
Advancement of single-port and reduced-port laparoscopic gastrectomy for gastric cancer: a systemic review
Introduction
Laparoscopic gastrectomy was first described in 1994 by Kitano (1). As accumulation of laparoscopic experiences and development of innovative instrument, laparoscopic gastrectomy has become widely accepted as a standard alternative treatment for early stage gastric cancer. Patients who have undergone laparoscopic gastrectomy have shown less postoperative pain, shorter hospital stays, better cosmetic results and similar oncological outcomes compared to patients who have undergone open gastrectomy (2-7). Originally, laparoscopic gastrectomy was performed using multiple ports and required a mini-laparotomy for extracorporeal anastomosis and specimen extraction. Recently, further operative modalities have been introduced to minimize the invasiveness of gastrectomy for better quality of life in patients with gastric cancer: reduced port laparoscopy and single port laparoscopy.
Single-port surgery has been reported as a feasible procedure for several abdominal operations, such as cholecystectomy (8), appendectomy (9), colectomy (10), hysterectomy (11), and urologic surgery (12). For gastric cancer, Omori et al. first introduced modified single port laparoscopic gastrectomy (SPLG) using one additional port for liver traction in 2011 (13). To date, a few reports have described that SPLG decreased postoperative pain in patients, provided the greatest cosmetic benefit for concealing the surgical scar within the umbilicus, and showed similar surgical safety to the conventional procedure (14-16). However, because laparoscopic gastrectomy with a systematic lymph node (LN) dissection is complex and has a steep learning curve (17), it has not been as widely adopted as other organ resections. SPLG is still not a well-established technique, and is rarely practiced. Moreover, single incision laparoscopic surgery is associated with increased levels of surgeon fatigue and frustration (18). Therefore, there is no consensus regarding the feasibility of using this technique compared to the conventional laparoscopic approach.
Because of the limitations of SPLG, reduced port laparoscopic gastrectomy (RPLG) had been first reported by Kunisaki et al. in 2012 (19). It is similar to the modified SPLG: The acting port and scope are inserted through the umbilicus using the same multi-channel port. RPLG enables surgeons to overcome some limitations of SPLG including interference of instruments during the procedure. Several studies have reported that RPLG showed similar oncologic safety, including the number of dissected LNs, as well as less postoperative pain and better cosmetic results compared to conventional laparoscopic gastrectomy (CLG) (20,21). RPLG was considered as extension of SPLG because it was sometimes performed using a single incision port with acting ports added (22).
The aim of this study was to review RPLG and SPLG as advanced surgical techniques for patients with gastric cancer in terms of safety and efficacy, and to suggest future directions.
Methods
Search strategies
This systematic review was designed based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting criteria followed by systematic review and meta-analysis. The electronic databases MEDLINE/PubMed and EMBASE/Ovid were searched using pre-specified terms (entries searched were published between January 1990 and November 2016). Reference lists of relevant articles and ongoing trial databases were also searched. Search keywords used were: gastrectomy; gastric cancer; laparoscopy, laparoscopic; reduced port; single port, single incision, single site, single, and one. Studies included were clinical trials except case reports of patients with gastric cancer undergoing RPLG or SPLG.
Inclusion and exclusion criteria
Inclusion criteria were (I) clinical studies that reported RPLG; (II) clinical studies that reported SPLG; (III) clinical studies that compared RPLG to SPLG; or (IV) clinical studies of patients with primary gastric cancer undergoing RPLG or SPLG. Duplicate publications, publications not written in English, and publications which did not provide sufficient data were excluded.
Study review and analysis
Two reviewers (JH Lee and SM Kim) independently assessed each trial. Statistical analysis could not be performed due to the small number of trials. Only one study conducted comparison of SPLG and RPLG (14); all others compared SPLG or RPLC to conventional laparoscopic approaches. Only pure SPLG without the use of any additional ports has regarded as SPLG; all other procedures were considered to be RPLG.
Results
Search results
The electronic search yielded 45 distinct titles up to November 2016, 37 of which appeared potentially relevant and were retrieved, but 27 of which failed to meet the inclusion criteria. A total of 10 clinical studies investigating RPLG or SPLG were included (Figure 1). There were seven studies (19-25) and two case reports for RPLG. For SPLG, three studies (14-16) and seven case reports had been published.
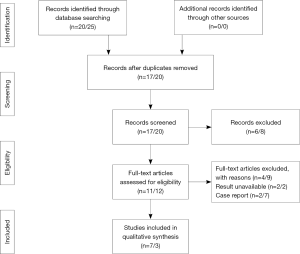
Description of included trials
Study characteristics for RPLG and SPLG are summarized in Tables 1 and 2, respectively. Seven studies for RPLG and three studies for SPLG included in this review were published between 2012 and 2016. The patient population in these studies underwent their operations between the years of 2009 and 2015. Included studies were performed in multiple countries and with single or multiple surgeon study designs.
Table 1
| Author | Year | No. | Male | Female | Age (y) | BMI (kg/m2) | Retrieved LN | LOS (d) | First diet (d) | Pain assessment | Operation time (min) | Blood loss (mL) | Complication (rate) | Trocar |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kunisaki et al. | 2012 | 20 | 8 | 12 | 67.4±11.1 | 21.1±2.1 | 36.8±16.8 | 15.6±7.9 | 5.1±1.1 | No significant | 278.8±36.2* | 66.0±46.4 | 3 (15.0) | SIL +2 |
| Kawamura et al. | 2013 | 30 | 19 | 11 | 69.2±11.4 | 22.3±3.6 | 39.5±28.5 | Not be examined | 3.5±0.5 | No significant | 178.2±46.5* | 61.8±125.1 | 0 | SIL +1 |
| Usui et al. | 2014 | 76 | 47 | 29 | 66.8±10.3 | 22.1±2.9 | 35.3/43.9* | 7.5±1.6 | Not be examined | Not be examined | 247.8±54.9 | 48.8±56.7 | 1 (1.3) | SIL +2 |
| Kashiwagi et al. | 2015 | 10 | 6 | 4 | 68.1±11 | 21.4±1.91 | 16.1±8.9 | 8.1±1.5 | 3.4±1.1 | No significant | 266.9±38.3 | 37.8±56.8 | 0 | SILS +1 |
| Kim et al. | 2015 | 102 | 63 | 39 | 52(29-84) | 23.4±2.8* | 36 [17–76] | 7 [7–23] | Not be examined | No significant | 121.1±19.3* | 91.4±68.4 | 16 (15.7) | 3 ports |
| Seo et al. | 2016 | 97 | 70 | 27 | 63.5±11.1 | 24.5±3.6 | 35.3±14.7 | 9.3±6.8 | 5.0±2.8 | Not be examined | 187.5±67.7* | 172.2±260.9 | 9 (9.3) | 3 ports |
| Jeong et al. | 2016 | 49 | 38 | 11 | 61.1±11.4 | 23.6±3.2 | 43±17 | 8.6±8.3 | 2.2±3.9 | Not be examined | 147±29* | 49±44 | 2 (4.1) | 3 ports |
Data are mean ± standard deviation or median value (range) or number (percentage). *, P<0.05 compared to conventional laparoscopic gastrectomy. RPLG, reduced port laparoscopic gastrectomy; BMI, body mass index; LN, lymph node; LOS, length of hospital stay.
Table 2
| Author | Year | No | Male | Female | Age (y) | BMI (kg/m2) | Retrieved LN | LOS (d) | First diet (d) | Pain assessment | Operation time (min) | Blood loss (mL) | Incision length (cm) | Complication (rate) | Ports | Camera |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ahn et al. | 2014 | 50 | 33 | 17 | 60.7±11.9 | 23.0±3.3 | 51.7±16.3 | 5.98±1.74 | 3.33±0.97 | VAS score POD #0, #1 significant* | 144.5±35.4 | 50.5±31.5* | 2.5 | 6 (12.0) | Glove | Rigid |
| Omori et al. | 2016 | 50 | 28 | 22 | 64.5±11.2 | 22.7±3.1 | 60.8±23 | 9.7±6.4 | 3.3±1 | Significant less analgesics* | 261.4±52.7 | 44.8±97.6* | 2.5–3 | 4 (8.0) | EZ access | Flexible |
| Kim et al. | 2016 | 48 | 22$ | 26$ | 53.5 [33–80] | 21.1±2.1$ | 35.5 [16–67] | 7 [7–24] | Not be examined | No significant different | 135.3±18.8 | 101.1±78.5 | 3–3.5 | 10 (20.8) | Handmade | Flexible |
Data are mean ± standard deviation or median value (range) or number (percentage). *, P<0.05 compared to conventional laparoscopic gastrectomy; $, P<0.05 compared to reduced. SPLG, single port laparoscopic gastrectomy; BMI, body mass index; LN, lymph node; LOS, length of hospital stay; VAS, visual analogue scale; POD, postoperative.
Patient characteristics
A total of 532 patients with gastric cancer were included in these studies: 384 who underwent RPLG, 148 who underwent SPLG. The mean age was from 52 to 68 years, and there was no significant difference in the mean age between RPLG or SPLG and CLG patients. Two studies [one (19) for RPLG and the other (14) for SPLG] showed opposite male to female ratios. For SPLG, two studies reported no significant difference in the number of patients from each gender compared to CLG patients (14,15). One author reported significantly lower body mass index (BMI) in patients who underwent RPLG or SPLG (14,20), while all others reported that patients who had RPLG or SPLG had similar BMIs compared to patients who had CLG. Four studies (19,21-23) out of seven studies for RPLG and all three studies for SPLG reported a mean BMI of less than 23.0 in patients. Japanese patients tend to show lower BMI than Korean patients.
Perioperative outcomes
No patients underwent conversion to open surgery, and only one patient who underwent SPLG (15) initially converted to CLG. Most studies showed a similar number of retrieved LNs in patients who underwent either RPLG or SPLG compared with CLG, although one study (23) reported RPLG resulted in significantly more LN dissections compared to CLG.
Interestingly, as SPLG had a similar mean operating time compared to CLG, operating times were significantly shorter than CLG in three RPLG studies (20,21,24) compared to CVG. Two studies (15,16) for SPLG reported lower blood loss during the operation, and there was no significant difference in blood loss during the operation between RPLG and CLG.
For the length of incision, studies using a single incision port reported from 2.5 to 3.5 cm at the start of the operation. Three studies (20,24,25) using three ports, including 5 or 12 mm trocars, had similar incision lengths required to remove the specimen. For all studies involving RPLG or SPLG, there was no reported significant difference in the length of hospital stay, recovery of bowel movement including time to first flatus or time to first meal, or presence of postoperative complications compared to CLG.
Postoperative pain
Postoperative pain was measured by most included studies, but there were some discrepancies in the type of pain recorded. Some of the trials reported on analgesic use postoperatively as a surrogate measure of pain (20-22). Moreover, there were widely varying analgesic regimens (timing, type of analgesia, and method of administration) among study groups. There were four studies (19-22) that evaluated postoperative pain among the seven studies for RPLG, and no significant differences in postoperative pain measures were observed between the two laparoscopic approaches. Two studies (15,16) reported that patients who had SPLG showed lower postoperative pain compared to patients who had CLG. Ahn et al. reported pain scores for patients who had SPLG or CLG across several postoperative days, and showed a linear decrease in pain with no significant difference beyond postoperative 1st day (16).
Surgical techniques
There was no consensus regarding the patient’s position (lithotomy vs. supine) or type of scope used (flexible vs. rigid) among surgical groups. Two groups (15,16) used a commercial single incision port and one group (14) used a handmade port for SPLG (Figure 2A). In RPLG, four groups (19,21-23) used a single port consisted of multiple trocars which can be used for SPLG, together with additional acting trocars (Figure 2B) while other groups used three trocars which is also used in CVG (Figure 2C).

Some investigators introduced simple surgical technical tips to make RPLG comfortable. Kim et al. (20) suggested that the camera is inserted into the left lateral port among the three port placements: right lateral, umbilicus, and left lateral side for RPLG. This approach provided a better operating view than the view from the umbilicus port during dissection of the suprapancreatic area, including number 5, 8, and 11 LN areas (Figure 3). The left lateral view method could be a convenient solution to this limited view, especially in patients with a redundant falciform ligament.
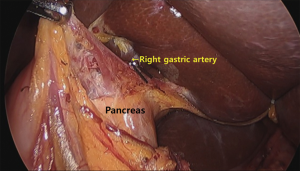
The use of a flexible scope provided several advantages, and was therefore used by several surgical teams for SPLG (14,15). The ability of bend the scope provided a better operating view and reduced instrument collisions. In addition, if the camera was located on the right side of the patient and bent to the left side for a better view, the right gastric artery was well identified when the supraduodenal vessels and tissues were dissected from the anterior side of the stomach.
Kim et al. (14) performed a transverse umbilical incision instead of a vertical umbilical incision to overcome a poor operation field and limited intra-abdominal space. The transverse umbilical incision can give a wider space in which to manipulate instruments, and prevents clashing of instruments. What is more, only a faint scar remained after several months from the surgery, because the direction of scarring matched that of the skin folds.
Ahn et al. (16) introduced the curved grasper which was more useful in handling tissue in the suprapancreatic LN dissection compared to the straight devices, if working points of two devices were too close.
Omori et al. (26) performed an intracorporeal triangular anastomotic technique (INTACT) characterized by a true end-to-end anastomosis, in contrast to the delta-shaped anastomosis made with RPLG and SPLG. Each arm of the linear stapler was inserted through holes in the remnant stomach and the duodenum, and then both ends were stitched together with the stapler. This first staple line would be at the bottom of the triangular anastomosis. The second and third lines were created by closure of the common hole and simultaneous removal of three staple lines (from stumps of the stomach and duodenum and the ventral side of the first anastomosis) using two linear staplers, respectively. This technique does not require additional mobilization and rotation of the stomach and duodenum during anastomosis. Thus, it may be applicable for patients with a relatively small remnant stomach.
Several kinds of thin forceps with a diameter of 2–3 mm, such as the MiniLap (Stryker, Kalamazoo, MI, USA) and the BJneedle (Nichion, Funabashi, Japan), have been developed. These forceps and a thin trocar could avoid conflict of instruments and result in better cosmetic results (23). A surgical nylon ligature with a straight needle proposed by Kashiwagi et al. could contribute to maintaining the visual field by lifting up the stomach without any additional surgical instruments (22). The lithotomy position of the patient might extend the range of motion of the operator when standing between the patient’s legs (15,16,21-23,25).
The use of gravity was recommended as a solution for the absence of assistant association with RPLG and SPLG (20), resulted in a falling away of the pancreas, making dissection around the celiac area easy.
Discussion
This study aimed to review the benefits and efficacy of SPLG and RPLG by analyzing seven studies for RPLG and three studies for SPLG published until the present. RPLG and SPLG showed no significant differences in the number of retrieved LNs, length of hospital stay, postoperative diet course and the presence of postoperative complications compared to CLG. There was no consistency about the effect of RPLG and SPLG on the operating time and postoperative pain.
Several studies showed a selection bias in terms of significantly different BMIs or opposite male-to-female ratios in the RPLG or SPLG group compared to the CLG group. RPLG and SPLG are novel procedures which are performed in situations involving restricted working space and instruments, so patients might be selected very carefully in the initial period. Less obese or female patients would be preferred by most operators as well as by experienced surgeons. In patients with low BMI, RPLG might be a better surgical option than CLG because in those patients, working ports of the assistant and the operator might interfere in movements each other during CLG.
There was controversy regarding the operating time in RPLG and SPLG. Considering these operations are performed without any assistant, it is natural that the operating time might be longer in RPLG and SPLG compared with CLG. However, only three studies (20,21,24) for RPLG reported significantly shorter operating time, while two studies (22,23) for RPLG and all studies for SPLG showed similar operating times compared to CLG. Just one study (19) for RPLG had a longer operating time than CLG, as expected. The reason for these disparities might be related to operator experience. Although RPLG and SPLG without additional help for traction would be a challenge to most surgeons, experienced laparoscopic surgeons tried to this type of approaches and they might perform more carefully than usual. This could also be the reason for the similar pattern between SPLG or RPLG and CLG in terms of the number of retrieved LNs and blood loss during the operation.
Laparoscopic gastrectomy is a complicated technique compared to open gastrectomy, and is associated with a steeper surgical learning curve. Kunisaki et al. found that experiencing 20 cases of laparoscopy assisted distal gastrectomy (LADG) was sufficient to achieve stable surgical outcomes including appropriate operation time and reduced blood loss (17). Moreover, it seems necessary for the surgeon to have sufficient experience in open gastrectomy before transition to the use of laparoscopic gastrectomy. In this review, three studies dealt with the learning curve: Usui et al. (23) and Kim et al. (20) reported no significant learning curve between RPLG and CVG, and An et al. (16) suggested the learning curve associated with SPLG could be overcome after a surgeon had performed 33 cases. However, the number of enrolled patients was too small to conclusively analyze the learning curve, and these reports came from skillful surgeons who had experienced a sufficient number of CLG cases prior to attempting SPLG or RPLG, as mentioned above. Further study of RPLG and SPLG should reveal details of the expected learning curve in the same way as when laparoscopic gastrectomy was firstly introduced following open gastrectomy. And SPLG which is more technically demanding due to the ergonomics of the crossing instruments may require a longer learning period compared to RPLG.
There was little consensus in the literature on which technique was superior in reducing post-operative pain. Although theoretically single port laparoscopic surgery reduces postoperative pain and improves postoperative recovery because of reduced trauma to the abdominal muscles and the parietal peritoneum compared with conventional laparoscopic surgery, four of seven studies on RPLG (19-22) and one of three studies on SPLG (14) had reported no significant difference in postoperative pain compared to CLG. Incisional pain might be dependent on various factors, including the number of ports, length of the incision, and other variable individual characteristics. The study conducted by Carter et al. (27) measured and reported the fascia incision size for appendectomy and demonstrated that the single port laparoscopy group experienced significantly more postoperative pain than conventional laparoscopic appendectomy. In laparoscopic gastrectomy, CLG and RPLG had the same umbilical incision for specimen retrieval; SPLG also had an umbilical incision for insertion of a single port. The overall length of fascial incision was actually from 2.5 to 3.5 cm.
Both RPLG and SPLG can be performed using newer instruments compared to conventional methods, and advanced instruments make the operation easier, especially for SPLG. Ahn et al. (16) reported that the specific commercial glove port (Nelis, Seoul, Korea) was more convenient and permitted each valve to have 2 to 12 mm instruments inserted with no air leakage. Kim et al. (14) suggested that the flexible scope provided a better operating view compared to the rigid scope. Further advanced instruments can overcome the technical limitations of SPLG. Robotic surgery, which provides more wide and fine motion by use of multiple joints, could be a solution for these limitations. In fact, single-port devices for robot-assisted surgery have already been developed, and these devices are expected to improve the ease with which single-incision operations are performed (28,29).
Some studies involved operations performed by a single-surgeon, while others employed a multi-surgeon design. This difference in study design most likely led to varying experience levels between the operating surgeons from the compared studies, which could have skewed the outcomes in terms of operating time and the number of retrieved LNs. The present review therefore has a methodological limitation, as it was based on the collation and comparison of data and outcomes from several heterogeneous studies.
Standardizing these techniques and shortening the learning curve associated with them are important issues to be addressed in the future. Additionally, the potentially higher cost of these new techniques should be taken into consideration when adopting them into existing healthcare systems. None of the trials performed an economic analysis to determine the financial costs or benefits aspect of RPLG and SPLG. The use of special ports in SPLG will need to be evaluated against the cosmetics and the quality of life to determine if SPLG is an overall more cost-effective procedure than CLG. More objective parameters for postoperative pain and cosmetic result are also needed to clarify the potential benefit of RPLG and SPLG. Finally, the incidence of umbilical port hernia in the larger incision made during SPLG should be checked with long-term follow up.
In conclusion, RPLG and SPLG are noteworthy as less invasive approaches with acceptable postoperative outcomes compared to CLG. However, these approaches are just extensions of CLG, which can be performed with the same instruments, and may not represent the final type of gastrectomy used for treatment of gastric cancer. RPLG or SPLG might be considered as a bridge technique from CLG to robotic single port surgery or natural orifice transluminal endoscopic surgery (NOTES).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Chikara Kunisaki) for the series “Advancement of Single-port, Reduced-port Laparoscopic Gastrectomy for Gastric Cancer” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2017.03.02). The series “Advancement of Single-port, Reduced-port Laparoscopic Gastrectomy for Gastric Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kitano S, Iso Y, Moriyama M, et al. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc 1994;4:146-8. [PubMed]
- Lee JH, Han HS, Lee JH. A prospective randomized study comparing open vs laparoscopy-assisted distal gastrectomy in early gastric cancer: early results. Surg Endosc 2005;19:168-73. [Crossref] [PubMed]
- Kim YW, Baik YH, Yun YH, et al. Improved quality of life outcomes after laparoscopy-assisted distal gastrectomy for early gastric cancer: results of a prospective randomized clinical trial. Ann Surg 2008;248:721-7. [Crossref] [PubMed]
- Huscher CG, Mingoli A, Sgarzini G, et al. Laparoscopic versus open subtotal gastrectomy for distal gastric cancer: five-year results of a randomized prospective trial. Ann Surg 2005;241:232-7. [Crossref] [PubMed]
- Kim HH, Han SU, Kim MC, et al. Prospective randomized controlled trial (phase III) to comparing laparoscopic distal gastrectomy with open distal gastrectomy for gastric adenocarcinoma (KLASS 01). J Korean Surg Soc 2013;84:123-30. [Crossref] [PubMed]
- Kitano S, Shiraishi N, Uyama I, et al. A multicenter study on oncologic outcome of laparoscopic gastrectomy for early cancer in Japan. Ann Surg 2007;245:68-72. [Crossref] [PubMed]
- Lee SW, Nomura E, Bouras G, et al. Long-term oncologic outcomes from laparoscopic gastrectomy for gastric cancer: a single-center experience of 601 consecutive resections. J Am Coll Surg 2010;211:33-40. [Crossref] [PubMed]
- Bresadola F, Pasqualucci A, Donini A, et al. Elective transumbilical compared with standard laparoscopic cholecystectomy. Eur J Surg 1999;165:29-34. [Crossref] [PubMed]
- Rispoli G, Armellino MF, Esposito C. One-trocar appendectomy. Surg Endosc 2002;16:833-5. [Crossref] [PubMed]
- Remzi FH, Kirat HT, Kaouk JH, et al. Single-port laparoscopy in colorectal surgery. Colorectal Dis 2008;10:823-6. [Crossref] [PubMed]
- Fanfani F, Fagotti A, Gagliardi ML, et al. Minilaparoscopic versus single-port total hysterectomy: a randomized trial. J Minim Invasive Gynecol 2013;20:192-7. [Crossref] [PubMed]
- Kaouk JH, Haber GP, Goel RK, et al. Single-port laparoscopic surgery in urology: initial experience. Urology 2008;71:3-6. [Crossref] [PubMed]
- Omori T, Oyama T, Akamatsu H, et al. Transumbilical single-incision laparoscopic distal gastrectomy for early gastric cancer. Surg Endosc 2011;25:2400-4. [Crossref] [PubMed]
- Kim SM, Ha MH, Seo JE, et al. Comparison of single-port and reduced-port totally laparoscopic distal gastrectomy for patients with early gastric cancer. Surg Endosc 2016;30:3950-7. [Crossref] [PubMed]
- Omori T, Fujiwara Y, Moon J, et al. Comparison of Single-Incision and Conventional Multi-Port Laparoscopic Distal Gastrectomy with D2 Lymph Node Dissection for Gastric Cancer: A Propensity Score-Matched Analysis. Ann Surg Oncol 2016;23:817-24. [Crossref] [PubMed]
- Ahn SH, Son SY, Jung DH, et al. Pure single-port laparoscopic distal gastrectomy for early gastric cancer: comparative study with multi-port laparoscopic distal gastrectomy. J Am Coll Surg 2014;219:933-43. [Crossref] [PubMed]
- Kunisaki C, Makino H, Yamamoto N, et al. Learning curve for laparoscopy-assisted distal gastrectomy with regional lymph node dissection for early gastric cancer. Surg Laparosc Endosc Percutan Tech 2008;18:236-41. [Crossref] [PubMed]
- Koca D, Yildiz S, Soyupek F, et al. Physical and mental workload in single-incision laparoscopic surgery and conventional laparoscopy. Surg Innov 2015;22:294-302. [Crossref] [PubMed]
- Kunisaki C, Ono HA, Oshima T, et al. Relevance of reduced-port laparoscopic distal gastrectomy for gastric cancer: a pilot study. Dig Surg 2012;29:261-8. [Crossref] [PubMed]
- Kim SM, Ha MH, Seo JE, et al. Comparison of Reduced Port Totally Laparoscopic Distal Gastrectomy (Duet TLDG) and Conventional Laparoscopic-Assisted Distal Gastrectomy. Ann Surg Oncol 2015;22:2567-72. [Crossref] [PubMed]
- Kawamura H, Tanioka T, Shibuya K, et al. Comparison of the invasiveness between reduced-port laparoscopy-assisted distal gastrectomy and conventional laparoscopy-assisted distal gastrectomy. Int Surg 2013;98:247-53. [Crossref] [PubMed]
- Kashiwagi H, Kumagai K, Monma E, et al. Dual-port distal gastrectomy for the early gastric cancer. Surg Endosc 2015;29:1321-6. [Crossref] [PubMed]
- Usui S, Tashiro M, Haruki S, et al. Triple-incision laparoscopic distal gastrectomy for the resection of gastric cancer: comparison with conventional laparoscopy-assisted distal gastrectomy. Asian J Endosc Surg 2014;7:197-205. [Crossref] [PubMed]
- Seo HS, Lee HH. Is the 5-ports approach necessary in laparoscopic gastrectomy? Feasibility of reduced-port totally laparoscopic gastrectomy for the treatment of gastric cancer: A Prospective Cohort Study. Int J Surg 2016;29:118-22. [Crossref] [PubMed]
- Jeong O, Park YK, Ryu SY. Early experience of duet laparoscopic distal gastrectomy (duet-LDG) using three abdominal ports for gastric carcinoma: surgical technique and comparison with conventional laparoscopic distal gastrectomy. Surg Endosc 2016;30:3559-66. [Crossref] [PubMed]
- Omori T, Masuzawa T, Akamatsu H, et al. A simple and safe method for Billroth I reconstruction in single-incision laparoscopic gastrectomy using a novel intracorporeal triangular anastomotic technique. J Gastrointest Surg 2014;18:613-6. [Crossref] [PubMed]
- Carter JT, Kaplan JA, Nguyen JN, et al. A prospective, randomized controlled trial of single-incision laparoscopic vs conventional 3-port laparoscopic appendectomy for treatment of acute appendicitis. J Am Coll Surg 2014;218:950-9. [Crossref] [PubMed]
- Petroni G, Niccolini M, Caccavaro S, et al. A novel robotic system for single-port laparoscopic surgery: preliminary experience. Surg Endosc 2013;27:1932-7. [Crossref] [PubMed]
- Horise Y, Nishikawa A, Sekimoto M, et al. Development and evaluation of a master-slave robot system for single-incision laparoscopic surgery. Int J Comput Assist Radiol Surg 2012;7:289-96. [Crossref] [PubMed]
Cite this article as: Kim SM, Lee JH. Advancement of single-port and reduced-port laparoscopic gastrectomy for gastric cancer: a systemic review. Ann Laparosc Endosc Surg 2017;2:71.


