Endoscopic resection of gastric submucosal tumor
Introduction
Submucosal tumors (SMT) are occasionally found in the esophagus and stomach during diagnostic endoscopy with reported incidence of 0.36% (1). There was no consensus on the management of incidental SMT as the histopathologic nature of such tumor is often uncertain. Attempt in obtaining adequate samples for tissue diagnosis is associated with risk of hemorrhage and perforation. The reported diagnostic yield was inconsistent and often disappointing (2). With the advancement of endoscopic instruments, numerous studies have reported the feasibility of endoscopic treatment of gastric SMT (2-5). We herein report endoscopic resection of a 3 cm SMT located in gastric antrum.
Case presentation
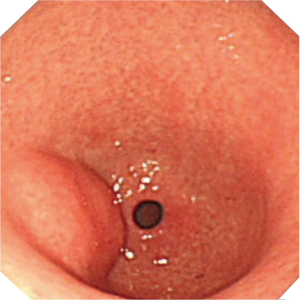
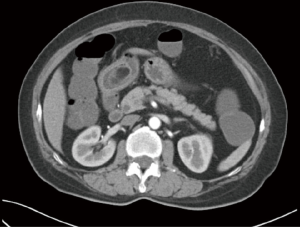
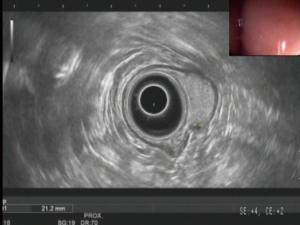
A 68-year-old woman presented with upper abdominal pain for 3 months. There was no constitutional symptom. Oesophagogastroduodenoscopy (OGD) was performed and a 3 cm bulge was noted at the antero-superior part of the antrum (Figure 1). The overlying mucosa was intact. There was no other mucosal lesion seen in the esophagus, stomach and proximal duodenum. Endoscopic ultrasound showed a hyperechoeic lesion located mostly at deep mucosal layer (Figure 2). CT scan showed a hypoenhancing lesion in antrum (Figure 3). The preoperative diagnosis was gastric wall lipoma. The patient was interviewed and different treatment options including conservative treatment, tumor biopsy and resection were discussed. She was well informed of the differential diagnosis, potential need of additional surgery in case of malignant tumor or incomplete resection. The patient opted for upfront resection of without preoperative biopsy of the tumor, as she worried about the suboptimal diagnostic yield of endoscopic biopsy and preferred tumor removal for definitive diagnosis. Endoscopic resection was considered suitable in view of the endoscopic ultrasound findings.
The procedure was performed under general anesthesia with endotracheal intubation. The patient was placed in left lateral position. An upper endoscope (GIF-Q260J; Olympus, Japan) with a water jet function was used and a transparent hood (D-201-11304; Olympus, Japan) was attached at its distal end. The electrosurgical unit used was ERBE (VIO 200D; Germany). The endoscopic instruments used were Dual knife (KD-650L; Olympus, Japan), injector (NM-200L-0423; Olympus, Japan) and snare (SD-210U-25; Olympus, Japan). Carbon dioxide insufflation was used during the whole procedure. The procedure started with circumferential marking with Dual knife at the margin of the target lesion. A mixture of 250 mL mannitol with adrenaline 1mg and methylene blue was prepared. A submucosal cushion was then created by injection of the above solution to the submucosal plane. It was followed by mucosal incision along the marking dots. A whitish tumor was exposed after completion of circumferential mucosal incision. Submucosal dissection was carried out and additional submucosal injections were used to facilitate separation of the tumor from muscularis propria (MP). Dissection was continued with the Dual knife at a direction parallel to the MP layer. The tumor was resected en bloc. Careful hemostasis was secured. The procedure was completed and the tumor was removed with a snare. Nasogastric tube was not inserted at the end of the procedure (Figure 4).

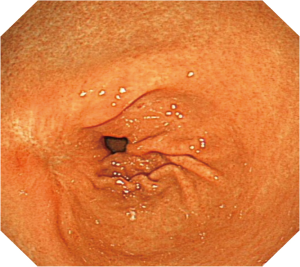
The endoscopic resection was completed in 90 minutes with minimal blood loss. The patient was managed according to a standardized postoperative protocol including intravenous proton pump inhibitor and metoclopramide for 2 days. A liquid diet was allowed on the first postoperative day and soft diet on the third day after surgery. There was no complication. The patient was discharged on the fourth day after surgery with oral proton pump inhibitor for 6 weeks. The pathology of the resected specimen confirmed the diagnosis of lipoma with clear resection margins. The wound healed well with a scar seen on reassessment OGD performed 6 months after the procedure (Figure 5).
Discussion
Gastric SMTs comprise tumors locating in the submucosa, muscularis mucosa or MP layers and are thus sometimes referred as subepithelial tumors (SETs). Incidental finding of SMT is increasingly encountered at diagnostic upper endoscopy. To formulate a management plan is difficult as the histopathological nature of the tumor is often uncertain. Tumors with malignant potential, include gastrointestinal stromal tumor (GIST), glomus tumors and carcinoids, are not uncommonly encountered. A tissue diagnosis is preferred but not always successful without resection of the tumor. Various methods of tissue sampling have been evaluated for SMTs, including the bite-on-bite biopsy, EUS-guided FNA and EUS-guided trucut biopsy. The data on diagnostic yield were inconsistent and many studied had reported disappointing results (2). Moreover, attempt in obtaining adequate samples for tissue diagnosis is associated with risk of hemorrhage and perforation. Even for small and clinically benign lesion, regular interval surveillance is commonly performed. This imposes emotional burden on patients and potentially delays diagnosis in case of malignancy. A safe and effective method for endoscopic removal of these tumors is desired.
With the rapid development of endoscopic imaging technique and endoscopic instruments, resection of submucosal lesions is feasible and increasingly performed worldwide. It allows a definitive histopathological diagnosis and is less invasive when comparing with surgical resection. Nonetheless, it is a time-consuming procedure and is associated with risk of perforation and bleeding. Endoscopic enucleation of gastric GIST is usually performed without excision of surrounding normal tissue. Therefore, complete resection of this potentially malignant lesion is not guaranteed (4). The reported complete resection rate ranged from 68–94% (2,7,8). Further surgical resection may be required.
Routine workup of gastric SMT includes OGD, endoscopic ultrasound and CT scan. Treatment options include conservative treatment with regular surveillance, endoscopic resection or surgical resection. The decision was made mainly based on the size and origin of tumor from which gastric layer. It was reported that perforation often occurs if ESD is performed for SMT arising from the MP layer. In a study of ESD for SETs originating in the MP layer, intraluminal SETs up to 5cm in size and without ulceration were included. ESD was performed for 145 gastric SETs in 144 patients. Perforation and intraoperative bleeding occurred in 21 patients (14.48%) and 7 patients (4.83%) respectively. These complications were successfully managed endoscopically (5). On the other hand, Chun et al. advocated that ESD should only be performed for those gastric SMT, originate from MP, with size less than 20 mm and a positive rolling sign (8).
For larger SMT with relatively extensive involvement of MP layer, the treatment options are endoscopic full-thickness resection or combined endoscopic-laparoscopic resection (9,10) depending on the availability of endoscopic and surgical specialists.
Endoscopic resection for SMTs is often a challenging procedure. Refined endoscopic technique is a basic requirement for performing ESD procedures. Ample experience in ESD for mucosal lesions is a prerequisite for performance of ESD for SMTs. Selection of endoscope is important. An endoscope with water jet function and a big working channel allows irrigation and adequate suction, which maintains a clear view throughout the procedure. Endoscopes with double bending sections provide flexibility and enable precise positioning during dissection of lesions at cardia or fundus. The selection of endoscopic knife depends on the preference of endoscopist and combination of knifes are sometimes required. Creation of a submucosal cushion by injection of sufficient volume of solution is essential for initial puncture and subsequent full incision of the mucosa. This greatly facilitates the exposure of SMT and makes further dissection easier. Further injection during procedure may facilitate separation of the SMT from MP layer. This allows dissection of tumor in a safe and precise manner. While the mucosal defect is not usually closed, careful hemostasis should be secured after resection of the tumor.
Conclusions
We report endoscopic resection of a gastric SMT located in antero-superior part of the gastric antrum. With careful dissection, the tumor was resected without perforation of gastric wall and a quick recovery was observed.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2017.05.08). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hedenbro JL, Ekelund M, Wetterberg P. Endoscopic diagnosis of submucosal gastric lesions. The results after routine endoscopy. Surg Endosc 1991;5:20-3. [Crossref] [PubMed]
- Bialek A, Wiechowska-Kozlowska A, Pertkiewicz J, et al. Endoscopic submucosal dissection for treatment of gastric subepithelial tumors (with video). Gastrointest Endosc 2012;75:276-86. [Crossref] [PubMed]
- Park YS, Park SW, Kim TI, et al. Endoscopic enucleation of upper-GI submucosal tumors by using an insulated-tip electrosurgical knife. Gastrointest Endosc 2004;59:409-15. [Crossref] [PubMed]
- Kim SY, Kim KO. Management of gastric subepithelial tumors: The role of endoscopy. World J Gastrointest Endosc 2016;8:418-24. [Crossref] [PubMed]
- He Z, Sun C, Wang J, et al. Efficacy and safety of endoscopic submucosal dissection in treating gastric subepithelial tumors originating in the muscularis propria layer: a single-center study of 144 cases. Scand J Gastroenterol 2013;48:1466-73. [Crossref] [PubMed]
- Chan FS, Liu ZH, Wu JW, et al. Video showing endoscopic resection of an antral submucosal tumor. Asvide 2017;4:241. Available online: http://www.asvide.com/articles/1550
- Lee IL, Lin PY, Tung SY, et al. Endoscopic submucosal dissection for the treatment of intraluminal gastric subepithelial tumors originating from the muscularis propria layer. Endoscopy 2006;38:1024-8. [Crossref] [PubMed]
- Chun SY, Kim KO, Park DS, et al. Endoscopic submucosal dissection as a treatment for gastric subepithelial tumors that originate from the muscularis propria layer: a preliminary analysis of appropriate indications. Surg Endosc 2013;27:3271-9. [Crossref] [PubMed]
- Inoue H, Ikeda H, Hosoya T, et al. Endoscopic mucosal resection, endoscopic submucosal dissection, and beyond: full-layer resection for gastric cancer with nonexposure technique (CLEAN-NET). Surg Oncol Clin N Am 2012;21:129-40. [Crossref] [PubMed]
- Mitsui T, Niimi K, Yamashita H, et al. Non-exposed endoscopic wall-inversion surgery as a novel partial gastrectomy technique. Gastric Cancer 2014;17:594-9. [Crossref] [PubMed]
Cite this article as: Chan FS, Liu ZH, Wu JW, Yang XF, Law S. Endoscopic resection of gastric submucosal tumor. Ann Laparosc Endosc Surg 2017;2:99.