
Reduced port surgery and reduced surgical staff surgery for gastric cancer
Introduction
Recently, laparoscopic surgery for the treatment of gastric cancer has spread widely throughout Japan. Initially, a laparoscopy-assisted gastrectomy required about 5 cm mini-laparotomy at the epigastrium for reconstruction after the gastrectomy. Now, several reconstructive techniques for intracorporeal anastomosis during a laparoscopic distal gastrectomy (LDG) have been reported (1,2). Laparoscopic reconstruction methods for use during a laparoscopic total gastrectomy (LTG) and a laparoscopic proximal gastrectomy have also been developed (3-5). Consequently, the required size of the mini-laparotomy has been reduced, and the position of the mini-laparotomy has been transferred from the epigastrium to the umbilicus. Reduced port gastrectomy techniques have been developed to minimize invasiveness by using an umbilical mini-laparotomy more effectively.
Here, we considered the number of surgical staff members required to perform a laparoscopic gastrectomy. In a conventional laparoscopic gastrectomy, three surgical staff members, consisting of an operator, an assistant operator, and a laparoscopist, are necessary. Scheduling three surgical staff members to attend every operation for gastric cancer, can be difficult at facilities performing large numbers of surgical operations. In this respect, a conventional laparoscopic gastrectomy is more disadvantageous than an open gastrectomy, which can be conducted by only two surgical staff members. With this in mind, we designed a new style of laparoscopic gastrectomy that can be conducted by only two surgical staff members, while also adopting the concept of RPS. This new procedure is known as a triple incision laparoscopic distal gastrectomy (TIL-DG); using this procedure, a LTG can be conducted by only two surgical staff members (reduced surgical staff-laparoscopic total gastrectomy: re-LTG).
Methods
Patients
During the period between April 2010 and June 2016, 121 patients (76 men and 45 women) underwent a TIL-DG. The indications for this procedure were as follows: histologically confirmed adenocarcinoma of the stomach; clinical stage I−III (cT1–cT4a, cN0–2) tumor according to the 14th Japanese Classification of Gastric Carcinoma (6); and a tumor location in the lower third or the middle third of the stomach.
During the period between November 2011 and December 2015, 39 patients underwent a LTG that was conducted by two surgical staff members (re-LTG). The indications for this procedure were clinical stage I–III (cT1–T4a, cN0–2) gastric cancer with a location in the upper third of the stomach.
After obtaining adequate informed consent, a TIL-DG and a Re-LTG were performed.
To assess the oncologic safety and feasibility of the TIL-DG and Re-LTG procedures, 59 patients who underwent a conventional LDG between May 2008 and January 2010 and 79 patients who underwent a conventional LTG between February 2010 and October 2014 were retrospectively selected.
Ideas for improving the operative field
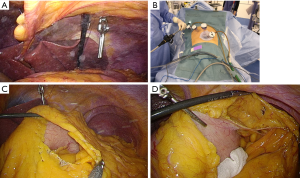
Our procedure is conducted by two surgeons. The assistant operator manipulates forceps and a laparoscope through a multichannel port located at the umbilicus. Thus, one pair of forceps, which was originally manipulated by the assistant operator, was removed, compared with the conventional laparoscopic surgery method. To compensate for this shortage, we developed a novel tool that we called “hanging forceps”. These forceps are based on ready-made detachable laparoscopic forceps (B Braun Aesculap, Germany). The detachable forceps were suspended from the upper abdominal wall by a thread (Figure 1A). After grasping the tissue, the thread was pulled and clamped. The operative field was created by adjusting the grasping point for the hanging forceps and by adjusting the force of the traction on the thread.
Operative procedure of TIL-DG
The patient was placed in a Fowler’s position with his or her legs apart. Initially, a 3-cm incision was made at the umbilicus and a multichannel port, which was created using an EZ access and two 12-mm trocars, was attached to the wound. Thereafter, a pneumoperitoneum was established, and two 5-mm trocars were inserted on the right side of the patient (Figure 1B). The operator performed all the procedures from the right side of the patient using the two 5-mm trocars. The assistant operator stood between the patient’s legs and manipulated a 10-mm flexible scope and rigid forceps through the multichannel port.
The greater omentum was dissected toward the splenic flexure using an ultrasonic scalpel. During these steps, the forceps in the operator’s left hand were inserted into the cavity of the omental bursa and the posterior wall of the stomach was grasped and pulled. This manipulation enabled the omental bursa to be pulled in a line. The other portion of the omental bursa was grasped by the forceps manipulated by the assistant operator and pulled in a counter direction to create a triangulation. We named this method “modified triangulation” (Figure 1C). The left gastroepiploic vessels were divided using a clip and the ultrasonic scalpel. If a suitable surgical field was difficult to create, the hanging forceps were attached to the posterior wall of the stomach (Figure 1D). The lymph nodes along the left gastroepiploic vessels (No. 4Sb) were dissected. Then the greater omentum was dissected toward the hepatic flexure and the pedicle of the right gastroepiploic vessels was hung using the hanging forceps (Figure 2A,B), and the infrapyloric lymph nodes (No. 6) were dissected (Figure 2C).
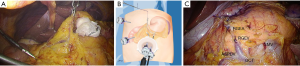
The duodenum was transected using a 60-mm endoscopic linear stapler, which was inserted into the abdominal cavity through the multichannel port. After transecting the duodenum, the right gastric artery was divided at its origin using a ligation and the ultrasonic scalpel; the suprapyloric lymph nodes (No. 5) were then dissected.
The pedicle of the left gastric vessels was pulled in a ventral direction using the hanging forceps (Figure 3A,B), and the lymph nodes along the common hepatic artery (No. 8a) and the celiac artery (No. 9) were dissected using the ultrasonic scalpel; the left gastric vessels were divided at their origins using a ligation, a clip and the ultrasonic scalpel. In cases requiring a D2 lymph node dissection, the lymph nodes along the proper hepatic artery (No. 12a) and the splenic artery (No. 11p) were also dissected (Figure 3C).
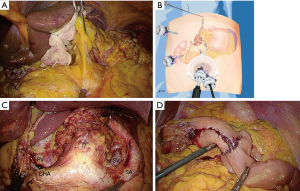
The lymph nodes along the lesser curvature (No. 1 and No. 3) were also dissected.
The distal two-thirds of the stomach were intracorporeally dissected using a 60-mm endoscopic linear stapler. An intraoperative endoscopy was occasionally performed to determine the gastric transection line. The dissected stomach was pulled out through the mini-laparotomy at the umbilical incision.
Reconstruction was performed using the Roux-en-Y method (Figure 3D). The jejuno-jejunal anastomosis was performed extracorporeally, while the gastrojejunal anastomosis was performed laparoscopically using a 60-mm endoscopic linear stapler. The common entry hole of the endoscopic linear stapler was closed laparoscopically using hand suturing (Figure 4).

Operative procedure of LTG conducted by two surgical staff members
After a multichannel port was attached to the umbilicus, the remaining four trocars were placed as follows: two ports were used as bilateral subcostal ports, one was used as a right mid-abdominal port, and one was used as an infra-xiphoid port.
Lymph node dissections along the greater curvature (No. 4as and No. 6), the lesser curvature (No. 1, No. 3 and No. 5), the hepatic artery (No. 8 and No. 12a), the celiac artery (No. 9), and the splenic artery (No. 11p) were performed as for the TIL-DG. During the dissection of the gastrosplenic ligament, the hanging forceps were attached to the posterior wall of the stomach, and the stomach was pulled in a ventral direction to create a better operative field (Figure 5A).
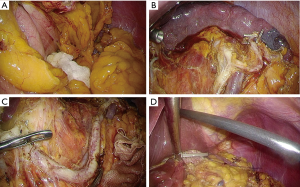
In the case of D2 lymph node dissection, No. 11d and No. 10 lymph node dissection was performed after the removal of the stomach (Figure 5B,C). Reconstruction was performed using the Roux-en-Y method and a circular stapler. An anvil head was attached to the abdominal esophagus using “Endo-PSI” (Figure 5D). The detailed procedure was reported in the Asian Journal of Endoscopic Surgery in 2016 (8) (Figure 6).

Clinical pathway
All the patients received the following clinical pathway for postoperative care. The nasogastric tube was removed in the operation room. Fluid intake was restarted on postoperative day (POD) 1, and a fluid diet was restarted on POD 2. Oral analgesic drugs were administered on POD 1, and epidural catheters were removed on POD 4. The infusion treatment was ended on POD 5, a drainage tube placed in the abdominal cavity was removed on POD 5, and the patient was discharged on POD 7 or POD 8.
Statistical method
Categorical data between the two groups were compared using the χ2 test or the Fisher exact test. Continuous variables were compared using the Student t-test and were expressed as the mean ± standard deviation. The level of significance was set at P<0.05. Statcel3 (The Useful Add-in Forms on Excel, 3rd ed.) was used for the statistical analyses.
Results
The patient characteristics of the TIL-DG group and of the LDG group are shown in Table 1. The mean patient age and the sex ratio were similar for the two groups. The short-term outcomes of both groups are shown in Table 2. Both groups were each classified into two subgroups according to the level of lymph node dissection (D1+ and D2). There were no significant differences in operation time, volume of blood loss, or postoperative hospital stay between the two groups. The number of retrieved lymph nodes in the TIL-DG group was higher than that in the LDG group for each of the subgroups. Intraoperative complications did not occur in either group, and no conversions to open surgery were required. The percentages of postoperative complications were 2.4% for the TIL-DG group and 3.4% for the LDG group. All the patients were treated conservatively. The histopathological findings are listed in Table 3. No significant differences in histopathological findings, including pT, pN, or p-stage, were observed among the subgroups.
Table 1
| Characteristics | TIL-DG group | LDG group | P value |
|---|---|---|---|
| Total | |||
| n | 121 | 59 | |
| Gender (male:female) | 76:45 | 34:25 | 0.50 |
| Age | 66.6±11.2 | 65.1±13.1 | 0.41 |
| Level D1+ | |||
| n | 48 | 35 | |
| Gender (male:female) | 37:11 | 17:18 | 0.007 (<0.05) |
| Age | 70.8±9.5 | 70.1±10.3 | 0.77 |
| Level D2 | |||
| n | 73 | 24 | |
| Gender (male:female) | 39:34 | 17:7 | 0.22 |
| Age | 64.0±11.4 | 57.8±13.4 | 0.03 (<0.05) |
TIL-DG, triple incision laparoscopic distal gastrectomy; LDG, laparoscopic distal gastrectomy.
Table 2
| Variables | TIL-DG group | LDG group | P value |
|---|---|---|---|
| Level D1+ | |||
| n | 48 | 35 | |
| Operation time (min) | 248.8±47.9 | 240.7±62.1 | 0.50 |
| Blood loss (g) | 45.1±51.2 | 70.0±60.5 | 0.04 (<0.05) |
| Number of dissected lymph nodes | 34.2±11.0 | 22.9±13.3 | 0.00006 (<0.05) |
| Postoperative hospital stay | 7.6± 2.1 | 8.7±4.0 | 0.11 |
| Intraoperative complication | 0 | 0 | |
| Postoperative complication | 2 (pseudomonas colitis: 1; pancreatic fistula: 1) | 1 (pancreatic fistula) | |
| Level D2 | |||
| n | 73 | 24 | |
| Operation time (min) | 268.0±49.1 | 262.3±52.6 | 0.62 |
| Blood loss (g) | 43.4±65.6 | 61.5±67.1 | 0.25 |
| Number of dissected lymph nodes | 44.1±16.8 | 31.6±12.5 | 0.001 (<0.05) |
| Intraoperative complication | 0 | 0 | |
| Postoperative complication | 1 (pancreatitis) | 1 (anastomotic bleeding) |
TIL-DG, triple incision laparoscopic distal gastrectomy; LDG, laparoscopic distal gastrectomy.
Table 3
| Variables | TIL-DG group | LDG group | P value |
|---|---|---|---|
| Level D1+ | |||
| n | 48 | 35 | |
| pT (1/2/3/4a) | 39/4/5/0 | 34/0/1/0 | 0.07 |
| pN (0/1/2/3) | 44/2/1/1 | 29/6/0/0 | 0.16 |
| p-stage (1A/1B/2A/2B/3A) | 38/4/3/1/2 | 30/4/0/1/0 | 0.41 |
| Level D2 | |||
| n | 73 | 24 | |
| pT (1/2/3/4a) | 50/8/8/7 | 17/5/2/0 | 0.29 |
| pN (0/1/2/3) | 46/17/5/5 | 15/7/2/0 | 0.58 |
| p-stage (1A/1B/2A/2B/3A/3B/3C) | 41/11/9/5/2/2/3 | 13/6/2/2/1/0/0 | 0.80 |
TIL-DG, triple incision laparoscopic distal gastrectomy; LDG, laparoscopic distal gastrectomy.
The patient characteristics of the re-LTG group and the LTG group are shown in Table 4. The mean patient age and the sex ratio were similar for the two groups.
Table 4
| Variables | Re-LTG group | LTG group | P value |
|---|---|---|---|
| Total | |||
| n | 39 | 79 | |
| Gender (male:female) | 33:6 | 65:14 | 0.75 |
| Age | 66.8±9.2 | 66.4±10.2 | 0.88 |
| Level D1+ | |||
| n | 10 | 35 | |
| Gender (male:female) | 8:2 | 31:4 | 0.48 |
| Age | 70.1±7.9 | 68.1±10.0 | 0.57 |
| Level D2 | |||
| N | 29 | 44 | |
| Gender (male:female) | 25:4 | 34: 10 | 0.34 |
| Age | 65.6±9.5 | 65.1±10.3 | 0.85 |
LTG, laparoscopic total gastrectomy.
The short-term outcomes of both groups are shown in Table 5. The re-LTG group and the LTG group were each classified into two subgroups according to the level of lymph node dissection (D1+ and D2). No significant differences in operation time, volume of blood loss, number of retrieved lymph nodes, or postoperative hospital stay were observed between the two groups. Intraoperative complications did not occur in either group, and no conversions to open surgery were required. The percentages of postoperative complications were 2.6% for the re-LTG group and 5.2% for the LTG group. Although, an anastomotic stenosis required endoscopic balloon dilation, all of the other complications were treated conservatively. The histopathological findings are listed in Table 6. No significant differences in histopathological findings, including pT, pN, or p-stage, were observed among the subgroups.
Table 5
| Variables | Re-LTG group | LTG group | P value |
|---|---|---|---|
| Level D1+ | 10 | 35 | |
| n | |||
| Operation time (min) | 290.4±43.0 | 309.1±49.4 | 0.29 |
| Blood loss (g) | 83.8±90.6 | 88.3±76.0 | 0.88 |
| Number of dissected lymph nodes | 39.9±31.2 | 32.6±9.9 | 0.26 |
| Postoperative hospital stay | 7.7± 1.2 | 9.4 ±4.3 | 0.22 |
| Intraoperative complication | 0 | 0 | |
| Postoperative complication | 0 | 3 (anastomotic stenosis) | |
| Level D2 | |||
| n | 29 | 44 | |
| Operation time (min) | 325.7±44.9 | 329.3±97.0 | 0.85 |
| Blood loss (g) | 87.5±99.3 | 99.8 ±77.0 | 0.56 |
| Number of dissected lymph nodes | 41.7 ±15.8 | 37.7±14.6 | 0.27 |
| Postoperative hospital stay | 8.1± 2.9 | 8.5±3.1 | 0.57 |
| Intraoperative complication | 0 | 0 | |
| Postoperative complication | 1 (pancreatic fistula) | 1 (pancreatic fistula) |
LTG, laparoscopic total gastrectomy.
Table 6
| Variables | Re-LTG group | LTG group | P value |
|---|---|---|---|
| Level D1+ | 10 | 35 | |
| n | |||
| pT (1/2/3/4a) | 8/0/0/2 | 28/3/2/2 | 0.36 |
| pN (0/1/2/3) | 8/1/0/1 | 25/6/3/1 | 0.55 |
| p-stage (1A/1B/2A/2B/3A/3B/3C) | 7/0/1/0/1/0/0/1 | 6/4/0/2/0/1/0 | 1.0 |
| Level D2 | |||
| n | 29 | 44 | |
| pT (1/2/3/4a) | 12/2/10/5 | 22/6/12/4 | 0.52 |
| pN (0/1/2/3) | 17/5/5/2 | 28/6/8/2 | 0.93 |
| p-stage (1A/1B/2A/2B/3A/3B/3C) | 10/2/6/2/3/3/1/2 | 21/5/3/5/6/3/0/1 | 0.22 |
LTG, laparoscopic total gastrectomy.
Discussion
Single-port surgery (SPS) and reduced-port surgery (RPS) for gastroenterological surgery are presently in the spotlight as next-generation laparoscopic surgical techniques. SPS and RPS for gastric cancer have recently been reported in several studies (10-12). We previously reported a “Triple Incision Laparoscopic Distal Gastrectomy” in 2014 (13). In this previous report, the indications for a TIL-DG procedure were limited to c-stage I–II gastric cancer, and 79 patients underwent a TIL-DG; the feasibility of this procedure was then evaluated based on the short-term outcomes. Thereafter, the indications were widened to included c-stage III, and TIL-DG was performed in 42 cases. In the present report, the short-term outcomes of these 121 cases, in total, were compared with those after conventional LDG. No significant differences in short-term outcomes, including the operation time and the intraoperative blood loss, were observed between the TIL-DG group and the conventional LDG group. The average number of retrieved lymph nodes in the TIL-DG group was higher than that in the conventional LDG group. These results, however, do not mean that a TIL-DG is superior to a conventional LDG in terms of oncological safety. The period required to perform TIL-DG differed from that required to perform LADG. The data for the TIL-DG group was also newer than that for the LDG group. Nevertheless, the TIL-DG procedure seems to be, at least, not inferior to a conventional LDG in terms of oncological safety.
The reason that there was no significant difference in short-term outcomes between the TIL-DG group and the LDG group is that the TIL-DG procedure, including lymph node dissection and reconstruction, is almost the same as that for a conventional LDG. The operator’s trocar placements, which are located on the right side of the patient, are the same as those for a conventional LDG. Consequently, the conflict between forceps that occurs during SPS or RPS can be avoided. The assistant operator manipulates forceps and a laparoscope through a multichannel port. Conflict between the forceps and the laparoscope is rare because the angle of approach to the target organ differs between the forceps and the 10-mm flexible laparoscope.
The “hanging forceps” were developed using an endovascular clip in 2010, since a similar commercial product was not available at that time. Recently, a similar tool called an “Internal Organ Retractor” has become commercially available (14). However, our method has the advantage of being able to change the traction power easily by altering the clamping point of the thread.
The “modified triangulation method” is also useful for reduced port surgery. Generally, the “triangulation method”, which involves creating an operating plane bordered by one pair of forceps belonging to the operator and two pairs of forceps belonging to the assistant operator, is considered a basic technique in conventional laparoscopic surgery. During reduced port surgery, however, this method is not easily accomplished. Consequently, the formation of a line of tissue using only one pair of forceps belonging to the operator is an advantage of the “modified triangulation method”.
At our facilities, conventional LDG is performed even now. Beginner operators must start their training by performing a conventional LDG with a D1+ lymphadenectomy for the treatment of early gastric cancer. Consequently, the number of D2 cases was larger than the number of D1+ cases in the TIL-DG group.
The advantages of a TIL-DG include not only cosmesis, but also a reduction in the number of surgical staff members that the procedure requires. This concept was, in turn, applied to LTG. Generally, LTG is not yet widely performed because of the difficulties associated with the mobilization of the stomach and with reconstruction. Especially, a D2 lymphadenectomy during LTG requires complicated techniques. We previously reported the use of an Endo-PSI in 2005 (15), an Endo-PSI-II in 2007 (16), and an Endo-PSI-slim in 2016 for performing an endoscopic esophagojejunal anastomosis using a circular stapler. The feasibility of a LTG for advanced gastric cancer was also reported in 2016 (8).
Although a re-LTG, which is performed by only two surgical staff members, is considered a relatively difficult procedure, these difficulties can be overcome through the effective use of the “hanging forceps”. This device is particularly useful during the mobilization of the greater curvature of the upper third of the stomach. No significant differences in the short-term outcomes of the Re-LTG group and of the LTG group were seen.
During the D2 lymph node dissection in the LTG group, a spleen-preserving hilar lymph node dissection was performed. This procedure is both complex and difficult. In recent years, however, laparoscopic techniques for lymph node dissection have been improved remarkably because of advancements in high-definition laparoscope systems and increasing knowledge of microscopic anatomy. Thus, a lymphadenectomy of the hilum of the spleen can now be performed safely. The difficulty of the re-LTG procedure is similar to that of a conventional LTG. The lymphadenectomy of the hilum of the spleen and along the distal splenic artery is completed after the total gastrectomy. Consequently, a good operative field that includes the anterior surfaces of the pancreas and spleen can be obtained even when performing a re-LTG.
Recently, a randomized controlled trial to evaluate the necessity of a splenectomy during a total gastrectomy for proximal advanced gastric carcinoma (JCOG0110) was completed, and no difference in overall survival was seen between a total gastrectomy with splenectomy and a spleen-preserving total gastrectomy (17). Thus, prophylactic splenectomy with the goal of lymph node dissection at the splenic hilum is likely to be performed less in the future, and LTG with a D2-No. 10 lymphadenectomy is likely to become more common in Japan.
Conclusions
A TIL-DG enables better cosmesis than a conventional LADG. Although, further examination of the long-term outcomes is required to evaluate the benefit of these procedures, both TIL-DG and re-LTG are feasible and safe procedures in terms of their short-term outcomes. The reduction in the number of surgical staff required to perform the procedure is also an attractive feature in Japan, where a shortage of surgeons could become a problem.
Acknowledgments
The authors wish to thank Shigeyuki Sakaguchi for preparing the schema.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Chikara Kunisaki) for the series “Advancement of Single-port, Reduced-port Laparoscopic Gastrectomy for Gastric Cancer” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2017.03.10). The series “Advancement of Single-port, Reduced-port Laparoscopic Gastrectomy for Gastric Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethnical committee and written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kanaya S, Gomi T, Momoi H, et al. Delta-shaped anastomosis in totally laparoscopic Billroth I gastrectomy: new technique of intraabdominal gastroduodenostomy. J Am Coll Surg 2002;195:284-7. [Crossref] [PubMed]
- Motoyama K, Kojima K, Hayashi M, et al. β-Shaped intracorporeal Roux-en-Y reconstruction after totally laparoscopic distal gastrectomy. Gastric Cancer 2014;17:588-93. [Crossref] [PubMed]
- Inaba K, Satoh S, Ishida Y, et al. Overlap method: novel intracorporeal esophagojejunostomy after laparoscopic total gastrectomy. J Am Coll Surg 2010;211:e25-9. [Crossref] [PubMed]
- Kinoshita T, Oshiro T, Ito K, et al. Intracorporeal circular-stapled esophagojejunostomy using hand-sewn purse-string suture after laparoscopic total gastrectomy. Surg Endosc 2010;24:2908-12. [Crossref] [PubMed]
- Nunobe S, Hiki N, Tanimura S, et al. Three-step esophagojejunal anastomosis with atraumatic anvil insertion technique after laparoscopic total gastrectomy. J Gastrointest Surg 2011;15:1520-5. [Crossref] [PubMed]
- Japanese Classification of Gastric Carcinoma March 2010 (The 14th Edition). Japanese Gastric Cancer Association.
- Usui S, Tashiro M, Haruki S, et al. Triple incision distal gastrectomy with D2 lymph node dissection. Asvide 2017;4:166. Available online: http://www.asvide.com/articles/1474
- Usui S, Tashiro M, Haruki S, et al. Spleen preservation versus splenectomy in laparoscopic total gastrectomy with D2 lymphadenectomy for gastric cancer: A comparison of short-term outcomes. Asian J Endosc Surg 2016;9:5-13. [Crossref] [PubMed]
- Usui S, Tashiro M, Haruki S, et al. Laparoscopic total gastrectomy with D2-No. 10 lymph node dissection. Asvide 2017;4:167. Available online: http://www.asvide.com/articles/1475
- Omori T, Oyama T, Akamatsu H, et al. Transumbilical single-incision laparoscopic distal gastrectomy for early gastric cancer. Surg Endosc 2011;25:2400-4. [Crossref] [PubMed]
- Kunisaki C, Makino H, Kimura J, et al. Application of reduced-port laparoscopic total gastrectomy in gastric cancer preserving the pancreas and spleen. Gastric Cancer 2015;18:868-75. [Crossref] [PubMed]
- Kawamura H, Tanioka T, Funakoshi T, et al. Dual-ports laparoscopy-assisted distal gastrectomy compared with conventional laparoscopy-assisted distal gastrectomy. Surg Laparosc Endosc Percutan Tech 2011;21:429-33. [Crossref] [PubMed]
- Usui S, Tashiro M, Haruki S, et al. Triple-incision laparoscopic distal gastrectomy for the resection of gastric cancer: comparison with conventional laparoscopy-assisted distal gastrectomy. Asian J Endosc Surg 2014;7:197-205. [Crossref] [PubMed]
- Paik ES, Lee YY, Roh CR, et al. Mature Cystic Teratoma Is a Good Indication for LESS Approach: Initial Experience of an Internal Organ Retractor (IOR) device or Barbed Suture for LESS Cystectomy. J Minim Invasive Gynecol 2015;22:S224. [Crossref] [PubMed]
- Usui S, Ito K, Hiranuma S, et al. Hand-assisted laparoscopic esophagojejunostomy using newly developed purse-string suture instrument "Endo-PSI". Surg Laparosc Endosc Percutan Tech 2007;17:107-10. [Crossref] [PubMed]
- Usui S, Nagai K, Hiranuma S, et al. Laparoscopy-assisted esophagoenteral anastomosis using endoscopic purse-string suture instrument "Endo-PSI (II)" and circular stapler. Gastric Cancer 2008;11:233-7. [Crossref] [PubMed]
- Sano T, Sasako M, Mizusawa J, et al. Randomized Controlled Trial to Evaluate Splenectomy in Total Gastrectomy for Proximal Gastric Carcinoma. Ann Surg 2017;265:277-83. [Crossref] [PubMed]
Cite this article as: Usui S, Tashiro M, Haruki S, Takiguchi N. Reduced port surgery and reduced surgical staff surgery for gastric cancer. Ann Laparosc Endosc Surg 2017;2:75.


