, et al. Retrorectal tumor: is biopsy risky? South Med J 1986;79:1437-9. [Crossref] [PubMed]
- Woodfield JC, Chalmers AG, Phillips N, et al. Algorithms for the surgical management of retrorectal tumours. Br J Surg 2008;95:214-21. [Crossref] [PubMed]
- Marinello FG, Targarona EM, Luppi CR, et al. Laparoscopic approach to retrorectal tumors: review of the literature and report of 4 cases. Surg Laparosc Endosc Percutan Tech 2011;21:10-3. [Crossref] [PubMed]
- Sharpe LA, Van Oppen DJ. Laparoscopic removal of a benign pelvic retroperitoneal dermoid cyst. J Am Assoc Gynecol Laparosc 1995;2:223-6. [Crossref] [PubMed]
- Canelles E, Roig JV, Cantos M, et al. Presacral tumors. Analysis of 20 surgically treated patients. Cir Esp 2009;85:371-7. [Crossref] [PubMed]
- Duclos J, Maggiori L, Zappa M, et al. Laparoscopic resection of retrorectal tumors: a feasibility study in 12 consecutive patients. Surg Endosc 2014;28:1223-9. [Crossref] [PubMed]
- Zhou JL, Wu B, Xiao Y, et al. A laparoscopic approach to benign retrorectal tumors. Tech Coloproctol 2014;18:825-33. [Crossref] [PubMed]
- Kye BH, Kim HJ, Cho HM, et al. Clinicopathological features of retrorectal tumors in adults: 9 years of experience in a single institution. J Korean Surg Soc 2011;81:122-7. [Crossref] [PubMed]
- Baek SK, Hwang GS, Vinci A, et al. Retrorectal Tumors: A Comprehensive Literature Review. World J Surg 2016;40:2001-15. [Crossref] [PubMed]
Cite this article as: Hernández Casanovas MP, Martinez MC, Bollo J, Balla A, Batista Rodríguez G, Balagué C, Targarona EM. Laparoscopic approach for retrorectal tumors—results of a series of 11 cases. Ann Laparosc Endosc Surg 2017;2:126.