
A randomised controlled trial comparing the use of omega-3 polyunsaturated fatty acid supplements versus very low calorie dietary restriction in obese Malaysian patients awaiting bariatric surgery
Introduction
Morbid obesity is a significant risk factor in development of non-alcoholic fatty liver disease (NAFLD). NAFLD is a term encompassing a spectrum of liver damage, ranging from steatosis alone to steatohepatitis, advanced fibrosis to cirrhosis (1,2).
NAFLD is defined pathologically by presence of steatosis with necroinflammatory activity mostly in lobular distribution with or without Mallory’s hyaline or fibrosis (3,4). Its clinical progression can be as advanced as hepatic failure secondary to cirrhosis and sometimes hepatocellular carcinoma (5-7). NAFLD is theorized to be a result of a “two-hit’ hypothesis. Insulin resistance and visceral obesity ultimately will increase intrahepatic triglyceride content culminating in NAFLD (8). In a minority of patients, further insult to the liver in the form of oxidative stress and inflammation will ultimately exacerbate steatosis to progress to fibrosis and ending in cirrhosis or hepatocellular carcinoma. Several studies have demonstrated a relationship between steatosis, body weight and body mass index (BMI) (9-11). NAFLD and obesity have a close relationship. It is estimated that 84% to 96% of obese patients will have NAFLD as diagnosed by liver biopsy (9,12,13). Within this cohort, 24% to 55% will have non-alcoholic steatohepatitis (NASH) with 12% having established fibrosis (9). Alanine transaminase (ALT) has been used as a marker to diagnose or monitor treatment for NASH. Weight loss has been associated with reductions in ALT in some patients (14).
Morphologically, NAFLD patients tend to have larger livers at the initial stages before progressing to cirrhosis or hepatocellular carcinoma. This has been demonstrated through various imaging modalities such as ultrasonography (US), computed tomography (CT) and magnetic resonance imaging (15,16). Of the various imaging modalities available, a meta-analysis done by Bohte et al. has shown that MRI is the best imaging modality in detecting and grading hepatic steatosis according to its severity (17).
Low calorie diet (LCD) or very low calorie diet (VLCD) has been shown to be effective in reducing liver size and reducing fat content intrahepatically (18-22). Current VLCDs are usually provided in the context of comprehensive treatment programs, during which usual food intake is completely replaced by specific foods or liquid formulas containing 800 kcal/d or less. Current VLCDs are generally safe when used under proper medical supervision in moderately and severely obese patients and are usually effective in promoting significant short-term weight loss, with concomitant improvement in obesity-related conditions. Serious complications of modern VLCDs are unusual, cholelithiasis being most common (22). Omega-3 polyunsaturated fatty acids (PUFAs) have also been shown to reduce hepatic steatosis and reduce the effects of NAFLD by influencing lipid metabolism and insulin sensitivity. PUFAs are derived from exogenous sources such as from fish oil, flaxseed and olive oil. It regulates gene transcription factors thus producing a beneficial impact on cardio-metabolic risk factors (23,24). Although low amounts of PUFAs do not seem to bring up any adverse issues, some side effects do occur. Most common is a fishy aftertaste. Gastrointestinal disturbances and nausea also were commonly reported side effects. Finally, although refined and concentrated omega-3 fatty acid products contain virtually no methyl-mercury and are very low in organochloride contaminants (25), less well-controlled preparations can contain appreciable amounts (26). PUFAs have been shown in animal models and human intervention trials that it improves the effects of NAFLD in terms of imaging and functional measurements. Antonio et al has demonstrated that PUFAs supplementation without dietary modification reduces liver volume by 20% (8). With the knowledge of both methods, i.e., VLCD and PUFAs as having a beneficial role in reducing liver volume, we compared them in the context of pre-operative hepatic volume reduction (HVR) in obese individuals awaiting laparoscopic bariatric surgery. The primary objective of our study was to compare the amount of HVR in the group receiving VLVD versus the group receiving omega-3-PUFA supplementation at the end of 30 days. The secondary objectives were to measure change in serum ALT levels and weight loss in both VLCD as well as the omega-3-PUFA group.
Methods
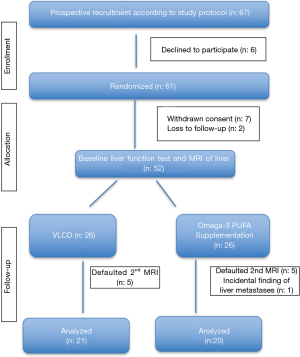
We conducted a single center, randomized controlled trial to compare reduction in hepatic volume between VLCD and omega-3 PUFA supplementation in obese individuals awaiting laparoscopic bariatric surgery. Participants were recruited from the Obesity Clinic of UKM Medical Centre. All patients aged 18–60 years old with BMI >30 kg/m2 who presented to the obesity clinic of University Kebangsaan Malaysia Medical Centre between January 2015 to January 2016 were recruited for this study. Patients with established diagnosis of liver cirrhosis, hepatocellular carcinoma, hepatitis B, hepatitis C and those with known allergy to omega-3 PUFA were excluded. Following informed consent, demographic details were recorded and a base line LFT and MRI was performed for all patients. Patients were then randomized using block randomization technique into two groups; omega-3-PUFA group or VLCD group for 30 days. The dietary plan for the VLCD group was personalized by a dietitian according to the patients’ regular meal but restricted to a limit of 800 kcal/day. Patients were encouraged to maintain a food diary. Those in the omega-3 supplementation group were given fish oil Capsules at a dose of 2,000 mg per day (to be taken in the morning). They were allowed to continue with their regular food intake with advice to avoid deep fried food items and sweet drinks. Weight loss parameters were again documented along with a repeat of liver function test and MRI at the end of 30 days (Figure 1). The patients were blinded from knowing the supplements assigned to others. The radiologist reviewing the MRI images was also blinded from information pertaining to the type of intervention.
Data analysis
A descriptive per protocol analysis was performed. Collected data was entered into the Statistical Package for the Social Sciences (SPSS) for analysis. A paired t-test or Wilcoxon Sign Rank test was used to analyze the different variables pre & post—intervention. P value of <0.05 was taken as the significant level. The type I error probability associated with this test of this null hypothesis is 0.05.
Results
Overall, this study involves morbidly obese patients with a mean age were 41 and 47 years respectively for VLCD and omega-3 PUFAs group. Majority of study participants were female (Table 1). HVR in VLCD group was (mean ± SD) 37.10±15.76 cm3, and 34.88±9.99 cm3 in the omega-3-PUFA group, with statistically similar effect when compared (P=0.29) (Table 2). Serum ALT remained unaffected (P=0.41) (Table 3). Both groups demonstrated weight loss (kg) at the end of day 30 (VLCD, 2.21±2.29; omega-3-PUFA, 2.85±4.62) with no statistically significant difference between the two groups (P=0.58) (Table 4). There was no statistically significant difference between the two groups when weight, liver size, ALT and BMI were compared at the onset and end of this study (Table 5). There was no issue of non-compliance to given therapy in both groups.
Table 1
| Characteristics | VLCD | Omega-3 PUFAs |
|---|---|---|
| Number (n) | 21 | 20 |
| Age (years) | ||
| Mean ± SD | 41.00±10.86 | 47.00±9.17 |
| Maximum | 64 | 61 |
| Minimum | 19 | 32 |
| Gender [%] | ||
| Male | 9 [42.86] | 3 [15] |
| Female | 12 [57.14] | 17 [85] |
| Weight, mean ± SD (kg) | 112.54±33.30 | 101.88±23.49 |
| BMI, mean ± SD (kg/m2) | 43.22±10.87 | 42.03±6.98 |
VLCD, very low calorie diet; PUFA, polyunsaturated fatty acid; BMI, body mass index.
Table 2
| Groups | Duration | Liver volume, mean ± SD (cm3) | P value |
|---|---|---|---|
| VLCD | Baseline | 1,647.04±387.73 | 0.293 |
| 30 days | 1,609.94±359.41 | – | |
| PUFAs | Baseline | 1,526.04±469.43 | 0.135 |
| 30 days | 1,491.17±416.86 | – |
There was no significant difference (P>0.05) of total liver volume between VLCD and omega PUFAs at baseline and 30 days of intervention. VLCD, very low calorie diet; PUFA, polyunsaturated fatty acid; BMI, body mass index.
Table 3
| Groups | Duration | Serum ALT, mean ± SD (IU/L) | P value |
|---|---|---|---|
| VLCD | Baseline | 41.05±79.20 | 0.359 |
| 30 days | 24.62±17.51 | – | |
| PUFAs | Baseline | 26.45±17.48 | 0.441 |
| 30 days | 25.06±17.93 | – |
There was no significant difference (P>0.05) of Serum ALT between VLCD and omega-3 PUFAs at baseline and 30 days of intervention. ALT, alanine transaminase; VLCD, very low calorie diet; PUFA, polyunsaturated fatty acid.
Table 4
| Groups | Duration | Weight, mean ± SD (kg) | P value |
|---|---|---|---|
| VLCD | Baseline | 112.54±33.30 | 0.001 |
| 30 days | 110.33±31.93 | – | |
| PUFAs | Baseline | 101.88±23.49 | 0.013 |
| 30 days | 99.04±22.70 | – |
There was a significant difference (P<0.05) of weight reduction in each group at baseline and 30 days of intervention. VLCD, very low calorie diet; PUFA, polyunsaturated fatty acid.
Table 5
| Outcome | VLCD, mean ± SD | PUFAs, mean ± SD | P value |
|---|---|---|---|
| Weight loss (kg) | 2.21±2.29 | 2.85±4.62 | 0.58 |
| Liver volume reduction (cm3) | 37.10±15.76 | 34.88±9.99 | 0.29 |
| BMI reduction (kg/m2) | 1.17±1.31 | 1.13±1.84 | 0.93 |
| Serum ALT reduction (IU/L) | 16.43±17.48 | 1.40±1.767 | 0.41 |
There was no significant difference (P>0.05) of weight loss, liver volume, body mass index reduction and Serum ALT reduction between VLCD and omega-3 PUFAs at baseline and 30 days of intervention. VLCD, very low calorie diet; PUFA, polyunsaturated fatty acid; BMI, body mass index; ALT, alanine transaminase.
Discussion
The importance of achieving HVR in patients planned for bariatric surgery is obvious. A heavy and large liver limits the operating space, bleeds easily and impairs exposure of the gastro-esophageal junction (GEJ), hiatus as well as the angle of his, thus adding to the complexity of surgery in an already challenging anatomy.
There has been various methods advocated to achieve HVR. VLCD advice is one such method. It is easily administered, widely practiced, has been extensively researched and does has a proven track record for achieving HVR in obese individuals undergoing bariatric surgery (20,21). Edholm et al. prescribed a four-week course of low calorie diet for fifteen of their morbidly obese patients prior to laparoscopic gastric bypass to assess reduction in liver volume and investigate if these changes would facilitate the surgery. At the end of week 4, the liver volume had decreased by 12% (P<0.001) and intrahepatic fat by 40% (P<0.001). There was significant improvement in hiatal exposure (P<0.05), reduction in psychological stress among operating surgeons (P<0.05) and a significantly lower complexity rating score for the surgery as given by the participating surgeons (P<0.05) (22). Unfortunately, opponents of VLCD would argue against this method as there are no consensus on standardization of the VLCD menu and because it has been linked to poor patient compliance. A failure rate of 13–20% has been reported in previous studies involving dietary restrictions due to inability of patients to fully comply and adhere to the advice (19,20).
On the other hand, omega-3 PUFA supplements have become increasingly popular over the years. It has been perceived as a more compliance-friendly method to achieve the desired pre-operative HVR in obese individuals prior to bariatric surgery as it does not require caloric restriction. Deemed “safe” by the European Food Safety Board in 2012 (27), they are already being widely used in prevention and treatment of a wide variety of cardiovascular, immunological, psychological and neurological disorders. Iannelli et al. assessed the effects of pre-operative omega-3 PUFA supplementation in obese individuals planned for laparoscopic roux-en-y gastric bypass. A total of 20 patients were administered the omega-3-PUFA supplement for a duration of 4 weeks without any specific dietary restrictions. A 20% decrease in hepatic volume was noted (P=0.002), giving rise to easier retraction of the left lobe of liver and easier access to the GEJ, thus facilitating surgery (8).
The beneficial effects of VLCD in achieving short term weight reduction has been linked to reduction in caloric intake and resting energy expenditure (28). Omega-3 PUFA supplementation on the other hand has not been largely associated with weight loss, even when used as an adjunct to lifestyle modification (29). Thus, the weight loss seen in our omega-3-PUFA group may highlight a new advantage of this dietary supplement. Various dosage have been used in previous reports (8,30) thus the possibility of this observed weight loss in our sample population being dose-dependent is not totally out of question and warrants further investigation.
Omega-3-PUFA supplements have been postulated to be able to prevent and reverse hepatic steatosis by reducing lipogenic gene expression, exerting anti-inflammatory action, reducing oxidative stress, increasing insulin sensitivity and improving glycemic control (31,32). This compounded with other advantages of the supplement could revolutionize the way clinicians utilize PUFA supplementation. Although liver biopsy remains the gold standard method of quantifying hepatic steatosis, the invasiveness of this procedure limits its application (33). MRI volumetry is the next best option of assessing “liver fatness”. It provides a quantitative assessment of hepatic steatosis thus of great importance in clinical practice. This quality enables MRI to measure any improvement i.e., reduction in hepatic steatosis with any treatment regime prescribed. Hepatic steatosis when analyzed using MRI shows hyperintense signal in T1 and a signal drop out during out of phase imaging. However, in a developing economy the cost factor may be a set back and hindrance. In trained hands, ultrasonography of the liver could prove to be a more feasible, sustainable and reproducible investigative tool to quantify steatosis or fibrosis.
There has been conflicting reports on the use of ALT as a biochemical marker for NAFLD. While Daniel et al has shown that an elevated transaminase level had a positive predictive value of 90% for NAFLD (34) and another study by Wang et al concluded that reduction in Serum ALT could represent a reduction in liver fat deposition (35), others claim that the levels could be normal despite histological changes (36). Despite its pitfalls, ALT is still being used to detect and monitor patients with NAFLD.
The strength of our study lies in the design of the study. From our extensive literature review, we were not able to find any other RCT comparing VLCD to omega-3-PUFA supplementation with aim of achieving pre-operative HVR among obese Asian individuals awaiting bariatric surgery. We believe that our study might be the first to do so. The MRI volumetry was performed by a single senior consultant radiologist who was blinded to the type of intervention, thus reducing interoperator variability and reporting bias.
The limitation of our study was the non-standardization of the low calorie diet menu. We have updated our current practice and now prescribe a standardised VLCD regime prior to surgery using a combination of Glucerna® Triple Care (Abbott) and Valens Myotein®. The milk formulation is taken 4 times a day and functions as a meal replacement strategy (766 kcal, 66 g protein in 24 h). The duration of the VLCD may vary between 2 to 4 weeks depending on the availability of our operating list. The small sample size and our inability to strictly monitor the compliance of our VLCD group could have also influenced the results of our study.
Conclusions
VLCD and Omega-3 PUFA supplementation are both useful methods in achieving reduction of liver volume in obese individuals awaiting laparoscopic bariatric surgery. HVR, reduction in body weight and that of serum ALT levels were seen in both groups although not statistically significant when compared between the two. A larger, multi-centered randomized control trial with cost-effectiveness analysis of both methods would be able to more accurately determine superiority of one method over the other.
Acknowledgments
Funding: The study was funded by UKM Research Grant (GUP 2014-067).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Wei-Jei Lee) for the series “Laparoscopic Metabolic Surgery for the Treatment of Type 2 Diabetes in Asia” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2017.06.12). The series “Laparoscopic Metabolic Surgery for the Treatment of Type 2 Diabetes in Asia” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 201Research 3). The study was approved by the Ethics Committee of UKM Medical Centre (FF 2014-270). Informed consent was obtained from patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- World Health Organization, Regional Office for the Western Pacific. The Asia-Pacific perspective: redefining obesity and its treatment. Sydney: Health Communications Australia, 2000.
- Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology 2003;37:1202-19. [Crossref] [PubMed]
- Ludwig J, Viggiano TR, McGill DB, et al. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clinic proceedings 1980;55:434-8. [PubMed]
- Schaffner F, Thaler H. Nonalcoholic fatty liver disease. Prog Liver Dis 1986;8:283-98. [PubMed]
- Adams LA, Lymp JF, St Sauver J, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology 2005;129:113-21. [Crossref] [PubMed]
- Ekstedt M, Franzén LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology 2006;44:865-73. [Crossref] [PubMed]
- Harrison SA, Torgerson S, Hayashi PH. The natural history of nonalcoholic fatty liver disease: a clinical histopathological study. Am J Gastroenterol 2003;98:2042-7. [Crossref] [PubMed]
- Iannelli A, Martini F, Schneck AS, et al. Preoperative 4-week supplementation with omega-3 polyunsaturated fatty acids reduces liver volume and facilitates bariatric surgery in morbidly obese patients. Obes Surg 2013;23:1761-5. [Crossref] [PubMed]
- Clark JM. The epidemiology of nonalcoholic fatty liver disease in adults. J Clin Gastroenterol 2006;40:S5-10. [PubMed]
- Gaba RC, Knuttinen MG, Brodsky TR, et al. Hepatic steatosis: correlations of body mass index, CT fat measurements, and liver density with biopsy results. Diagn Interv Radiol 2012;18:282-7. [PubMed]
- Park BJ, Kim YJ, Kim DH, et al. Visceral adipose tissue area is an independent risk factor for hepatic steatosis. J Gastroenterol Hepatol 2008;23:900-7. [Crossref] [PubMed]
- Beymer C, Kowdley KV, Larson A, et al. Prevalence and predictors of asymptomatic liver disease in patients undergoing gastric bypass surgery. Arch Surg 2003;138:1240-4. [Crossref] [PubMed]
- Luyckx FH, Desaive C, Thiry A, et al. Liver abnormalities in severely obese subjects: effect of drastic weight loss after gastroplasty. Int J Obes Relat Metab Disord 1998;22:222-6. [Crossref] [PubMed]
- Ahmed MH BC. Non-alcoholic steatohepatitis. Metabolic Syndrome 2005;279-303.
- Sanford NL, Walsh P, Matis C, et al. Is ultrasonography useful in the assessment of diffuse parenchymal liver disease? Gastroenterology 1985;89:186-91. [Crossref] [PubMed]
- Siegelman ES, Rosen MA. Imaging of hepatic steatosis. Semin Liver Dis 2001;21:71-80. [Crossref] [PubMed]
- Bohte AE, van Werven JR, Bipat S, et al. The diagnostic accuracy of US, CT, MRI and 1H-MRS for the evaluation of hepatic steatosis compared with liver biopsy: a meta-analysis. Eur Radiol 2011;21:87-97. [Crossref] [PubMed]
- Fris RJ. Preoperative low energy diet diminishes liver size. Obes Surg 2004;14:1165-70. [Crossref] [PubMed]
- Benjaminov O, Beglaibter N, Gindy L, et al. The effect of a low-carbohydrate diet on the nonalcoholic fatty liver in morbidly obese patients before bariatric surgery. Surg Endosc 2007;21:1423-7. [Crossref] [PubMed]
- Lewis MC, Phillips ML, Slavotinek JP, et al. Change in liver size and fat content after treatment with Optifast very low calorie diet. Obes Surg 2006;16:697-701. [Crossref] [PubMed]
- Colles SL, Dixon JB, Marks P, et al. Preoperative weight loss with a very-low-energy diet: quantitation of changes in liver and abdominal fat by serial imaging. Am J Clin Nutr 2006;84:304-11. [PubMed]
- Edholm D, Kullberg J, Haenni A, et al. Preoperative 4-week low-calorie diet reduces liver volume and intrahepatic fat, and facilitates laparoscopic gastric bypass in morbidly obese. Obes Surg 2011;21:345-50. [Crossref] [PubMed]
- Kris-Etherton PM, Harris WS, Appel LJ, et al. American Heart Association. omega-3 fatty acids and cardiovascular disease: new recommendations from the American Heart Association. Arterioscler Thromb Vasc Biol 2003;23:151-2. [Crossref] [PubMed]
- Schwartz ML, Drew RL, Chazin-Caldie M. Factors determining conversion from laparoscopic to open Roux-en-Y gastric bypass. Obes Surg 2004;14:1193-7. [Crossref] [PubMed]
- Hilbert G, Lillemark L, Balchen S, et al. Reduction of organochlorine contaminants from fish oil during refining. Chemosphere 1998;37:1241-52. [Crossref] [PubMed]
- Jacobs MN, Santillo D, Johnston PA, et al. Organochlorine residues in fish oil dietary supplements: comparison with industrial grade oils. Chemosphere 1998;37:1709-21. [Crossref] [PubMed]
- EFSA Panel on Dietetic Products NAA. Scientific Opinion related to the Tolerable Upper Intake Level of eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) and docosapentaenoic acid (DPA). EFSA Journal 2012;10:2815. [Crossref]
- Foster GD, Wadden TA, Feurer ID, et al. Controlled trial of the metabolic effects of a very-low-calorie diet: short- and long-term effects. Am J Clin Nutr 1990;51:167-72. [PubMed]
- DeFina LF, Marcoux LG, Devers SM, et al. Effects of omega-3 supplementation in combination with diet and exercise on weight loss and body composition. Am J Clin Nutr 2011;93:455-62. [Crossref] [PubMed]
- Parker HMJN, Burdon CA, Cohn JS, et al. omega-3 supplemen- tation and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol 2012;56:944-51. [Crossref] [PubMed]
- de Assis AM, Rech A, Longoni A, et al. Ω3-Polyunsaturated fatty acids prevent lipoperoxidation, modulate antioxidant enzymes, and reduce lipid content but do not alter glycogen metabolism in the livers of diabetic rats fed on a high fat thermolyzed diet. Mol Cell Biochem 2012;361:151-60. [Crossref] [PubMed]
- Shapiro H, Tehilla M, Attal-Singer J, et al. The therapeutic poten- tial of long-chain omega-3 fatty acids in nonalcoholic fatty liver disease. Clin Nutr 2011;30:6-19. [Crossref] [PubMed]
- Tanaka N, Sano K, Horiuchi A, et al. Highly purified eicosapentaenoic acid treatment improves nonalcoholic steatohepatitis. J Clin Gastroenterol 2008;42:413-8. [Crossref] [PubMed]
- Daniel S, Ben-Menachem T, Vasudevan G, et al. Prospective evaluation of unexplained chronic liver transaminase abnormalities in asymptomatic and symptomatic patients. Am J Gastroenterol 1999;94:3010-4. [Crossref] [PubMed]
- Wang RT, Koretz RL, Yee HF. Is weight reduction an effective therapy for nonalcoholic fatty liver? A systematic review. Am J Med 2003;115:554-9. [Crossref] [PubMed]
- Mofrad P, Contos MJ, Haque M, et al. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology 2003;37:1286-92. [Crossref] [PubMed]
Cite this article as: Zainal Abidin ZA, Kosai NR, Mohd Taher M, Ghoneim I, Shuhaili MA, Yaacob NY, Rajan R. A randomised controlled trial comparing the use of omega-3 polyunsaturated fatty acid supplements versus very low calorie dietary restriction in obese Malaysian patients awaiting bariatric surgery. Ann Laparosc Endosc Surg 2017;2:112.


