
Pure single-port laparoscopic proximal gastrectomy using a novel double-flap technique
Current trends in the field of surgery are moving toward the use of less-invasive procedures for the treatment of early gastric cancer to provide a better quality of life for patients who have received a gastrectomy (e.g., function preserving and minimally invasive surgery) (1-4). Proximal gastrectomy is currently accepted by surgeons as the most favorable operative approach for the treatment of early gastric cancer located in the upper third of the stomach due to its advantages over total gastrectomy approaches with respect to preserving the reservoir function of the remnant stomach and for preventing the development of hormonal and nutritional deficiencies (5-7). Esophagogastrostomy is a theoretically optimal reconstructive method following proximal gastrectomy because it is technically simple and preserves normal bowel integrity. However, the procedure can increase the risk for postoperative complications, such as reflux esophagitis, gastric stasis, and anastomotic stenosis (8-10). Therefore, additional procedures, such as conventional fundoplication and reconstruction of the His angle, should be performed when conducting an esophagogastrostomy to decrease reflux esophagitis rates and to treat reflux symptoms (11-13). However, these procedures do not provide satisfactory results in preventing such complications.
Kamikawa developed an original valvuloplastic esophagogastrostomy procedure for preventing postoperative reflux esophagitis in 1998, whereby the distal esophagus and anastomotic site were implanted into the submucosal layer and covered with an H-shaped submuscular flap in open approaches (14). Using this reconstruction procedure, an ideal pseudo-fornix and shape can be formed for the esophagogastric junction that resembles original cardia, which may in turn facilitate the delivery of suitable anti-reflux functions.
Recently, conventional multi-port laparoscopic approaches have been applied to proximal gastrectomy with this reconstruction method for the treatment of early gastric cancer to reduce the invasiveness of surgical procedures (15-17). To maximize the advantages of laparoscopic surgery techniques, surgeons have attempted to use more minimally invasive surgical approaches. Since single-incision laparoscopic distal gastrectomy for gastric cancer, in which the procedure was performed via a single transumbilical incision to minimize abdominal trauma, was first reported in 2011 (18), several studies have reported the technical feasibility, improved cosmesis results, reduced levels of postoperative pain, shortened recovery times, and acceptable oncological outcomes compared to multi-port laparoscopic approaches (19-21). However, no reports have described a single-incision laparoscopic proximal gastrectomy method involving esophagogastrostomy for anti-reflux procedures because the approach is technically demanding due to the reduced number of access ports, the limited scope view, and conflictions among laparoscopic devices.
We have devised a novel technique for esophagogastrostomy after proximal gastrectomy in single-port laparoscopic approaches, in which all reconstructive procedures are performed through the use of continuous barbed sutures (V-Loc™90 Device, Medtronic, Minneapolis, MN). In the present report, we describe in detail this single-port laparoscopic proximal gastrectomy with a novel double-flap esophagogastrostomy and evaluate the safety and feasibility of the procedure.
Methods
Patients and data collection
From August 2014 to February 2017, 44 patients consecutively underwent laparoscopic proximal gastrectomy for gastric carcinoma or esophagogastric junctional adenocarcinoma (32 patients underwent double-flap esophagogastrostomy, 12 underwent double tract reconstruction, and 1 underwent a simple esophagogastrostomy) at our institution according to a prospectively collected gastric cancer database and electronic medical records. Indications of single-port laparoscopic proximal gastrectomy include clinical T1N0 gastric cancer patients of whom the remnant stomach is more than two third and the length of the esophageal invasion of cancer is less than 1cm. TMN classifications were based on the Japanese Classification of Gastric Carcinoma—3rd English Edition (22). We retrospectively analyzed 4 patients (3 men and 1 woman) who had consecutively undergone single-port laparoscopic proximal gastrectomy procedures based on the modified double-flap esophagogastrostomy for the treatment of clinical stage I gastric carcinoma from May 2016 and December 2016. All procedures of this surgery were performed by a single surgeon (Dr. O) who had experienced over 400 single-port laparoscopic gastrectomies for the treatment of gastric cancers. In addition, he has performed over 30 esophagogastrostomy with double flap technique in multiport laparoscopic proximal gastrectomy.
Data were prospectively collected and loaded into a computer database (age, gender, tumor location, pathological findings, reconstruction method used, operative outcomes, intraoperative and postoperative complications, and conversions and their causes). Postoperative parameters (time to flatus, time to oral intake, and postoperative hospital stay) were collected from medical records. Contrast radiography data were examined on the fourth postoperative day. Written informed consent was obtained from all the patients before surgery.
Surgical procedures
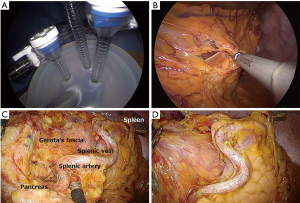
We previously reported our surgical approach for single-incision laparoscopic gastrectomy for D1 and D2 lymph node dissection (23,24). In brief, the patient is placed in the supine position with his or her legs spread open. The surgeon is positioned between the patient’s legs, and an assistant stand on each side of the patient. A transumbilical laparotomy is created through a 2.5 to 3.0 cm vertical umbilical incision, and a wound-sealing device is applied. Single-incision laparoscopic surgery is performed using a commercially available access-port (EZ access; Hakko, Nagano, Japan) (Figure 1A). During the procedure, a pneumoperitoneum is established with carbon dioxide insufflations at a pressure level of approximately 8 to 12 mmHg according to body type. A 10 mm flexible high-definition scope (Endoeye flexible HD camera system; Olympus Medical Systems Corp) is used to visualize the surgical fields. We mainly use a conventional straight grasper and ultrasonic coagulation cutting device (Harmonic scalpel, Ethicon Endosurgery, Cincinnati, OH, USA) to perform gastric mobilization and lymph node dissection. The greater omentum is dissected approximately 4 cm from the gastroepiploic artery (Figure 1B). The left gastroepiploic vessels are transected after clipping for the dissection of station no. 4sb lymph nodes. The gastrosplenic ligament is transected using laparoscopic coagulating shears for the dissection of station no. 4sa lymph nodes. During a station no. 8a dissection, the common hepatic artery and bifurcations of the proper hepatic and gastroduodenal arteries are exposed along the plane of the periarterial plexus for lymph node dissection. The left gastric artery is divided after double clipping, and station no. 7 and 9 lymph nodes are consecutively dissected from the periarterial plexus (Figure 1C). During a station no. 11p dissection, lymph nodes are dissected in the dorsal plane where the splenic vein or pancreas can be identified. No. 11d lymph nodes are dissected along the distal pancreatic artery toward the splenic hilum when necessary (Figure 1D). After achieving gastric mobilization and adequate lymphadenectomy, the esophagus and stomach are staple-transected using a linear stapling device (ECHELON FLEX Powered ENDOPATH Stapler, Ethicon Endosurgery, Cincinnati, OH, USA). Then, the resected specimen is extracted through a small incision.
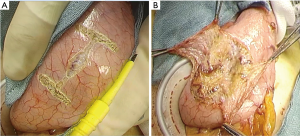
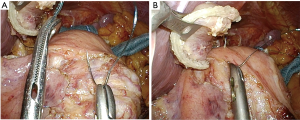
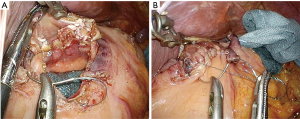
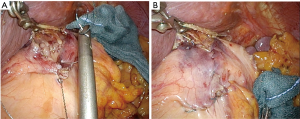
The reconstruction procedure is described as follows. An H-shaped seromuscular flap (2.5×3.5 cm) located 5 cm from the top is created on the anterior wall of the gastric remnant extracorporeally (Figure 2). The stomach is opened approximately 5 mm from the lower edge of the mucosal ‘casement’ to perform an esophagogastrostomy, and stay sutures are created along both edges of the opening window to make markings if necessary. The stomach is inserted into the peritoneal cavity, and the pneumoperitoneum is re-established to perform an intracorporeal anastomosis. All reconstruction tasks are completed using barbed sutures to perform the procedures simply (even during the single-incision laparoscopic approaches). First, the posterior esophageal wall is fixed to the stomach at the cranial edge of the submucosal window via continuous suturing using barbed sutures (V-Loc™90 Device, Medtronic, Minneapolis, MN, USA) (Figures 3,4). This step is the most cumbersome and difficult of the reconstruction method, as the procedure is often performed in narrow areas of the uppermost abdominals or lower mediastinum. The use of continuous and locked barbed sutures make this difficult step easier to perform. Second, the esophagus is opened proximal to the staple line of the stump, and the posterior wall of the esophagus and the gastric mucosa are then sutured by continuous barbed sutures. Layer-to-layer suturing via continuous V-Loc sutures is performed between the anterior esophagus and stomach along the lower edges of the flap to complete the esophagogastrostomy (Figure 5). Finally, the anastomosis and distal esophagus are covered with the seromuscular flap via 3-barbed suturing (Figure 6). We typically use the original ‘Ogami’ technique whereby the needle is grasped by the needle holder parallel to the instrument axis (as when placing one’s palms together in prayer) and is rotated and pushed to suture (Figure 4).

Results
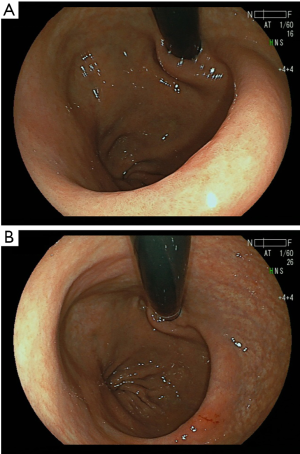
All proximal gastrectomies with the double-flap esophagogastrostomy were performed using the single-port laparoscopic approach in the R0 status with no open conversions or transfusions (Table 1). The median operative time was 275 minutes (range, 267–326 minutes). The median blood loss was 12.5 mL (range, 0–50 mL,). Liquid and soft diets were resumed over 2 and 3 postoperative days, respectively. The median first flatus and defecation times were 3 and 4 postoperative days, respectively (Table 2). The contrast radiography results from the third and fourth postoperative days reveal that in all four cases, contrast medium was not regurgitated into the esophagus when the head was tilted 30 degrees downward. Concerning postoperative morbidities, no complications, such as anastomotic leakage/stenosis or pancreatitis/pancreatic fistula, were observed. The postoperative course was excellent, and the postoperative hospital stay was 8 days for all patients. None of the patients experienced postoperative gastroesophageal reflux symptoms during the 6-month follow-up period. This reconstruction method created the pseudo-fornix and an ideal shape for the esophagogastric junction that resembled the original cardia, demonstrated by the upper gastrointestinal endoscopic examination at 3 to 6 months after operation (Figure 7). The upper gastrointestinal endoscopic examination showed no anastomotic stenosis and esophagitis. Although, one patient, who suffered no reflux esophagitis, has administered antacid agent such as proton pump inhibitors for the treatment of the NSAIDS-induced gastric ulcer, other patients could be free from antacid agent administration at about 3 months postoperative day. The average ratio of body weight loss at 1, 3, 6 months postoperative (relative to preoperative body weight) was 4.8%, 8.2%, and 7.7%, respectively. There was no mortality or recurrence in this series.
Table 1
| Characteristics | Value |
|---|---|
| Median age [range]; years | 73.5 [71–80] |
| Gender: male/female | 3/1 |
| ASA, 1/2/3 | 0/2/2 |
| Median BMI (range); kg/m2 | 22.2 (14.7–23.8) |
| Main lesion | |
| Upper | 4 |
| Extent of lymph node dissection | |
| D1+/D2 | 4/0 |
| Clinical TMN classification | |
| cT1bN0M0 | 4 |
| Pathological TMN classification | |
| pT1bN0M0 | 3 |
| pT2N0M0 | 1 |
| Curability | |
| R0/R1 | 4/0 |
| Completion rate | 4/4 (100%) |
BMI, body mass index; ASA, American Society of Anesthesiologists; TNM staging was based on the Japanese Classification of Gastric Carcinoma (3nd English Edition) (15).
Table 2
| Operative outcomes | Value |
|---|---|
| Operative time, Median [range], minutes | 275 [267–326] |
| Estimated blood loss, Median [range], mL | 12.5 [0–50] |
| Conversion to multiport LPG/ open procedure | 0/0 |
| Time to first flatus, days, median [range] | 3 [1–4] |
| Time to oral intake, liquids, days | 2 |
| Time to oral intake, solid diet, days | 4 |
| Postoperative hospital stay, days | 8 |
| Postoperative complications | 0 |
| Anastomotic leakage/stenosis | 0 |
| Pancreatitis/pancreatic fistula | 0 |
| Abdominal abscess | 0 |
| Gastroesophageal reflux symptoms | 0 |
| Reflux esophagitis | 0 |
Discussion
The esophagogastrostomy is theoretically a desirable reconstruction method after proximal gastrectomy for the treatment of early gastric cancer located in the upper portion of the stomach because the method preserves intestinal integrity and the physiological function of the gastric remnant, which yields better postoperative nutritional outcomes. However, the rates of late postoperative adverse events (e.g., reflux esophagitis and anastomotic stricture) are relatively high, even when fundoplication procedures are applied to the anastomotic site. Kamikawa’s valvuloplastic esophagogastrostomy approach, which involves supporting strong anti-reflux function by implanting the esophagus into the submuscular tunnel of the stomach, seems to be a promising reconstruction procedure for proximal gastrectomy (14). Double-flap valvuloplastic esophagogastrostomy should be performed with hand-sewn sutures (without stapling devices) to achieve a flexible anastomosis to create a one-way check valve (15). Thus, while the anastomosis of the posterior wall is performed by continuous suturing, the anastomosis of the anterior wall is performed by creating interrupted sutures to maintain the flexible anastomosis of the original technique. This reconstruction method is extremely complicated and time-consuming to perform even when using multi-port laparoscopic approaches because it involves applying multi-step, skilled laparoscopic hand-sewn sutures and ligation techniques to perform numerous interrupted sutures, leading to prolonged operative times (15-17,25).
We have devised a simple approach for valvuloplastic esophagogastrostomy that involves using barbed sutures and knotless, self-anchoring surgical sutures. We used continuous barbed sutures during every phase to more easily perform the reconstruction procedures and shorten the reconstruction period. This novel technique facilitates proximal gastrectomy with valvuloplastic esophagogastrostomy even in single-port laparoscopic approaches. In addition, the ‘Ogami’ holding approach to intracorporeal suturing is exceedingly useful when performing the reconstruction procedures based on single-port laparoscopic approaches whereby the axis of the needle holder is positioned parallel to the direction of suturing. In our study, the median operative time was 275 min when using a single-incision laparoscopic approach (including 50 min for reconstruction procedures), which was similar to that in multi-port laparoscopic approaches (data not shown). In addition, our data is far shorter than operative times reported previously in multi-port laparoscopic proximal gastrectomy with conventional double flap technique (342–387 minutes, medians) (15,16,22). There was one case report on single-port proximal gastrectomy with double tract reconstruction for early gastric cancer (26). Double tract reconstruction procedures necessitate three anastomoses including esophagojejunostomy, gastrojejunostomy, and jejunojejunostomy, each of which are technically complicated and time consuming in single-port laparoscopic approaches (350 minutes). The resulting short- and mid-term surgical outcomes were excellent (no anastomotic leakages, strictures, reflux esophagitis, or gastroesophageal reflux symptoms). Furthermore, the ratio of body weight loss at 1, 3, 6 months postoperative (relative to preoperative body weight), which was 4.8%, 8.2%, and 7.7%, respectively, may be minimal compared to other reconstruction procedures. Thus, single-port laparoscopic proximal gastrectomy procedures based on modified double-flap techniques are safe and feasible with acceptable short- and mid-term results.
However, our study included limited number of cases (n=4) and relatively short-terms follow up; therefore, further research is warranted to confirm the safety and feasibility of single-port laparoscopic proximal gastrectomy with the modified continuous suturing technique compared to multi-port laparoscopic proximal gastrectomy.
In summary, double-flap single-port laparoscopic proximal gastrectomy procedures are technically feasible and safe when applying our modified technique. By the use of above mentioned procedures, the expert laparoscopic surgeons could achieve a safe operation, and the approach constitutes an attractive surgical approach to proximal gastrectomy for the treatment of early gastric cancer. Prospective randomized trials that compare single-port laparoscopic gastrectomy approaches to conventional laparoscopic gastrectomy techniques are still required to further establish this innovative and minimally invasive surgical approach.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Chikara Kunisaki) for the series “Advancement of Single-port, Reduced-port Laparoscopic Gastrectomy for Gastric Cancer” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2017.06.01). The series “Advancement of Single-port, Reduced-port Laparoscopic Gastrectomy for Gastric Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kitano S, Iso Y, Moriyama M, et al. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc 1994;4:146-8. [PubMed]
- Katai H, Sasako M, Fukuda H, et al. Safety and feasibility of laparoscopy-assisted distal gastrectomy with suprapancreatic nodal dissection for clinical stage I gastric cancer: a multicenter phase II trial (JCOG 0703). Gastric Cancer 2010;13:238-44. [Crossref] [PubMed]
- Katai H, Mizusawa J, Katayama H, et al. Short-term surgical outcomes from a phase III study of laparoscopy-assisted versus open distal gastrectomy with nodal dissection for clinical stage IA/IB gastric cancer: Japan Clinical Oncology Group Study JCOG0912. Gastric Cancer 2017;20:699-708. [Crossref] [PubMed]
- Hiki N, Nunobe S, Kubota T, et al. Function-preserving gastrectomy for early gastric cancer Ann Surg Oncol 2013;20:2683-92. [Crossref] [PubMed]
- Braga M, Zuliani W, Foppa L, et al. Food intake and nutritional status after total gastrectomy: results of a nutritional follow-up. Br J Surg 1988;75:477-80. [PubMed]
- Adachi Y, Katsuta T, Aramaki M, et al. Proximal gastrectomy and gastric tube reconstruc tion for early cancer of the gastric cardia. Dig Surg 1999;16:468-70. [Crossref] [PubMed]
- Nozaki I, Hato S, Kobatake T, et al. Long-term outcome after proximal gastrectomy with jejunal interposition for gastric cancer compared with total gastrectomy. World J Surg 2013;37:558-64. [Crossref] [PubMed]
- Nakamura M, Yamaue H. Reconstruction after proximal gastrectomy for gastric cancer in the upper third of the stomach: a review of the literature published from 2000 to 2014. Surg Today 2016;46:517-27. [Crossref] [PubMed]
- Ahn SH, Lee JH. Comparative study of clinical outcomes between laparoscopy-assisted proximal gastrectomy (LAPG) and laparoscopy-assisted total gastrectomy (LATG) for proximal gastric cancer. Gastric Cancer 2013;16:282-9. [Crossref] [PubMed]
- Aihara R, Mochiki E, Ohno T, et al. Laparoscopy-assisted proximal gastrectomy with gastric tube reconstruction for early gastric cancer. Surg Endosc 2010;24:2343-8. [Crossref] [PubMed]
- Sakuramoto S, Yamashita K, Kikuchi S, et al. Clinical experience of laparoscopy-assisted proximal gastrectomy with Toupet-like partial fundoplication in early gastric cancer for preventing reflux esophagitis. J Am Coll Surg 2009;209:344-51. [Crossref] [PubMed]
- Ichikawa D, Komatsu S, Okamoto K, et al. Evaluation of symptoms related to reflux esophagitis in patients with esophagogastrostomy after proximal gastrectomy. Langenbecks Arch Surg 2013;398:697-701. [Crossref] [PubMed]
- Nakamura M, Nakamori M, Ojima T, et al. Reconstruction after proximal gastrectomy for early gastric cancer in the upper third of the stomach: an analysis of our 13-year experience. Surgery 2014;156:57-63. [Crossref] [PubMed]
- Kamikawa Y, Kobayashi T, Ueyama S. A new antireflux procedure in esophagogastrostomy after proximal gastrectomy. Shokakigeka 2001;24:1053-60.
- Kuroda S, Nishizaki M, Kikuchi S, et al. Double-Flap Technique as an Antireflux Procedure in Esophagogastrostomy after Proximal Gastrectomy. J Am Coll Surg 2016;223:e7-13. [Crossref] [PubMed]
- Muraoka A, Kobayashi M, Kokudo Y. Laparoscopy-Assisted Proximal Gastrectomy with the Hinged Double Flap Method. World J Surg 2016;40:2419-24. [Crossref] [PubMed]
- Hosoda K, Yamashita K, Moriya H, et al. Laparoscopically Assisted Proximal Gastrectomy with Esophagogastrostomy Using a Novel "Open-Door" Technique: LAPG with Novel Reconstruction. J Gastrointest Surg 2017;21:1174-80. [Crossref] [PubMed]
- Omori T, Oyama T, Akamatsu H, et al. Transumbilical single-incision laparoscopic distal gastrectomy for early gastric cancer. Surg Endosc 2011;25:2400-4. [Crossref] [PubMed]
- Park DJ, Lee JH, Ahn SH, et al. Single-port Laparoscopic Distal Gastrectomy With D1+β Lymph Node Dissection for Gastric Cancers. Surg Laparosc Endosc Percutan Tech 2012;22:e214-6. [Crossref] [PubMed]
- Ahn SH, Son SY. Pure single-port laparoscopic distal gastrectomy for early gastric cancer: comparative study with multi-port laparoscopic distal gastrectomy. J Am Coll Surg 2014;219:933-43. [Crossref] [PubMed]
- Omori T, Fujiwara Y, Moon J, et al. Comparison of Single-incision and Conventional Multiport Laparoscopic Distal Gastrectomy with D2 Lymph Node Dissection for Gastric Cancer: A Propensity Score–Matched Analysis. Ann Surg Oncol 2016;23:817-24. [Crossref] [PubMed]
- Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011;14:101-12. [Crossref] [PubMed]
- Omori T, Masuzawa T, Akamatsu H, et al. A simple and safe method for Billroth I reconstruction in single-incision laparoscopic gastrectomy using a novel intracorporeal triangular anastomotic technique. J Gastrointest Surg 2014;18:613-6. [Crossref] [PubMed]
- Omori T, Nishida T. Distal Gastrectomy. In: Mori T, Dapri G, editors. Reduced Port Laparoscopic Surgery. Tokyo: Springer, 2014:183-95.
- Hayami M, Hiki N, Nunobe S, et al. Clinical Outcomes and Evaluation of Laparoscopic Proximal Gastrectomy with Double-Flap Technique for Early Gastric Cancer in the Upper Third of the Stomach. Ann Surg Oncol 2017;24:1635-42. [Crossref] [PubMed]
- Lee CM, Park DW, Jung DH, et al. Single-Port Laparoscopic Proximal Gastrectomy with Double Tract Reconstruction for Early Gastric Cancer: Report of a Case. J Gastric Cancer 2016;16:200-6. [Crossref] [PubMed]
Cite this article as: Omori T, Moon JH, Yanagimoto Y, Sugimura K, Miyata H, Yano M. Pure single-port laparoscopic proximal gastrectomy using a novel double-flap technique. Ann Laparosc Endosc Surg 2017;2:123.


