Videoscopic inguinal lymphadenectomy: a novel approach for melanoma groin metastases
It is with great pleasure that we accept the invitation to comment on the manuscript entitled “Safety and Feasibility of Minimally Invasive Inguinal Lymph Node Dissection in Patients With Melanoma (SAFE-MILND): Report of a Prospective Multi-institutional Trial” by Jakub et al. (1). This study is a multi-institutional prospective trial of 87 patients with melanoma groin metastases undergoing video-assisted inguinal lymphadenectomy (VIL). After a structured training program, a group of melanoma surgeons from US high volume centers were enabled to perform the minimally invasive approach to the groin with a very favorable safety and morbidity profile. The number of excised lymph nodes met the benchmarks of the main oncological guidelines and evidence based studies. The study confirms that melanoma surgeons after a specific training program can safely and adequately perform VIL.
Lymphadenectomy is recommended for patients with melanoma who have a positive sentinel lymph node biopsy or clinically positive nodal metastasis. All surgeons dealing with groin metastases know that inguinal lymphadenectomy is burdened by significant morbidity, which includes wound infection and dehiscence, lymphatic collection and limb lymphoedema (2-4). These complications are feared by surgeons and patients, and this has led to a substantial reduction in the indication for and extent of surgery.
We should bear in mind that a complication in itself is often a risk factor for another complication. Wound ischemia and seroma collection under the incision can contribute to infection, which is always related to multiple factors. The incision of the skin in the groin plays a significant role in determining wound complication (5). Surgical incision should permit full access to the groin, allowing an effective clearance of groin lymph nodes and a safe control of bleeding and lymphatic leak. One critical point is the blood supply of the groin area, which is maintained by three main collaterals: the epigastric, the circumflex iliac and the external pudendal arteries. These vessels lie in the Camper fascia, run parallel to the skin creases and the inguinal ligament and are generally transected by the classic vertical incision of open lymphadenectomy, creating cutaneous areas at risk of ischemia that predispose infection and dehiscence. Therefore, a careful skin flap preparation, including at least 2–3 mm of subcutaneous fat, plays a pivotal role in preventing wound edge ischemia (6). Although no significant advantage with respect to the vertical incision is demonstrated, a double incision technique may also be considered in some cases where wound healing is considered at risk in presence of multiple risk factors (7).
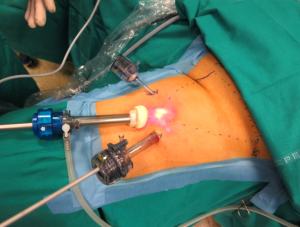
One of the most promising approaches to reduce wound related morbidity after groin dissection would simply be to avoid skin incision and the video-assisted groin dissection technique is the most valuable approach towards this goal (Figure 1). VIL is performed via three ports: one at the apex of the femoral triangle, a second medial to the adductors, and the third lateral to the Sartorius muscle. No inguinal incision is utilized. A standard inguinal lymphadenectomy is carried out, removing the contents of the femoral triangle and 5 cm up onto the external oblique aponeurosis with or without preservation of the saphenous vein.
Although the level of evidence is based mainly on retrospective studies with different histologies (melanoma and tumours from the anogenital area), the complications profile after VIL seems very encouraging and the video-assisted approach to the groin has gained interest among surgical oncologists (8). For melanoma, the technical feasibility, complications and midterm oncological outcome have been verified for both inguinal and inguinoiliac video-assisted lymphadenectomy (Table 1).
Table 1
| Study | Video-assisted lymphadenectomy | Procedures | Conversion rate (%) | Wound complication rate (%) | Lymph node excised | Local recurrence rate (%) |
|---|---|---|---|---|---|---|
| Abbott et al. (9) | Inguinal | 13 | 7.7 | 1.8 | 13 | Not reported |
| Martin et al. (10) | Inguinal | 40 | 10.0 | 15 | 13 | 2.5 |
| Sommariva et al. (11) | Inguinal and iliac | 24 | 16.5 | 4 | 21 | 8.3 |
| Jakub et al. (1) | Inguinal | 87 | 11.5 | 23 | 11 | Not reported |
The study by Jakub et al. makes a very important contribution to this field. As for other innovative surgical approaches, safety issues are very important; thus, only after an adequate training period is a surgeon able to verify the feasibility of the technique himself. The complications profile seems acceptable, with 26% suffering from grade III adverse event, and no reported grade IV–V events. Differently from gynecologists and urologists, not all melanoma surgeons are confident with video-assisted techniques. Although, in this study, all 12 participating surgeons had no previous experience on minimally invasive lymphadenectomy, no data are provided on their experience and skills in other video-assisted surgical procedures. This may have affected the results, as the learning curve of surgeons skilled in laparoscopy or thoracoscopy can be very different from those without this experience.
The most valuable result of the study is that adequately trained surgeons can remove the same number of lymph nodes with respect to the number reported after open dissection performed in high volume centers. Mean, median and range values (11.6, 12 and 3–23, respectively) are absolutely in line with those reported after open surgery in large single institution or multi-institutional series (12,13). This aspect reflects the quality of dissection, even if the oncological results after VIL should be better defined within prospective series with more patients and longer follow-up. Evaluating melanoma specific survival and lymphatic basin recurrence in patients who underwent video-assisted dissection should therefore be further investigated and compared with the results of open lymphadenectomy.
One of the most unpleasant complications of groin lymphadenectomy is lower limb lymphoedema, which occurs in about one-third of patients (14). In the present study, only 3% of patients experienced serious grade III lymphedema. VIL can lower the incidence and gravity of lymphoedema as subcutaneous lymphatic flow can be preserved by avoiding extensive skin incision. Moreover, wound infection represents a risk factor for lymphoedema; thus, the lower frequency of this complication after VIL may translate into less lymphoedema (15).
The best way to compare video-assisted with open lymphadenectomy should be a randomized controlled trial, but this attempt has already failed in the US (Keith Delman, personal communication) due to the reluctance of patients to be included in a trial where one arm of the study (open surgery) is characterized by a very high morbidity profile. In our opinion, the most practical way to obtain more data on video assisted groin dissection is to prospectively collect, according to a shared protocol, data from international institutions performing VIL for melanoma, where indication for surgery, intra-operative parameters (e.g., length of surgery), outcomes (e.g., length of hospitalization, drain duration, post-operative complications, leg lymphedema) and oncological outcome (e.g., number of lymph nodes, recurrence) could been addressed and clarified. Our institution (Veneto Institute of Oncology of Padova, Italy) is planning a multi-institutional prospective data collection project in collaboration with the Mayo Clinic of Rochester and the Winship Cancer Center of Atlanta. This international registry on VIL should give more evidence in favour of this innovative technique and allow us to better define its potential benefit for patient outcomes.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Laparoscopic and Endoscopic Surgery. The article did not undergo external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2017.06.10). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jakub JW, Terando AM, Sarnaik A, et al. Safety and Feasibility of Minimally Invasive Inguinal Lymph Node Dissection in Patients With Melanoma (SAFE-MILND): Report of a Prospective Multi-institutional Trial. Ann Surg 2017;265:192-6. [Crossref] [PubMed]
- Chang SB, Askew RL, Xing Y, et al. Prospective assessment of postoperative complications and associated costs following inguinal lymph node dissection (ILND) in melanoma patients. Ann Surg Oncol 2010;17:2764-72. [Crossref] [PubMed]
- Stuiver MM, Westerduin E, ter Meulen S, et al. Surgical wound complications after groin dissection in melanoma patients - a historical cohort study and risk factor analysis. Eur J Surg Oncol 2014;40:1284-90. [Crossref] [PubMed]
- Spillane AJ, Saw RP, Tucker M, et al. Defining lower limb lymphedema after inguinal or ilio-inguinal dissection in patients with melanoma using classification and regression tree analysis. Ann Surg 2008;248:286-93. [Crossref] [PubMed]
- Spratt J. Groin dissection. J Surg Oncol 2000;73:243-62. [Crossref] [PubMed]
- Kean J, Hough M, Stevenson JH. Skin excision and groin lymphadenectomy: techniques and outcomes. Lymphology 2006;39:141-6. [PubMed]
- Spillane AJ, Tucker M, Pasquali S. A pilot study reporting outcomes for melanoma patients of a minimal access ilio-inguinal dissection technique based on two incisions. Ann Surg Oncol 2011;18:970-6. [Crossref] [PubMed]
- Sommariva A, Pasquali S, Rossi CR. Video endoscopic inguinal lymphadenectomy for lymph node metastasis from solid tumors. Eur J Surg Oncol 2015;41:274-81. [Crossref] [PubMed]
- Abbott AM, Grotz TE, Rueth NM, et al. Minimally invasive inguinal lymph node dissection (MILND) for melanoma: experience from two academic centers. Ann Surg Oncol 2013;20:340-5. [Crossref] [PubMed]
- Martin BM, Etra JW, Russell MC, et al. Oncologic outcomes of patients undergoing videoscopic inguinal lymphadenectomy for metastatic melanoma. J Am Coll Surg 2014;218:620-6. [Crossref] [PubMed]
- Sommariva A, Pasquali S, Cona C, et al. Videoscopic ilioinguinal lymphadenectomy for groin lymph node metastases from melanoma. Br J Surg 2016;103:1026-32. [Crossref] [PubMed]
- Spillane AJ, Haydu L, McMillan W, et al. Quality assurance parameters and predictors of outcome for ilioinguinal and inguinal dissection in a contemporary melanoma patient population. Ann Surg Oncol 2011;18:2521-8. [Crossref] [PubMed]
- Rossi CR, Mozzillo N, Maurichi A, et al. Number of excised lymph nodes as a quality assurance measure for lymphadenectomy in melanoma. JAMA Surg 2014;149:700-6. [Crossref] [PubMed]
- Shaitelman SF, Cromwell KD, Rasmussen JC, et al. Recent progress in the treatment and prevention of cancer-related lymphedema. CA Cancer J Clin 2015;65:55-81. [Crossref] [PubMed]
- Abbas S, Seitz M. Systematic review and meta-analysis of the used surgical techniques to reduce leg lymphedema following radical inguinal nodes dissection. Surg Oncol 2011;20:88-96. [Crossref] [PubMed]
Cite this article as: Sommariva A, Rossi CR. Videoscopic inguinal lymphadenectomy: a novel approach for melanoma groin metastases. Ann Laparosc Endosc Surg 2017;2:108.