Secrets for successful laparoscopic antireflux surgery: surgical technique
Introduction
Plication of the stomach fundus around the distal esophagus to prevent gastroesophageal reflux was first reported by Rudolf Nissen in 1956 (1). Such a fundoplication evolved over the ensuing years, initially performed by laparotomy. The laparoscopic performance of the Nissen fundoplication was first described by Dallemagne in 1991 (2). This minimally invasive approach offered improved exposure to the esophageal hiatus, resulting in significantly less pain and morbidity than its open counterpart. Consequently, there has been an increased application of the procedure over the past several decades for the treatment of gastroesophageal reflux disease (GERD) (3).
At the most fundamental level, a “Nissen fundoplication” is defined as complete circumferential wrapping of the stomach fundus fixed around the posterior aspect of the distal esophagus. There have been several iterations of the operation over the years, but most surgeons now perform a “short floppy” fundoplication, as popularized by DeMeester, meaning a complete mobilization of the fundus with a wrap that extends no more than 2.5 cm along the esophagus (4). The practicing surgeon should recognize that although critical technical principles that contribute to a successful fundoplication are conserved among fundoplication experts, precise procedural details tend to vary per surgeon preference. Essential technical principles that should be carefully followed to ensure good long-term functional outcomes are listed in Table 1. The surgical details and technique related to these principles, as well as tips, tricks, and pitfalls, will be expounded upon in the following text. An instructional video of a laparoscopic Nissen fundoplication, as performed, narrated, and edited by the authors, accompanies this text and can be used as a visual reference (Figure 1).
Table 1
| The esophagus must be protected from injury during dissection |
| Mediastinal esophageal mobilization must allow at least 3 cm of esophagus caudad to the hiatus |
| The anterior and posterior vagal trunks must be protected from injury |
| The fundus (rather than any other part of the stomach) must be plicated around the esophagus rather than around the proximal stomach |
| There must be no tension on the fundoplication, either axial or rotational |
| Approximation of the right and left crura should lie such that they touch the walls of the empty esophagus without tension |

Pre-operative preparation
Patient positioning is crucial to prevent neuromuscular injury in the patient and protect the surgeon from fatigue and sub-optimal ergonomics. The patient’s torso is cradled in a vacuum bean bag mattress and his or her legs are abducted and secured in a padded split-leg position. This minimizes potential traction injury by extending the knees, as the patient will be in steep reverse Trendelenburg throughout the operation. The sharp incline of the patient allows passive inferior retraction of the abdominal viscera by gravity, while also bringing the operative surface of the patient up to the surgeon’s level, allowing him or her to stand straight up with arms in the neutral position. The patient’s right arm is padded and tucked to facilitate the laparoscopic liver retractor on that side of the operating table. Slightly angled video monitors are positioned flanking the patient’s shoulders at eye level.
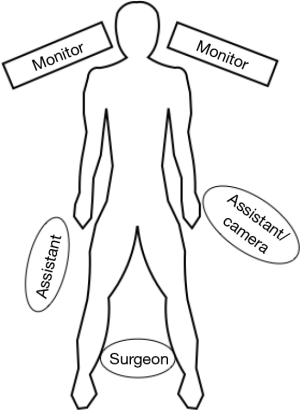
The surgical team is set up as in Figure 2. The surgeon stands between the patient’s legs facing directly forward. The first assistant stands next to the patient’s right leg. If a separate camera driver is available, he or she can sit adjacent to the patient’s left leg, to minimize fatigue and discomfort. If not, the first assistant must stand to the left of the patient, manipulating a grasper placed through a port in the left subcostal region while holding the camera with the left hand.
Equipment
Specific instrumentation facilitates success of the laparoscopic Nissen fundoplication, as listed on our equipment preference card in Table 2. A 30° (our preference) or 45° angled laparoscope offers dynamic visualization of the shifting views requisite for dissection, such as peering around the esophagus to take posterior attachments high in the mediastinum or visualization of the retrogastric space during fundic mobilization. A self-retaining atraumatic liver retractor is quite necessary to give consistent gentle retraction of the left lateral segment and exposure of the hiatus; types of liver retractors varies per surgeon preference. We use several different atraumatic laparoscopic instruments for retraction and dissection, typically with Babcock-type and DeBakey-type tips. A laparoscopic energy device is used for a significant portion of the dissection, as it allows a seamless transition between blunt sweeping of tissues and coagulation. Again, this is variable among surgeons; we prefer ultrasonic coagulating shears. A flexible gastroscope should always be present in the operating room for easy endoscopic evaluation of the manipulated anatomy.
Table 2
| Veress needle |
| Angled 30° laparoscope 5 or 10 mm |
| Atraumatic self-retaining liver retractor |
| Atraumatic grasping and dissecting instruments (Babcock-type, DeBakey-type tips) |
| Hemostasis instruments: harmonic shears or bipolar coagulating device |
| Flexible gastroscope |
Procedure
Abdominal access and port placement
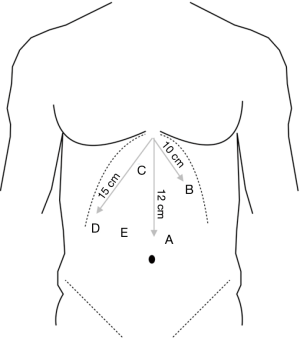
The abdominal cavity is entered with an open technique or with the Veress needle (our preference). Placement of ports is contingent on the number of operative assistants, but typically follows that displayed and described in Figure 3. This configuration splits the laparoscope between the surgeon’s hands, allowing a direct approach and permitting accurate perception of three dimensional relationships. Of note, we generally secure the liver retractor before placing the surgeon’s left-handed instruments, as the location of this port varies slightly based on the retracted position of the liver. Also, if only one assistant is present, his or her working port is placed left subcostal, 15 cm from the xiphoid out laterally from the camera. This allows the assistant to work the camera in the left hand while assisting the surgeon with the right hand.
Hiatal dissection
The operation begins with the assistant retracting the stomach inferiorly and to the left. This places the gastrohepatic ligament on tension, which is then entered and divided with an energy source up to the right crus of the diaphragm. The hepatic branch of the vagus nerve is often sacrificed with this maneuver; care is taken to avoid a major replaced left hepatic artery. The assistant then retracts the epiphrenic fat pad caudad, allowing transverse division of the phrenoesophageal membrane, being mindful of the underlying esophagus and anterior vagal trunk.
We start the esophageal dissection by developing the plane between the esophagus and right crus. This is best identified by grasping a substantial bite of the lateral right crus with the surgeon’s left hand and retracting laterally. A blunt-tipped instrument can then be insinuated at the medial aspect of the crus and opened in a vertical direction; alternatively, ultrasonic shears may be used to open the overlying peritoneum at the same location. This space can then be opened with gentle blunt sweeps of the esophagus and periesophageal tissue to the left. The posterior vagus nerve should be identified at this point and used to mark the posterolateral dissection plane. Blunt mobilization is then carried along the right side up around the anterior aspect of the distal esophagus, and then up cephalad as far as possible.
As dissection is taken further up into the mediastinum, caution is taken to stay in the plane outside of the pleura, which may cross midline or adhere to the lateral aspect of the esophagus depending on the degree of periesophageal inflammation. As dissection continues in a clockwise direction anteriorly and to the left, the surgeon must be cognizant of the pericardium, and gently separate it from the esophagus. The surgeon’s left hand then continues around to elevate the left crus away from the esophagus and develop this space. At this point, the anterior vagus nerve should be identified, used as a dissection plane, and swept back toward the esophagus. Thus, the mediastinal dissection is complete, mobilizing the esophagus away from the pleura, aorta, both crura, and maintaining both vagus nerves protected alongside the esophagus.
Mobilization of the fundus
Once the esophagus is freed from the mediastinum, attention is turned to creation of a tension-free wrap. One of the most important principles in creating a “floppy” fundoplication is complete fundic mobilization. To achieve this, the stomach is grasped just off the greater curve on its anterior surface 10–15 cm inferior to the angle of His and superior to the gastroepiploic vessels. The gastrosplenic ligament is placed under tension superolaterally and entered with ultrasonic shears. This is best done by dividing a short gastric vessel close to the stomach wall. The lesser sac is then entered and the camera is oriented to facilitate easy separation of the short gastric vessels close to their insertion on the stomach. The dissection plane is carried cephalad up to the previously mobilized left crus. To complete fundic mobilization the edge of the fundus is retracted medially and anteriorly to visualize and divide high retrogastric vessels and gastropancreatic attachments. The periesophageal attachments to the medial border of the left crus are then dissected away, as was performed on the right side. This joins the two dissection planes, completing circumferential distal esophageal mobilization.
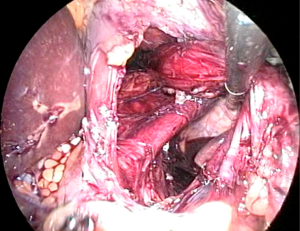
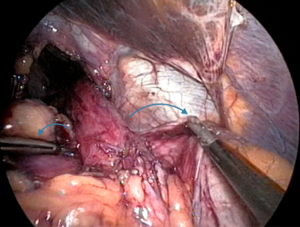
At this point, the stomach and esophagus can be elevated anteriorly to view the retroesophageal space and ensure there are no remaining posterior attachments (Figure 4). With this window created, a Penrose drain is often placed encircling the esophagogastric junction (EGJ) to allow for atraumatic traction for the remainder of the case. We tend to skip this step and instead use the epiphrenic fat pad or fundus itself as a functional handle. With the surgeon maintaining anterior inferior traction on the EGJ to expose the window as visualized from the patient’s right, the assistant carefully inserts a grasper from right to left in this space. The camera view is then modified to allow the surgeon to hand the apex of the fundus to the assistant, who then slowly pulls it back through to the right. Once the fundus is completely pulled through, it is released and observed for tension. If the fundus retracts behind the esophagus back to its native state, fundic mobilization is inadequate and further division of attachments must be performed. If the fundus does not recoil, the surgeon then performs the “shoe shine” maneuver (Figure 5), ensuring the future wrap slides easily and lacks undue twist or redundancy. Similarly, traction must be released on the EGJ, and at least 2.5 cm of tension-free intra-abdominal esophagus should be measured for a successful subsequent fundoplication. If adequate esophageal length is not evident, the surgeon should pursue additional esophageal mobilization or an esophageal lengthening procedure.
Crural closure
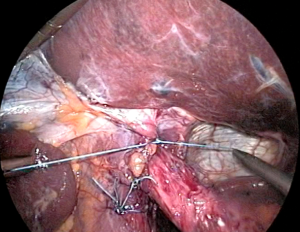
With the EGJ mobilized, the crura can be clearly exposed for re-approximation. The closure proceeds posterior to anterior, recreating the diaphragmatic hiatus back to its native shape as an inverted teardrop. The assistant retracts the EGJ anterior and to the left to allow the surgeon to proceed with intracorporeal suturing. We prefer to use a heavy braided suture on an SH needle in an interrupted fashion. It is important to take large bites and incorporate the intra-abdominal crural fascia, as this is more durable and resists tearing more than suturing the muscle body alone. The first stitch is placed about 1 cm anterior to the crural junction, and proceeds anteriorly, for a total of usually 2–3 posterior crural stitches. We also typically place one or more sutures anterior to the esophagus (Figure 6). Most hiatal hernias recur anteriorly, and these sutures should stimulate scarring in this area, helping to prevent recurrence. The relative number of anterior and posterior sutures varies; the surgeon must assess whether the esophagus is being angulated as the sutures are placed. When finished, the crura should lightly touch the walls of the empty esophagus, with enough slack to allow a 5-mm instrument to be placed between the two.
Fundoplication
At this point in the operation we advocate placing a 56–60 French bougie dilator prior to creating the fundoplication to help prevent narrowing the EGJ. We do not place a dilator before this point as they stiffen the esophagus and hinder access to the space posterior to the EGJ needed for hiatal closure. Once the dilator has been inserted, the wrap can be simulated before suturing by grasping both sides of the fundus and approximating them around the esophagus, gauging tension while doing so.
By simulating the wrap in this manner, a point is chosen on the lateral edge of the fundus on the left to suture to a corresponding point on the edge of the wrapped fundus to the right of the esophagus. Again, we prefer intracorporeal suturing techniques with large gauge, braided permanent suture on an SH needle, taking deep seromuscular bites of the fundus. The first stitch joins fundus to fundus alone, to minimize tension on future sutures. Once this is tied, the position of the wrap may be assessed, as it may be slid proximal or distal on the esophagus or rotated slightly. When this is satisfactory, the next suture may be placed, incorporating the anterior muscularis of the esophagus to the right of the anterior vagus between bites of fundus, securing the wrap to the esophagus without tension. Although care is taken while knotting all these stitches, the one that includes the esophagus is the most important. It is key to take a substantial enough bite of esophagus so that it does not tear easily, but also to avoid taking a full-thickness bite. We generally place three total sutures to secure the wrap, approximately 1 cm apart, to achieve a fundoplication length of about 2.5 cm.
When suturing is completed, the dilator is removed and the wrap is assessed for laxity by passing a 5-mm diameter instrument between the left side of the wrap and esophagus. We re-measure the wrap and also ensure the position is on the distal esophagus rather than proximal stomach. We orient the wrap so that the fundic suture line lays to the right of the anterior vagus nerve, although this varies among centers (Figure 7).
Concluding the operation
The esophageal hiatus and crural closure are assessed once more. The liver retractor is removed and the liver capsule is examined for tears or bleeding. The field is aspirated and examined for hemostasis. Ports are removed under direct visualization. Fascia is closed on ports that result in a fascial defect greater than 5 mm below the costal margin after desufflation of the abdomen.
Tips, tricks, and pitfalls
Ergonomics
The ergonomics of laparoscopic surgery are often undervalued by the novice. Optimizing monitors, ports, and patient positioning in advanced laparoscopic procedures is crucial to minimize not only fatigue and pain during the case (which can adversely affect the technical aspects of the fundoplication), but also to ensure the successful long-term operative career of the surgeon. The goal is to achieve a “neutral” position for the surgeon. Monitors should be placed at or below the surgeon’s eye level. The table should be low enough that the surgeon’s shoulders are not actively flexed. Step stools can be used as an adjunct, but are often not necessary when the patient is in steep reverse Trendelenburg. Elbows should be maintained close to the body as much as possible to avoid rotator cuff strain, and frequent “mini-breaks”, consisting of stretching, refocusing of the eyes, and resetting of the neutral position should be considered throughout the procedure to minimize surgeon discomfort and maximize operative endurance.
Placing ports too low
In the obese patient or an individual with a long torso, it is tempting to use the umbilicus as a landmark or place ports as a proportionate extrapolation of what is pictured as the ideal patient anatomy in the mind’s eye. This can lead to placing the ports too inferiorly on the patient, making the entire operation a struggle to reach the hiatus. Avoid this pitfall by using the measurements from the xiphoid provided.
Dissection of a large hernia sac
To effectively deal with a large hernia sac, the right crus is elevated anteriorly with the surgeon’s left hand as the assistant grasps high on the hernia sac medial to the crus and retracts caudally and to the left. An energy device is used to divide the sac about 1 cm medial to the crural muscle, retaining endoabdominal fascia on the crus. The plane may then be opened between the sac and mediastinum. While the assistant retracts the sac, the surgeon bluntly sweeps the hernia sac medially and inferiorly away from the right pleura. This should be an avascular plane, allowing blunt dissection to be carried anteriorly to the left side of the hiatal orifice. The anterior and posterior vagus nerves should be quickly identified and maintained on the esophagus. They may then be used as planes for dissection, brushing other structures away from them. Dissection to the left side of the aorta posteriorly is also avoided to prevent damaging the left pleura.
Once the hernia sac is reduced, it may have considerable bulk interfering with the operation. We attempt to excise as much of the hernia sac as possible; however, while doing so the surgeon must be vigilant not to injure the vital structures to which the sac is adherent, including the vagus nerves or the true wall of the stomach or esophagus. If the hernia sac to the right of the esophagus is elevated to the right and caudally, the anterior vagus nerve is usually placed on tension, allowing identification. The sac to the left of this nerve can then be elevated and divided. The posterior sac may be dissected in a similar manner, always with direct visualization of the posterior vagus. Leaving some hernia sac is better than damaging the nerves or the tissue at the EGJ.
Inadvertent injury to the pleura
Inadvertent injury to the pleura occurs more often with dissection of a large hiatal hernia and is the result of a tear in the pleura. Insufflating CO2 then creates a capnothorax at insufflation pressure. If this occurs, the anesthetist should be notified, as hypotension and elevated airway pressure may result. Hypotension almost always resolves with reduction in the pneumoperitoneum and elevation of positive pressure ventilation. At the end of the case, if a capnothorax has occurred we typically aspirate the mediastinum through the hiatal closure during desufflation while the anesthetist delivers vital capacity breaths. In cases with extensive scarring, such as in a re-operation, a ball valve effect may allow development of a true tension pneumothorax. In this case, the surgeon should enlarge the pleural hole to create a common cavity. We do not routinely obtain chest X-rays post-operatively in these cases, unless the patient’s physiologic parameters would prompt otherwise.
Injury to the splenic capsule
The fundus of the stomach and the spleen are often very close when taking the most superior short gastric vessels. The pitfall when dissecting this space is to tear the splenic capsule. The surgeon should err toward damaging the gastric wall as opposed to the spleen if a choice must be made, as the fundic dissection line can be mobilized, evaluated, and repaired much easier than the spleen. Many surgeons attempt to push the gastric wall cephalad against the diaphragm, offering inadequate exposure and no room for dissection. A more advantageous technique is to insinuate an atraumatic instrument posterior to the fundic dissection line, open the jaws parallel to the stomach wall, and elevate the fundus anteriorly while turning the jaws slightly in a clockwise manner to retract the fundus in a ventral direction, away from the diaphragm and spleen. This works best when inferolateral traction is exerted on the rest of the stomach by the assistant.
Inadequate intra-abdominal esophageal length
From a laparoscopic perspective, one can easily make the mistake of over-estimating the length of intra-abdominal esophagus. To prevent this, we ensure there is no caudad retraction on the EGJ or tension on the stomach whatsoever, and measure the intra-abdominal esophageal length to 2.5 cm, which corresponds to the fully open jaws of an atraumatic grasper (Figure 8). Other precautions include decreasing the intra-abdominal pressure slightly so the diaphragm is not pushed superiorly. If esophageal length is inadequate, increased caudad retraction of the EGJ may allow more proximal hiatal mobilization of the esophagus up to the inferior pulmonary vein. If the intra-abdominal esophageal length remains less than 2.5 cm after additional mediastinal dissection, an esophageal lengthening procedure will be needed.
Difficulty in placing crural sutures
Poor posterior visualization may be remedied by anterior traction on the EGJ and an angled laparoscope. Decreasing the intra-abdominal pneumoperitoneum pressure in turn diminishes tension on the diaphragm and can help facilitate closure. Difficulty in securely fashioning intracorporeal knots often suggests the closure may be under too much tension. One corrective measure is a relaxing incision at the lateral aspect of the right crus with a mesh patch of the resulting defect. It is also important not to overly tighten the crural closure anteriorly, resulting in esophageal angulation. Crossing and pulling both suture ends after stitch placement and before knotting allows assessment of tissue approximation and tension before committing the suture.
Esophageal perforation while placing dilator
This is a rare occurrence, and can be avoided with appropriate caution. Ensuring the dilators are appropriately malleable and not “expired” is important. Some surgeons encourage scrubbing out to place the dilator themselves. With experienced anesthesia colleagues and frequent communication, we do not feel this time-consuming practice is necessary. The anesthesiologist passes the dilator slowly, calling out the distance of insertion while the surgeon maintains the EGJ in a non-angulated position. Any unusual pressure or difficulty in advancement halts the procedure, as the dilator can also double back on itself and bow out, tearing the esophagus, without seeming to have advanced significantly.
Difficulty with fundoplication sutures
A wrap sutured too tight can lead to dysphagia; one created too loose can lead to both a hiatal hernia and recurrent reflux. This delicate balance may be accomplished with several finer points. The shoe shine maneuver is key to recognize and prevent redundancy of the fundus. We also find that placing the most caudad suture first allows the wrap to be positioned just proximal to the EGJ. Placing the proximal suture first seems to make it easier to mistakenly create the wrap too high on the esophagus, which may allow the distal esophageal mucosa to be exposed to gastric acid. Again, while suturing the fundoplication, bites should be seromuscular, as full thickness suturing may facilitate gastric ulcers at the site of the stitch. Intraoperative endoscopy should demonstrate a “stacked coins” appearance on retroflexed view to confirm a normal Nissen fundoplication. This may be utilized as routine practice or in select cases depending on the appearance of the wrap.
Post-operative management
Postoperatively, we routinely schedule anti-nausea medications with additional forms of anti-emetics available on patient request to prevent early retching and straining of the wrap. We also schedule intravenous anti-inflammatories to diminish the need for narcotics (which in turn may also propagate nausea). We allow patients sips of liquids the evening of the operation, with overnight monitoring and progression to a full liquid diet the following morning. If the patient tolerates subsequent advancement to a mechanical soft diet for lunch, they are discharged soon after. We keep patients on this soft diet for two to four weeks after the operation depending on the extent of initial dysphagia the patient experiences. Retching, vomiting, or undue chest pain in the early postoperative period mandates a water-soluble contrast swallow study. In the case of an early acute herniation, we take the patient immediately back to the operating room for re-repair.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Fernando A. M. Herbella) for the series “Secrets for Successful Laparoscopic Antireflux Surgery” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2017.03.06). The series “Secrets for Successful Laparoscopic Antireflux Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Liebermann-Meffert D. Rudolf Nissen (1896-1981)-perspective. J Gastrointest Surg 2010;14:S58-61. [Crossref] [PubMed]
- Dallemagne B, Weerts JM, Jehaes C, et al. Laparoscopic Nissen fundoplication: preliminary report. Surg Laparosc Endosc 1991;1:138-43. [PubMed]
- Wang YR, Dempsey DT, Richter JE. Trends and perioperative outcomes of inpatient antireflux surgery in the United States, 1993-2006. Dis Esophagus 2011;24:215-23. [Crossref] [PubMed]
- Demeester TR, Johnson LF, Kent AH. Evaluation of current operations for the prevention of gastroesophageal reflux. Ann Surg 1974;180:511-25. [Crossref] [PubMed]
- Deschner BW, Soper NJ. Summary of the technical aspects of Nissen fundoplication. Asvide 2017;4:173. Available online: http://www.asvide.com/articles/1481
Cite this article as: Deschner BW, Soper NJ. Secrets for successful laparoscopic antireflux surgery: surgical technique. Ann Laparosc Endosc Surg 2017;2:82.