Laparoscopic left lateral sectionectomy for living liver donation: the Ghent University experience
Introduction
Minimally invasive liver surgery has been widely adopted for the treatment of different liver diseases. Compared to open liver surgery, this has the advantages of reducing complications, postoperative pain, and recovery (1-3). Further developments have demonstrated its technical feasibility for living donor hepatectomy (4-7). The first laparoscopic living donor liver transplantation (LDLT) was described in 2002, and since then, this has taken some time for acceptance because of technical difficulties and for the skills required to perform it (5). Later, specialized centers have performed minimally invasive donor hepatectomy with either the hybrid or pure technique (6-10).
Different types of graft harvesting, including left lateral sectionectomy, left and right lobes have been reported (11-15). While the left lateral sectionectomy (including hyper reduced segments for infants), is the graft of choice for pediatric LDLT, larger grafts as the full left or full right lobes are considered for adult recipients.
Comparative studies of conventional and minimally invasive techniques for living donor hepatectomy have been reported (16-18). However, because of the small number of reports available and because of the small series, it is still not yet clear what is more beneficial to the donor. According to the 2nd International Consensus Conference on Laparoscopic Liver Surgery, such procedures are classified as Balliol 2b, meaning that institutional oversight is needed and a registry to determine short and long term outcomes in both the donor and the recipient should be provided (19).
Left lateral sectionectomy: surgical technique
The donor work up is standardly performed to assess the anatomy. The modal arterial anatomy (a4 from the left hepatic artery), the replaced left from the gastric artery or the a4 from the right hepatic artery are all considered for graft procurement. Biliary anatomical variations for the left liver are very uncommon: double or multiple biliary ducts could eventually be found if the dissection line is behind the umbilical ligament (trans-umbilical approach-T-U technique). A double separate vein draining segment II into the middle hepatic vein (HV) could be considered as a contraindication for living donor hepatectomy, however, making a separate drainage into one common patch is not a major issue. The CT volumetry of segments II–III including the arterial reconstruction and the cholangio-MRCP are routinely performed.
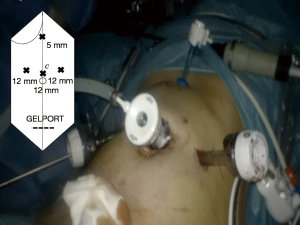
The donor is placed in a supine position. Usually, four trocars are placed on the upper abdominal quadrants, and an 8–10 cm suprapubic incision is performed in order to place a Gelport device (Applied Medical, USA) (Figure 1).
Middle and left HV confluence is identified by intraoperative ultrasonography, dissection of the hilum to expose the left hepatic artery and the left portal vein is performed with scissors and bipolar forceps. The left triangular ligament is divided with a high-energy instrument (Thunderbeat, Olympus), the Arantius ligament is dissected and cut with the exposure of the groove of the middle and left HV. An umbilical tape can be placed between the left and the middle HV. Further, the hilum is gently dissected skeletonizing the left artery eventually preserving the branch for segment IV. In case of small arterial size and a modal arterial anatomy (a4 originating from the left artery) the decision to include a4 into the graft should be considered.
Parenchymal dissection is performed with the ultrasonic dissector and without Pringle maneuver (20). The transection line could be at the level of the round ligament (T-U approach) or 1-cm on the medial side of this (trans-hilar approach). The difference is that in the first case it is possible to preserve, in most of the cases, the a4 and the segmental biliary duct (b4). However, the risk to have multiple ducts for biliary anastomosis is higher (21).
Non-resorbable clips (Hem-o-Lock, TFX Medical Ltd., Durham, USA) are placed on intra-hepatic vessels. Alternatively, sealing of small vessels (up to 5–7 mm) could also be done by means of high-energy instruments.
The site of transection of the left hepatic duct is close to the rex recessus and does not require a real-time cholangiography because of the distance from the biliary confluence so that, even in case of a b6–7 duct (right posterolateral) draining into the left duct, there is no risk of biliary injury. The left hepatic bile duct is secured with a couple of titanium clips or with sutures. Afterwards, we divide and cut the portal vein branches to the caudate lobe from the left portal branch, and the bile duct tributaries sealing them with high-energy instruments. Usually we avoid clips in this area to facilitate the position of the stapler on the left portal branch. Preservation of the portal branches to the caudate lobe is possible however a shorter left portal vein branch should be anticipated.
After administration of systemic heparin (5,000 units), the left hepatic artery is clipped on the remnant side and divided. Then, a stapler division of the left portal vein (Endo TA 30 mm, Covidien, Mansfield, USA) and the left HV after exposing the confluence of middle HV (Endo GIA 60 mm curved, Covidien) is performed. The manual graft extraction through the suprapubic incision is usually done by putting the graft into a plastic bag previously introduced through the Gelport system.
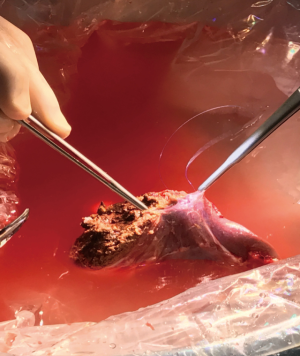
The graft is flushed on the back table with 1–2 L of HTK solution (UW or IGL-1 are also currently used) (Figure 2). No heparin antagonist is given to the donor to avoid the risk of possible pro-thrombotic effects (Figure 3).

Results
From January 2009 to March 2017, 11 pediatric LDLT with a pure laparoscopic approach for donor hepatectomy have been performed in our institution. Neither conversions nor surgical revisions have been recorded so far. Donor characteristics are depicted in Table 1. The transection line followed the T-U approach. The first warm ischemia was around 4 minutes and the total cold ischemia less than 3 hrs. An ERAS protocol haw been implemented even in case of laparoscopic donor hepatectomy. Dietary intake has been allowed from the first post-operative day. Complications have been recorded in 2 (16.6%) donors: 1 necrosis of segment IV needing antibiotic therapy and one fluid collection on the section edge treated conservatively. Analgesics drugs have been administered through a central line during the first 48 hrs.
Table 1
| Characteristics | Mean age (y ± SD) | Parental | Graft volume (mL3 ± SD) | Peak AST (U/L ± SD) | Peak ALT (U/L ± SD) | OP time (min. ± SD) | Blood losses (ML ± SD) | Real GW (g ± SD) |
|---|---|---|---|---|---|---|---|---|
| Outcome | 29.2±6.9 | 6 father, 2 uncles, 2 aunts, 1 mother | 208.9±67.5 | 318±182 | 273.4±156.4 | 237±99 | 70±41 | 210.8±52.3 |
SD, standard deviation; AST, aspartate aminotransferase; ALT, alanine aminotransferase; OP, operative time; GW, graft weight.
The median length of hospital stay was 4 days. Major indications in children were: Biliary atresia (n=8), primary oxaluria, cholestatic syndrome and multifocal HCC on a cirrhotic liver from unknown origin (one case each).
One child died because of a fungal sepsis following retransplantation due to graft dysfunction. Most likely the subjacent infection has been the reason of the dysfunction of both grafts leading to patient’s death. Biliary complications requiring percutaneous dilations and/or revision of the anastomosis have been recorded in 4 (36%).
Discussion
According to our experience, laparoscopic donor hepatectomy for pediatric LDLT is safe and feasible allowing few complications and an earlier return to daily activity. To date there are only two comparative single center studies showing the feasibility and safety of laparoscopic left lateral sectionectomy for pediatric LDLT (23,24). Recently, a comparative study between laparoscopic living liver and kidney donor surgeries showed, interestingly, a significant lower number of minor complications in liver donors compared to the others; major complications were, however, identical. A comparable CCI was observed between liver and kidney donors with complicated postoperative outcome (25). This study is the first validation of laparoscopic donor hepatectomy, and suggests that the laparoscopic approach along the open could become a standard of care in the hands of experts, as for donor nephrectomy.
Donor morbidity is intensely evaluated in the Western countries where living donor is considered not as first-choice. This is why the split grafts are proposed to children with end-stage liver diseases, although this can vary according to center’s policy.
Laparoscopic living donor hepatectomy must be considered as the ultimate evolution of the minimally invasive approach to the liver. The concept of applying the laparoscopic technique to a living donor is attractive because it can further reduce donor complications ensuring the best grafts to diseased children in a timely fashion. Unfortunately, two main disadvantages should be considered: learning curve of laparoscopy and the specific experience of partial liver transplants from living donors. The learning curve is mainly depending on the background in advanced laparoscopic surgery that facilitates laparoscopic hepatobiliary procedures (provided one has already gained experience in open hepatobiliary surgery and liver transplantation). To date, more than 600 pure laparoscopic hepatectomy have been done at the Ghent University Hospital including major resections and resections in the posterosuperior segments. In our opinion there are two major critical points in the laparoscopic procurement of segment 2–3 grafts: the small size of the left hepatic artery (if the a4 patch is not considered) including the risk of intima damage during laparoscopic dissection of the hilum and the possibility to have two or more biliary ducts for the anastomosis with the consequent higher risk of late stenosis in the recipients (especially in case of the T-U approach).
In conclusion, our experience proves the feasibility of laparoscopic left lateral sectionectomy for pediatric LDLT. Although seems that laparoscopic LLS could be considered as standard practice in highly specialized centers, the potential of this technique in lowering donor morbidity rates and, especially, its reproducibility should be further validated.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Giovanni Battista Levi Sandri) for the series “Laparoscopic Liver Surgery” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2017.04.09). The series “Laparoscopic Liver Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ciria R, Cherqui D, Geller DA, et al. Comparative Short-term Benefits of Laparoscopic Liver Resection: 9000 Cases and Climbing. Ann Surg 2016;263:761-77. [Crossref] [PubMed]
- Koffron AJ, Auffenberg G, Kung R, et al. Evaluation of 300 minimally invasive liver resections at a single institution: less is more. Ann Surg 2007;246:385-92; discussion 92-4. [Crossref] [PubMed]
- Montalti R, Berardi G, Laurent S, et al. Laparoscopic liver resection compared to open approach in patients with colorectal liver metastases improves further resectability: Oncological outcomes of a case-control matched-pairs analysis. Eur J Surg Oncol 2014;40:536-44. [Crossref] [PubMed]
- Berardi G, Tomassini F, Troisi RI. Comparison between minimally invasive and open living donor hepatectomy: A systematic review and meta-analysis. Liver Transpl 2015;21:738-52. [Crossref] [PubMed]
- Cherqui D, Soubrane O, Husson E, et al. Laparoscopic living donor hepatectomy for liver transplantation in children. Lancet 2002;359:392-6. [Crossref] [PubMed]
- Koffron AJ, Kung R, Baker T, et al. Laparoscopic-assisted right lobe donor hepatectomy. Am J Transplant 2006;6:2522-5. [Crossref] [PubMed]
- Kurosaki I, Yamamoto S, Kitami C, et al. Video-assisted living donor hemihepatectomy through a 12-cm incision for adult-to-adult liver transplantation. Surgery 2006;139:695-703. [Crossref] [PubMed]
- Choi HJ, You YK, Na GH, et al. Single-port laparoscopy-assisted donor right hepatectomy in living donor liver transplantation: sensible approach or unnecessary hindrance? Transplant Proc 2012;44:347-52. [Crossref] [PubMed]
- Scatton O, Katsanos G, Boillot O, et al. Pure laparoscopic left lateral sectionectomy in living donors: from innovation to development in France. Ann Surg 2015;261:506-12. [Crossref] [PubMed]
- Suh KS, Yi NJ, Kim T, et al. Laparoscopy-assisted donor right hepatectomy using a hand port system preserving the middle hepatic vein branches. World J Surg 2009;33:526-33. [Crossref] [PubMed]
- Rotellar F, Pardo F, Benito A, et al. Totally Laparoscopic Right Hepatectomy for Living Donor Liver Transplantation: Analysis of a Preliminary Experience on 5 Consecutive Cases. Transplantation 2017;101:548-54. [Crossref] [PubMed]
- Samstein B, Griesemer A, Cherqui D, et al. Fully laparoscopic left-sided donor hepatectomy is safe and associated with shorter hospital stay and earlier return to work: A comparative study. Liver Transpl 2015;21:768-73. [Crossref] [PubMed]
- Soubrane O, Perdigao Cotta F, Scatton O. Pure laparoscopic right hepatectomy in a living donor. Am J Transplant 2013;13:2467-71. [Crossref] [PubMed]
- Troisi RI, Wojcicki M, Tomassini F, et al. Pure laparoscopic full-left living donor hepatectomy for calculated small-for-size LDLT in adults: proof of concept. Am J Transplant 2013;13:2472-8. [Crossref] [PubMed]
- Yu YD, Kim KH, Jung DH, et al. Laparoscopic live donor left lateral sectionectomy is safe and feasible for pediatric living donor liver transplantation. Hepatogastroenterology 2012;59:2445-9. [PubMed]
- Han HS, Cho JY, Yoon YS, et al. Total laparoscopic living donor right hepatectomy. Surg Endosc 2015;29:184. [Crossref] [PubMed]
- Kasahara M, Sakamoto S, Shigeta T, et al. A 7-cm upper midline incision for living donor left lateral hepatectomy: singe-center consecutive 70 donor experience. Transplantation 2012;93:e33-4. [Crossref] [PubMed]
- Takahara T, Wakabayashi G, Nitta H, et al. The first comparative study of the perioperative outcomes between pure laparoscopic donor hepatectomy and laparoscopy-assisted donor hepatectomy in a single institution. Transplantation 2017; [Epub ahead of print]. [Crossref] [PubMed]
- Wakabayashi G, Cherqui D, Geller DA, et al. Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg 2015;261:619-29. [PubMed]
- Troisi RI, Van Huysse J, Berrevoet F, et al. Evolution of laparoscopic left lateral sectionectomy without the Pringle maneuver: through resection of benign and malignant tumors to living liver donation. Surg Endosc 2011;25:79-87. [Crossref] [PubMed]
- de Ville de Goyet J, di Francesco F, Sottani V, et al. Splitting livers: Trans-hilar or trans-umbilical division? Technical aspects and comparative outcomes. Pediatr Transplant 2015;19:517-26. [Crossref] [PubMed]
- Troisi RI, Berardi G. Left lateral LD Hamburg technique. Asvide 2017;4:243. Available online: http://www.asvide.com/articles/1552
- Kim KH, Jung DH, Park KM, et al. Comparison of open and laparoscopic live donor left lateral sectionectomy. Br J Surg 2011;98:1302-8. [Crossref] [PubMed]
- Soubrane O, Cherqui D, Scatton O, et al. Laparoscopic left lateral sectionectomy in living donors: safety and reproducibility of the technique in a single center. Ann Surg 2006;244:815-20. [Crossref] [PubMed]
- Soubrane O, de Rougemont O, Kim KH, et al. Laparoscopic Living Donor Left Lateral Sectionectomy: A New Standard Practice for Donor Hepatectomy. Ann Surg 2015;262:757-61; discussion 61-3. [Crossref] [PubMed]
Cite this article as: Troisi RI, Berardi G. Laparoscopic left lateral sectionectomy for living liver donation: the Ghent University experience. Ann Laparosc Endosc Surg 2017;2:100.